Abstract
Diabetes Insipidus (DI) is a heterogeneous clinical syndrome of disturbance in water balance, characterized by polyuria (urine output > 4 ml/kg/hr), polydypsia (water intake > 2 L/m2/d) and failure to thrive. In children, Nephrogenic DI (NDI) is more common than Central DI (CDI), and is often acquired. The signs and symptoms vary with etiology, age at presentation and mode of onset. Neonates and infants with NDI are severely affected and difficult to treat. Diagnosis is based on the presence of high plasma osmolality and low urinary osmolality with significant water diuresis. Water deprivation test with vasopressin challenge, though has limitations, is done to differentiate NDI and CDI and diagnose their partial forms. Measurement of urinary aquaporin 2 and serum copeptin levels are being studied and show promising diagnostic potential. Magnetic Resonance Imaging (MRI) pituitary helps in the etiological diagnosis of CDI, absence of posterior pituitary bright signal being the pathognomic sign. If pituitary stalk thickening of < 2 mm is present, these children need to be monitored for evolving lesion. Neonates and young infants are better managed with fluids alone. Older children with CDI are treated with desmopressin. The oral form is safe, highly effective, with more flexibility of dosing and has largely replaced the intranasal form. In NDI besides treatment of the underlying cause, use of high calorie low solute diet and drugs to ameliorate water excretion (thiazide, amelioride, indomethacin) are useful. Children with NDI however well treated, remain short and have mental retardation on follow up.
Keywords: Diabetes insipidus, polydipsia, polyuria
INTRODUCTION
Regulation of water intake and excretion helps maintain extracellular fluid tonicity, within a narrow range, which is crucial for proper cell functions. Maintenance of water balance is primarily dependent on an intact thirst mechanism, vasopressin synthesis and renal tubular responsiveness to vasopressin action.[1] Diabetes insipidus (DI) is a heterogeneous clinical syndrome of disturbance in water balance characterized by the passage of large volumes of dilute urine and the presence of an inordinate thirst. In children three pathophysiologic mechanisms give rise to polydypsia and polyuria:
Central (vasopressin sensitive, hypothalamic, neurogenic) DI caused by defective vasopressin synthesis and/or secretion.
Nephrogenic (vasopressin resistant) DI caused by defective renal tubular response to vasopressin action.
Primary polydypsia due to compulsive water drinking (psychogenic) or defective thirst mechanism (dipsogenic).
In children, Nephrogenic DI (NDI) is more common than Central DI (CDI) and is most often acquired.[2] X linked NDI accounts for 90% of all the congenital forms of NDI.[1] The various causes of DI are shown in Table 1.
Table 1.
Etiology of diabetes insipidus in children

APPROACH TO DIAGNOSIS
Clinical features
Polyuria, defined as quantified urine output of more than 4 ml/kg/hr in children (more than 6 ml/kg/hr in neonates) and polydypsia, defined as water intake of more than 2 L/m2/d (or more than 5 L/d) and failure to thrive or growth retardation are essential features of DI.[3] Although the signs and symptoms of DI vary with the etiology, the loss of excess of free water, excessive thirst, dehydration, and hypernatremia manifests differently in different age groups depending on their ability to replenish water. Infants may present with a vigorous suck, vomiting, recurrent episodes of fever without an apparent cause, excessive crying, irritability, weight loss, constipation, and excessively wet diapers. Younger children often manifest with primary enuresis and difficulty in toilet training. Older children characteristically have high urine output and nocturia leading to disturbed sleep and easy fatigability.
Polydypsia may be intense or uncontrollable and may involve craving for ice. Anorexia due to preference of water over food results in loss of weight and failure to thrive. Irritability, fatigue due to disturbed sleep and affection of linear growth is seen in all age groups. Seizures due to severe hypernatremia, hyperosmolar dehydration and potential hypoxia may lead to neurological sequel in the form of intracranial bleed and developmental delay. Death due to hypovolemic shock or hypernatremic seizure has been reported.[1,3,4] The age at presentation and onset of polyuria and polydypsia varies [Table 2].
Table 2.
