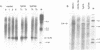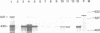Abstract
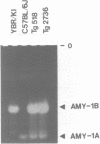
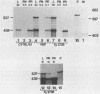
Two distinct mouse amylase cDNAs, corresponding to the genes Amy-1.1 and Amy-1.2, have been isolated from a YBR/Ki parotid cDNA library. A cosmid clone containing the intact Amy-1.1 gene from strain YBR/Ki, including both the parotid and liver promoters, was transferred to the germ line of C57BL/6J mice. Two independent transgenic lines were produced. The transferred genes are organized as a 4-copy autosomal locus in line Tg518 and an 8-copy Y-linked locus in line Tg2736. Serum of both transgenic lines contained high levels of the AMY1B isozyme encoded by the transferred gene. Transcripts were detected in liver and, at a lower level, in several other tissues including white and brown fat. The anticipated expression in parotid was not observed. Constructs containing 270 or 540 bp of the 5' flanking region of the parotid promoter, cloned upstream of the chloramphenicol acetyltransferase (CAT) structural gene, were also not expressed in transgenic mice. The results suggest that sequences located more than 5 kb upstream of the Amy-1 parotid promoter and/or more than 10 kb downstream from the structural gene are required for parotid-specific expression. The results also demonstrate that non-parotid sources can produce a normal level of AMY1 in serum. Liver is the probable source of AMY1 in serum of these transgenic mice.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloor J. H., Meisler M. H., Nielsen J. T. Genetic determination of amylase synthesis in the mouse. J Biol Chem. 1981 Jan 10;256(1):373–377. [PubMed] [Google Scholar]
- Cereghini S., Blumenfeld M., Yaniv M. A liver-specific factor essential for albumin transcription differs between differentiated and dedifferentiated rat hepatoma cells. Genes Dev. 1988 Aug;2(8):957–974. doi: 10.1101/gad.2.8.957. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- Dranginis A., Morley M., Nesbitt M., Rosenblum B. B., Meisler M. H. Independent regulation of nonallelic pancreatic amylase genes in diabetic mice. J Biol Chem. 1984 Oct 10;259(19):12216–12219. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Wiebauer K., Caldwell R. M., Samuelson L. C., Meisler M. H. Concerted evolution of human amylase genes. Mol Cell Biol. 1988 Mar;8(3):1197–1205. doi: 10.1128/mcb.8.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio D. L., Wiebauer K., Dranginis A., Samuelson L. C., Treisman L. O., Caldwell R. M., Antonucci T. K., Meisler M. H. Evolution of the amylase multigene family. YBR/Ki mice express a pancreatic amylase gene which is silent in other strains. J Biol Chem. 1985 Nov 5;260(25):13483–13489. [PubMed] [Google Scholar]
- Hagenbüchle O., Schibler U., Petrucco S., Van Tuyle G. C., Wellauer P. K. Expression of mouse Amy-2a alpha-amylase genes is regulated by strong pancreas-specific promoters. J Mol Biol. 1985 Sep 20;185(2):285–293. doi: 10.1016/0022-2836(85)90404-8. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Tosi M., Schibler U., Bovey R., Wellauer P. K., Young R. A. Mouse liver and salivary gland alpha-amylase mRNAs differ only in 5' non-translated sequences. Nature. 1981 Feb 19;289(5799):643–646. doi: 10.1038/289643a0. [DOI] [PubMed] [Google Scholar]
- Hardon E. M., Frain M., Paonessa G., Cortese R. Two distinct factors interact with the promoter regions of several liver-specific genes. EMBO J. 1988 Jun;7(6):1711–1719. doi: 10.1002/j.1460-2075.1988.tb03000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth J. P. Altered salivary amylase gene in the mouse strain BXD-16. Heredity (Edinb) 1982 Feb;48(Pt 1):127–135. doi: 10.1038/hdy.1982.13. [DOI] [PubMed] [Google Scholar]
- Hjorth J. P. Genetic variation in mouse salivary amylase rate of synthesis. Biochem Genet. 1979 Aug;17(7-8):665–682. doi: 10.1007/BF00502125. [DOI] [PubMed] [Google Scholar]
- Hjorth J. P., Lusis A. J., Nielsen J. T. Multiple structural genes for mouse amylase. Biochem Genet. 1980 Apr;18(3-4):281–302. doi: 10.1007/BF00484242. [DOI] [PubMed] [Google Scholar]
- Kugler W., Wagner U., Ryffel G. U. Tissue-specificity of liver gene expression: a common liver-specific promoter element. Nucleic Acids Res. 1988 Apr 25;16(8):3165–3174. doi: 10.1093/nar/16.8.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- Meisler M. H., Antonucci T. K., Treisman L. O., Gumucio D. L., Samuelson L. C. Interstrain variation in amylase gene copy number and mRNA abundance in three mouse tissues. Genetics. 1986 Jul;113(3):713–722. doi: 10.1093/genetics/113.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Rosenberg M. P., Keller S. A., Meisler M. H. Tissue-specific and insulin-dependent expression of a pancreatic amylase gene in transgenic mice. Mol Cell Biol. 1987 Jan;7(1):326–334. doi: 10.1128/mcb.7.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Rosenberg M. P., Keller S. A., Ting C. N., Meisler M. H. Insulin response of a hybrid amylase/CAT gene in transgenic mice. J Biol Chem. 1988 Nov 15;263(32):16519–16522. [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Samuelson L. C., Keller P. R., Darlington G. J., Meisler M. H. Glucocorticoid and developmental regulation of amylase mRNAs in mouse liver cells. Mol Cell Biol. 1988 Sep;8(9):3857–3863. doi: 10.1128/mcb.8.9.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelson L. C., Wiebauer K., Gumucio D. L., Meisler M. H. Expression of the human amylase genes: recent origin of a salivary amylase promoter from an actin pseudogene. Nucleic Acids Res. 1988 Sep 12;16(17):8261–8276. doi: 10.1093/nar/16.17.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Schibler U., Pittet A. C., Young R. A., Hagenbüchle O., Tosi M., Gellman S., Wellauer P. K. The mouse alpha-amylase multigene family. Sequence organization of members expressed in the pancreas, salivary gland and liver. J Mol Biol. 1982 Mar 5;155(3):247–266. doi: 10.1016/0022-2836(82)90004-3. [DOI] [PubMed] [Google Scholar]
- Shaw P., Sordat B., Schibler U. The two promoters of the mouse alpha-amylase gene Amy-1a are differentially activated during parotid gland differentiation. Cell. 1985 Apr;40(4):907–912. doi: 10.1016/0092-8674(85)90350-2. [DOI] [PubMed] [Google Scholar]
- Townes T. M., Lingrel J. B., Chen H. Y., Brinster R. L., Palmiter R. D. Erythroid-specific expression of human beta-globin genes in transgenic mice. EMBO J. 1985 Jul;4(7):1715–1723. doi: 10.1002/j.1460-2075.1985.tb03841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebauer K., Gumucio D. L., Jones J. M., Caldwell R. M., Hartle H. T., Meisler M. H. A 78-kilobase region of mouse chromosome 3 contains salivary and pancreatic amylase genes and a pseudogene. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5446–5449. doi: 10.1073/pnas.82.16.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]