Clinical characteristics of patient presenting with centran and nephrogenic diabetes insipidus
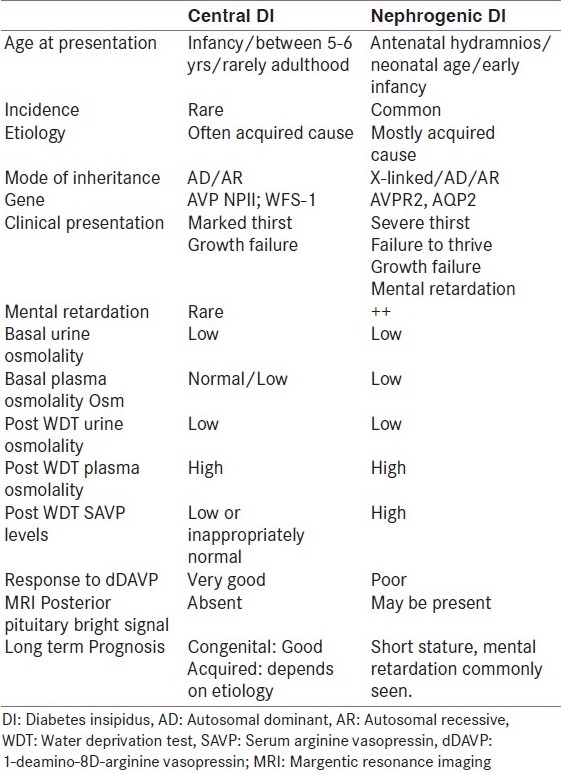
Central DI
The onset is usually abrupt in CDI due to trauma or neurosurgical intervention and is gradual in other non-trauma conditions. The age at presentation varies. Severe neonatal forms are rarely described in children with CDI. The familial autosomal dominant form of CDI presents by 5-6 years of age but may manifest as late as the third decade.[5] The familial autosomal recessive form manifests in infancy and the age of presentation of Wolfram syndrome Diabetes Insipidus, Diabetes Mellitus, Optic Atrophy, and Deafness syndrome (DIDMOAD) may be variable.[4,5] Developmental defects of midline brain structures like septo-optic-dysplasia, holoprosencephaly, pituitary hypoplasia and stalk defect, corpus callosum agenesis etc may present early. These children also have defective thirst perception.[6] Trauma to the base of brain, even minor, can cause transient or permanent CDI and 50% of children with fracture of sella turcica develop CDI which may be delayed as late as 1 month post injury.[7]
Neoplasms causing CDI may present with neurological features like headache, visual disturbance and other pituitary hormone deficiencies if associated. Tuberculosis, sarcoidosis, langerhan cell histiocytosis, lymphocytic hypophysitis are common infiltrative lesions, the latter accounting for > 50% cases of idiopathic CDI. Meningitis or encephalitis due to meningococci, cryptococcus, listeria, cytomegalovirus involving the base of brain may present as transient CDI.[4] Neurosurgical intervention more so in the suprasellar area, causes CDI acutely with the characteristic “triple phase” response. The first phase of transient DI, lasting 0.5 to 2 days, is attributed to axonal shock and/or edema interfering with vasopressin secretion followed by anti diuretic phase, lasting 10 -14 days, due to unregulated release of vasopressin. Finally the phase of permanent DI sets in if more than 90% of vasopressin secreting neurons have been destroyed. Marked antidiuretic phase portends a permanent CDI.[4,8] CDI is a pathognomonic marker of hypoxic brain death and 15% of these children recover their brain functions.[9]
Nephrogenic DI
In children acquired causes of NDI are more common.[2] Congenital forms are more severe and difficult to treat.[1,4] Congenital X linked NDI is essentially a disease of males and can rarely affect females due to extreme lyonization of X chromosome.[4,10] Congenital NDI (X-linked, autosomal dominant, autosomal recessive forms) develops early and manifests as early as the first week of life. Severe forms present in utero as polyhydramnios and premature birth. In some children polyuria and polydypsia may be seen after weaning from breast milk as the low sodium and protein content in breast milk causes a lesser solute load for the kidneys to excrete.[4] Thirst is more severe than CDI and difficult to satisfy. Children may present with recurrent episodes of fever, vomiting, dehydration and often get treated for sepsis. Marked growth failure is seen. Unlike CDI, mental retardation is seen with variable severity in children with NDI attributed to intracranial calcification.
Acquired cause of NDI is essentially due to drugs- Lithium, demeclocyclin, foscarnet, clozapine, amphoterecin B, methicillin and rifampin. Hypercalcemia and hypokalemia also causes excess free water excretion, with the exact mechanism being unknown.[1,4] Mobile fecoliths may be palpable in the abdomen along with an enlarged bladder and occasionally enlarged ballotable kidneys due to hydronephrosis.[2,4]
Primary polydypsia is usually gradual in onset. Absence of nocturia, need to drink water in the night, lack of interference in daily activity and normal growth are pointers to absence of a pathological cause.
Laboratory diagnosis
The initial step in the diagnosis of DI is to ascertain the presence of polyuria which can be established with an accurate 24 hours urine output measurement either by direct collection or indirectly by weighing the diaper in smaller children and infants. Urine output more than 4 ml/kg/hr in infants and children and more than 6 ml/kg/hr in newborn is suggestive of polyuria. Once polyuria is established, it is necessary to rule out solute diuresis i.e glucosuria, hypercalciuria or uremia by urine analysis and biochemistry. Measurement of serum potassium and calcium concentrations is also important to exclude the possibility of polyuria secondary to hypokalemia or hypercalcemia.
Presence of polyuria in the absence of solute diuresis should raise the suspicion of DI. CDI and NDI could manifest as partial or complete forms. The first morning urine specific gravity is a useful screening test and urinary specific gravity of more than 1.010 makes the diagnosis of DI less likely. In young infants, finding a distinction between normal and pathological inability to concentrate urine may be difficult because infants generally exhibit a constitutional hyposthenuria. Early morning measurement of simultaneous serum osmolality, urine osmolality and serum electrolyte is essential in pediatric age group while assessing a case of suspected DI. Urine osmolality of more than 800 mOsm /kg with a serum osmolality of less than 270 mOsm/kg rules out the diagnosis of DI, whereas dilute urine with an osmolality of less than 300 mOsm/kg, and a serum osmolality of more than 300 mOsm/kg effectively establishes the diagnosis.
If the initial serum osmolality is less than 300 mOsm/kg, a water deprivation test is done to confirm the diagnosis and 1-deamino-8D-arginine vasopressin (dDAVP) test is done to distinguish between CDI and NDI. Measure of post water deprivation osmolality is an “indirect test” of vasopressin sufficiency, whereas measurement of vasopressin (AVP) levels post water deprivation is a “direct test” of vasopressin action. Water Deprivation Test (WDT) is a potentially life threatening test and should be performed only in the centers with expertise [Annexure 1].
Annexure 1.
Water deprivation test

Various studies suggest that the combination of the water deprivation test and direct AVP determination would allow the diagnosis of more than 95% of all cases of DI correctly.[11,12] The dehydration test has been questioned as the diagnostic reference standard for several reasons. First, the long dehydration period may provide not only an osmotic but also a volume stimulus.[13] Second, it has been shown that dehydration alone may induce considerable AVP-independent urine concentration.[14] Chronic polyuria per se impairs the renal medullary concentrating gradient and down-regulates aquaporin-2 synthesis, and hence response to desmopressin can be attenuated in patients with DI.[15,16]
Direct AVP measurement is recommended to overcome these limitations.[12] but there are caveats. It is required that whenever the direct test is applied, the osmotic stimulus has to be pursued until hypertonic levels of serum osmolality have been attained (>295 mOsm/kg). AVP assay has failed to be a diagnostic reference standard to date due to its methodological limitations- a very short half life of 10 -30 minutes, high pre analytical instability and high turn around time in most laboratories.[17] Measurement of Aquaporin-2 excretion is being used to differentiate NDI and CDI. The urinary excretion of Aquaporin-2 increases following desmopressin administration in CDI, while there is no increase in NDI.[2,4,18] More recently plasma copeptin levels have been studied as a surrogate marker of AVP. Copeptin, the C-terminal part of the AVP precursor, co-secreted with AVP is much easier to measure. It was demonstrated that plasma copeptin is a valuable surrogate of AVP release in patients with the DI, exhibiting a promising diagnostic potential.[19,20]
Magnetic resonance imaging pituitary
MRI pituitary is an important tool for assessment of etiology of CDI, and should always be performed after gadolinium injection, to check for abnormal enhancement within the stalk. It is also important to perform MRI after the patient is well hydrated as the intensity of posterior pituitary bright signal is inversely related to the degree of dehydration. MRI findings have been heterogenous and the most common feature is the absence of posterior pituitary bright signal.[21] However in familial CDI, when evaluated during infancy or early childhood, and chronic neurogenic hypernatremia, the bright signal remains visible. Besides the bright signal, the size of the adenohypophysis and pituitary stalk thickness (PST) are of important diagnostic value. The pituitary stalk is considered enlarged if at least part of the stalk is found to have a diameter of > 2.0 mm[22] PST is observed in almost one third of children with CDI, and may be the first sign of a germinoma (15%) or of stalk infiltration, as in Langerhans cell histiocytosis (LCH - 15%). CDI with PST remains idiopathic (70%) in most cases.[22–24] Spontaneous regression of PST has been observed in some children. Changes in the size of PST and in the size of the adenohypophysis are observed during the first 2 or 3 years, and remain unchanged thereafter. The natural history of idiopathic CDI with PST is unpredictable. Determination of tumor markers in blood and Cerebrospinal fluid (CSF) helps in diagnosis. In idiopathic PST, repeat MRI and tumor marker estimation is done every 3 – 6 months during the first 3 years. Subsequently, MRI evaluation is performed once per year for 2 years and every 2 – 5 years thereafter, depending on the size and progression of the lesion. Biopsy is not recommended if PST is less than1.7 mm in diameter and is well delimited.[22,25]
Renal sonography
It helps rule out primary renal disorders like polycystic kidney disease, ureteral obstruction etc. Massive hydronephrosis and mega ureters are seen in children with polyuria- polydypsia of long standing duration.
Gene testing
Gene testing for the familial forms of CDI and NDI are not commercially available.
TREATMENT
The therapeutic goals are primarily to reduce polyuria and decrease the thirst so that the child is able to grow adequately and maintain a normal life style. The specific therapy depends on the etiology.
This could be achieved by:
Providing free access to water
Dietary management to optimize free water excretion.
Therapy with vasopressin analogue – desmopressin in CDI
Therapy with drugs to enhance water reabsorption in NDI
Treatment of the underlying cause as in NDI.
It is necessary to provide inpatient care to these children especially those presenting with dehydration, hypernatremia and significant failure to thrive.
Free access to water should be allowed in children with DI. This facilitates maintenance of tonicity if the thirst mechanism is intact; however, it is very inconvenient to drink lots of fluids. Long standing excess fluid intake may cause hydronephrosis and hydroureter and may also lead to fluorosis if the florid content of the water is high.[4,26] Fluids alone can be a management strategy in very young infants and neonates. As they have a high obligatory oral fluid intake, vasopressin therapy may cause hyponatremia. Hence it is better to manage them with fluids alone. It is indeed very challenging to manage these babies who are chronically thirsty and show poor growth and parents have difficulty coping with the large amount of fluid intake and urine output and avoiding the life threatening complication of hyponatremia. Occasionally, the use of thiazide (hydrochlorthiazide 2-4 mg/kg/dose) twice daily with or without amelioride has been recommended to ameliorate the symptoms. Parenteral dDAVP in CDI has been used in some centers in a dose of 0.02 – 0.8 μg/dose subcutaneously once or twice a day; however, this is not yet an approved standard of care.[4,27,28]
Dietary management
Modification in the diet is helpful in decreasing solute load to the kidneys and has been shown to be useful especially in NDI. Diet with low sodium, low protein with high calories providing a high calorie: solute ratio is recommended. It is recommended to restrict oral sodium intake to 1 meq/kg/d and protein intake 2 g/kg/d. It is more prudent to restrict salt than proteins which are essential for growth[2,29] [Table 3].
Table 3.
Interpretation of water deprivation test

Vasopressin and its analogue
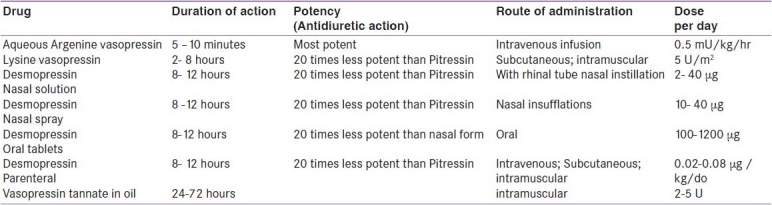
In older children with CDI aqueous vasopressin, lysine vasopressin, de-amino, D arginine vasopressin (dDAVP) may be used to minimize water excretion [Table 4]. Desmopressin (1-deamino-8-D-arginine vasopressin, dDAVP) is the current drug of choice for long-term therapy of CDI.[2,4,30] This synthetic analogue has more specific antiduretic action, negligible pressor activity and a longer half life than the native molecule. It can be given parenterally, orally, or intranasally. Oral tablets although 20 folds less potent than the intranasal form, are highly effective and safe in children, with more flexibility of dosing and have largely replaced the intranasal form.[4,31,32] The recommended dose off dDAVP is 100- 1200 μg /day in three divided doses orally; 2-40 μg once or twice a day intranasally; and, 0.1-1 μg parenterally.[4] There is a large variability in action amongst individuals and hence the duration between doses needs to be determined in each patient. It is a safe practice to allow a short period of diuresis between two doses. Dilutional hyponatremia, headache, hypertension and nasal congestion are some of the side effects occasionally seen. Vasopressin tannate in oil is also used in the dose of 2-5 U intramuscluar every 25 - 72 hours. Lysine vasopressin is used if shorter duration of action of 2 - 8 hours is needed [Table 4].
Table 4.
Vasopressin and vasopressin analogues
Perioperative management of Central DI[4,18,33]
In children acute onset of CDI either during or soon after neurosurgery is managed with care to avoid hyponatremia. As fluid intake more than 1 L/m2/d periopertatively results in dilutional hyponatremia; and, restriction of fluids to 2/3 normal maintenance is recommended. Onset of DI is suspected if the child becomes polyuric with a serum sodium of >145mEq/l and the Serum Osmolality of >300mOs/kg, wherein an intravenous infusion of aqueous vasopressin in the dose of 0.5 mU/kg/hr is started and titrated every 10 minute as to maintain a urine output of less than 2 ml/kg/hr and a stable rate of intravenous fluid and vasopressin infusion is maintained. This exercise needs frequent monitoring of the serum electrolytes (hourly), urine output,vital signs and post operatively must be carried out in the Intensive Care Unit (ICU). It is advisable to switch to oral fluids as soon as possible as the fluid intake will then be driven by thirst and serum osmolality is better maintained. Intravenous dDAVP should not be used because of its long half life.
A known case of diabetes insipidus undergoing surgery[4,33]
If a known case of DI requires surgery and needs prolonged oral fluid restriction, the usual dose of desmopressin is withheld prior to surgery. The child is kept on 1 L/m2/d of restricted intravenous fluids. When the effect of the previous desmopressin dose wanes off and CDI sets in, intravenous aqueous vasopressin is started as per the perioperative CDI protocol described above.
A known case of Central DI receiving hydration therapy[4,34]
If a child with CDI is to receive high volume of fluid as part of hydration accompanying chemotherapy or forced dieresis, management of DI is difficult. The antidiuretic therapy needs to be discontinued and fluid intake should be increased to 3-5 L/m2/d which will help maintain the tonicity. As such a high fluid volume may compound the nephrotoxicity of anti cancer drugs, and a low dose aqueous vasopressin (0.08 - 0.1 mU/kg/hr) infusion is recommended with fluid volume of 3 L/m2/d to achieve better osmolar control and confer renal protection.
Other management in Central DI
As glucocorticoids are essential for insertion of water channels independent of vasopressin, cortisol deficiency inhibits free water clearance and adequate replacement if required helps achieve better control of DI. Decreased bone mineral density has been reported in children with CDI central diabetes insipidus.[35,36] Desmopressin replacement treatment does not help prevent or revert bone status abnormalities. In one adult study, osteoporosis (33.3%) and osteopenia (55.5%) was commonly seen. Serum osteocalcin (OC) levels were decreased, whereas levels of urinary cross-linked N telopeptide of type 1 collagen were significantly increased. This study also showed significant improvement in bone mineral density (BMD) after treatment with oral alendronate.[36]
Treatment of Nephrogenic DI[2,4,18]
In NDI, commonly due to acquired cause, treatment of the underlying cause is essential. NDI is a severe form of DI and difficult to treat. Despite adequate control, growth retardation and mental retardation is often found on long term follow up. Besides providing access to free water and diet, and providing a high calorie to solute ratio, the therapeutic options are mostly to ameliorate the sodium and water loss in the urine. Hydrochlorthiazide in a dose of 2 - 4 mg/kg/day in divided doses is the most useful therapy in NDI. This drug works by enhancing sodium excretion at the expense of water. Amelioride given additionally or alone has similar effect but is useful in preventing hypokalemia. Dose used is 0.3 mg/kg/d. Indomethacin (2 mg/kg/d) further enhances proximal tubular water reabsorption, but has significant side effects. The combination of thiazide and amelioride is the most commonly used regimen. High dose dDAVP along with indomethacin has been found useful in animal studies with V2 receptor mutations.
CONCLUSION
DI is not a very common pediatric endocrine disorder and NDI is more common than CDI. The clinical presentation varies with age of onset and underlying etiology. Water deprivation test is useful in establishing a diagnosis of DI and helps differentiating between NDI and CDI. Desmopressin is the drug of choice for CDI therapy, the oral formulation being more preferred. Treatment of NDI is essentially to treat the underlying cause, and drugs like thiazide, indomethacin help decrease water excretion.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ranadive SA, Rosenthal SM. Pediatric disorders of water balance. Endocrinol Metab Clin North Am. 2009;38:663–72. doi: 10.1016/j.ecl.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colaco P. Disorder of water and electrolyte homeostasis. In: Desai MP, Menon PS, Bhatia V, editors. Pediatric Endocrine Disorders. 2nd ed. New Delhi: Orient Longman Pvt Ltd; 2008. pp. 445–57. [Google Scholar]
- 3.Saborio P, Tipton GA, Chan JC. Diabetes Insipidus. Pediatr Rev. 2000;21:122–9. doi: 10.1542/pir.21-4-122. quiz 129. [DOI] [PubMed] [Google Scholar]
- 4.Muglia LJ, Majzoub JA. Disorders of the posterior pituitary. In: Sperling MA, editor. Pediatric Endocrinology. 3rd ed. Philadelphia: Saunders; 2008. pp. 356–73. [Google Scholar]
- 5.Siggaard C, Christensen JH, Corydon TJ, Rittig S, Robertson GL, Gregersen N, et al. Expression of three different mutations in arginine vasopressin gene suggests genotype-phenotype correlation in familial neurohypophyseal diabetes insipidus kindreds. Clin Endocrinol (Oxf) 2005;63:207–16. doi: 10.1111/j.1365-2265.2005.02327.x. [DOI] [PubMed] [Google Scholar]
- 6.Masera N, Grant DB, Stanhope R, Preece MA. Diabetes insipidus with impaired osmoregulation in septo-optic dysplasia and agenesis of corpus callosum. Arch Dis Childhood. 1994;70:51–6. doi: 10.1136/adc.70.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Defoer F, Mahler C, Dua G, Appel B. Posttraumatic diabetes insipidus. Acta Anaesthesiol Belg. 1987;38:397–402. [PubMed] [Google Scholar]
- 8.Seckl JR, Dunger DB, Lightman SL. Neurohypophyseal peptide function during early postoperative diabetes insipidus. Brain. 1987;110:737. doi: 10.1093/brain/110.3.737. [DOI] [PubMed] [Google Scholar]
- 9.Barzilay Z, Smoekh E. Diabetes insipidus in severely brain damaged children. J Med. 1987;19:47–53. [PubMed] [Google Scholar]
- 10.Moses AM, Sangani G, Miller JL. Proposed cause of marked vasopressin resistance in a female with an X linked recessive V2 receptor abnormality. J Clin Endocrinol Metab. 1995;80:1184–8. doi: 10.1210/jcem.80.4.7714087. [DOI] [PubMed] [Google Scholar]
- 11.Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17:471–503. doi: 10.1016/s1521-690x(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 12.Robertson GL. Diabetes insipidus. Endocrinol Metab Clin North Am. 1995;24:549–72. [PubMed] [Google Scholar]
- 13.Baylis PH, Robertson GL. Plasma vasopressin response to hypertonic saline infusion to assess posterior pituitary-function. J R Soc Med. 1980;73:255–60. doi: 10.1177/014107688007300408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gellai M, Edwards BR, Valtin H. Urinary concentrating ability during dehydration in the absence of vasopressin. Am J Physiol. 1979;237:F100–4. doi: 10.1152/ajprenal.1979.237.2.F100. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Wang W, Kwon TH, Isikay L, Wen JG, Marples D, et al. Downregulation of AQP1, -2, and -3 after ureteral obstruction is associated with a long-term urine-concentrating defect. Am J Physiol Renal Physiol. 2001;281:F163–71. doi: 10.1152/ajprenal.2001.281.1.F163. [DOI] [PubMed] [Google Scholar]
- 16.Workeneh B, Balakumaran A, Bichet DG, Mitch WE. The dilemma of diagnosing the cause of hypernatremia: Drinking habits vs diabetes insipidus. Nephrol Dial Transplant. 2004;19:3165–7. doi: 10.1093/ndt/gfh479. [DOI] [PubMed] [Google Scholar]
- 17.Katan M, Morgenthaler NG, Dixit KC, Rutishauser J, Brabant GE, Muller B, et al. Anterior and posterior pituitary function testing with simultaneous insulin tolerance test and a novel copeptin assay. J Clin Endocrinol Metab. 2007;92:2640–3. doi: 10.1210/jc.2006-2046. [DOI] [PubMed] [Google Scholar]
- 18.Ghirardello S, Malattia C, Scagnelli P, Maghenie M. Current perspectives on the pathogenesis of central diabetes insipidus. J Pediatr Endocrinol Metab. 2005;18:631–45. doi: 10.1515/jpem.2005.18.7.631. [DOI] [PubMed] [Google Scholar]
- 19.Fenske W, Quinkler M, Lorenz D, Zopf K, Haagen U, Papassotiriou J, et al. Copeptin in the differential diagnosis of the polydypsia-polyuria syndrome--Revisiting the direct and indirect water deprivation tests. J Clin Endocrinol Metab. 2011;96:1506–15. doi: 10.1210/jc.2010-2345. [DOI] [PubMed] [Google Scholar]
- 20.Ulmer H, Schwarz S, Hasibeder WR, Friesenecker BE, Dunser MW. Copeptin and arginine vasopressin concentrations in critically ill patients. J Clin Endocrinol Metab. 2006;91:4381–6. doi: 10.1210/jc.2005-2830. [DOI] [PubMed] [Google Scholar]
- 21.Maghnie M, di Lorgi N, Bernasconi S. Genetic forms of central diabetes Insipidus. In: Deal C, editor. London, Rimedica: MRI in congenital Hypopituitarism; 2007. pp. 99–109. [Google Scholar]
- 22.Léger J, Velasquez A, Garel C, Hassan M, Czernichow P. Thickened pituitary stalk on magnetic resonance imaging in children with central diabetes insipidus. J Clin Endocrinol Metab. 1999;84:1954–60. doi: 10.1210/jcem.84.6.5745. [DOI] [PubMed] [Google Scholar]
- 23.Maghnie M, Cosi G, Genovese E, Manca-Bitti ML, Cohen A, Zecca S, et al. Central diabetes insipidus in children and young adults. N Engl J Med. 2000;343:998–1007. doi: 10.1056/NEJM200010053431403. [DOI] [PubMed] [Google Scholar]
- 24.Alter CA, Bilaniuk LT. Utility of magnetic resonance imaging in the evaluation of the child with central diabetes insipidus. J Pediatr Endocrinol Metab. 2002;15:681–7. doi: 10.1515/jpem.2002.15.s2.681. [DOI] [PubMed] [Google Scholar]
- 25.Czernichow P, Garel C, Léger J. Thickened pituitary stalk on magnetic resonance imaging in children with central diabetes insipidus. Horm Res. 2000;53(suppl 3):61–4. doi: 10.1159/000023536. [DOI] [PubMed] [Google Scholar]
- 26.Seow WK, Thomsett MJ. Dental fluorosis as a complication of hereditary diabetes insipidus: Studies of six affected patients. Pediatr Dent. 1994;16:128–32. [PubMed] [Google Scholar]
- 27.Rivkees SA, Dunbar N, Wilson TA. The management of central diabetes insipidus in infancy: Desmopressin, low renal solute load formula, thiazide diuretics. J Pediatr Endocrinol Metab. 2007;20:459–69. doi: 10.1515/jpem.2007.20.4.459. [DOI] [PubMed] [Google Scholar]
- 28.Blanco EJ, Lane AH, Aijaz N, Blumberg D, Wilson TA. Use of subcutaneous DDAVP in infants with central diabetes insipidus. J Pediatr Endocrinol Metab. 2006;19:919–25. doi: 10.1515/jpem.2006.19.7.919. [DOI] [PubMed] [Google Scholar]
- 29.Blalock JT, Gerron G, Quiter E, Rudman D. Role of diet in the management of vasopressin-responsive and resistant diabetes Insipidus. Am J Clin Nutr. 1977;30:1070–6. doi: 10.1093/ajcn/30.7.1070. [DOI] [PubMed] [Google Scholar]
- 30.Lam KS, Wat MS, Choi KL, Ip TP, Pang RW, Kumana CR. Pharmacokinetics, pharmacodynamics, long-term efficacy, and safety of oral 1-deamino-8-D-arginine vasopressin in adult patients with central diabetes insipidus. Br J Clin Pharmacol. 1996;42:379–85. doi: 10.1046/j.1365-2125.1996.39914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cunnah D, Ross G, Besser GM. Management of cranial diabetes insipidus with oral desmopressin (DDAVP) Clin Endocrinol (Oxf) 1986;24:253–7. doi: 10.1111/j.1365-2265.1986.tb03265.x. [DOI] [PubMed] [Google Scholar]
- 32.Williams TD, Dunger DB, Lyon CC. Antidiuretic effect and pharmokinetics of oral 1-desamino-8-D-arginine vasopressin: 1. Studies in adults and children. J Clin Endocrinol Metab. 1986;63:129–35. doi: 10.1210/jcem-63-1-129. [DOI] [PubMed] [Google Scholar]
- 33.Wise-Faberowski L, Soriano SG, Ferrari L. Perioperative management of diabetes in children. J Neurosurg Anesthesiol. 2004;16:14–22. doi: 10.1097/00008506-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Bryant WP, O’Marcaigh AS, Ledger GA, Zimmerman D. Aqueous vasopressin infusion during chemotherapy in patients with diabetes insipidus. Cancer. 1994;74:2589–98. doi: 10.1002/1097-0142(19941101)74:9<2589::aid-cncr2820740929>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 35.Pivonello R, Colao A, Di Somma C, Facciolli G, Klain M, Faggiano A, et al. Impairment of bone status in patients with central diabetes insipidus. J Clin Endocrinol Metab. 1998;83:2275–80. doi: 10.1210/jcem.83.7.4987. [DOI] [PubMed] [Google Scholar]
- 36.Pivonello R, Faggiano A, Di Somma C, Klain M, Filippella M, Salvatore M, et al. Effect of a short-term treatment with alendronate on bone density and bone markers in patients with central diabetes insipidus. J Clin Endocrinol Metab. 1999;84:2349–52. doi: 10.1210/jcem.84.7.5816. [DOI] [PubMed] [Google Scholar]


