Anterior discoid resection is associated with a shorter operative time, lower blood loss, shorter hospital stay, and lower rate of anastomotic strictures than laparoscopic anterior resection is in the treatment of rectal endometriosis.
Keywords: Endometriosis, Anastomosis, Laparoscopy
Abstract
Objective:
To compare laparoscopic anterior discoid resection (ADR) with low anterior resection (LAR).
Methods:
This is a retrospective review of a cohort (Canadian Task Force classification II-2) of patients undergoing laparoscopic ADR or LAR at a university hospital. Chart review and telephone questionnaires were conducted to examine long-term outcomes. Preoperative and operative findings, short- and long-term outcomes were compared. SF-12 quality of life scores, need for further interventions, and overall satisfaction were also compared.
Results:
Twenty-two patients underwent laparoscopic ADR (n=8) or LAR (n=14) for rectosigmoid endometriosis between January 2001 and December 2009. Mean follow-up time was 41.26 months (range, 14 to 70). Patients undergoing laparoscopic ADR had significantly less blood loss and shorter operative time and hospital stay. Patients who required LAR had a significantly higher rate of mucosal involvement (61.5% v. 0%). No statistically significant difference was found in the size, depth of invasion, location of lesions, or operative complications. Fifty percent of the LAR group had several lesions as opposed to 12.5% of the ADR group. Median age was significantly higher in patients who required LAR (39) than in patients who required ADR (32). Three patients in the LAR group (21.4%) had anastomotic strictures; 2 required dilation. The ADR group had consistently higher increments of improvement in bowel symptoms and dyspareunia. Overall satisfaction rate with the procedures was 93.3%. SF-12 scores were comparable between the 2 groups.
Conclusion:
ADR compared with LAR is associated with decreased operative time, blood loss, and hospital stay and a lower rate of anastomotic strictures. Other outcomes and satisfaction rates are comparable between the 2 procedures.
INTRODUCTION
Rectovaginal endometriosis is one of the most advanced and complex forms of the disease affecting approximately 3.8% to 37% of patients with endometriosis.1,2 The rectum and sigmoid colon are affected in 74% of cases of bowel endometriosis, the rectovaginal septum in 12% of cases, and the remainder involves the appendix (3%), the cecum (2%), the ileum, and other parts of the small and large bowel.3–5
Patients present with a wide range of symptoms. Superficial disease can be asymptomatic; however, patients often present with severe symptoms, such as dyschezia, dysmenorrhea, dyspareunia, and chronic pelvic pain, along with a spectrum of bowel symptoms like diarrhea, constipation, bloating, or cyclic rectal bleeding. The triad of dysmenorrhea, dyspareunia, and bowel symptoms was found to be 80% sensitive for diagnosing bowel endometriosis.1,6–8 Multifocal bowel involvement is common, affecting 25% to 34% of patients.2,9
Although medical and hormonal therapy have been found to be effective for improving the pain symptoms associated with rectal endometriosis, the relief is usually transient and symptoms generally recur once medical therapy is discontinued.10 Due to persistent or recurrent pain, and the marked anatomic distortion caused by deep infiltrating rectovaginal endometriosis, surgery is considered the treatment of choice for symptomatic disease.11 Moreover, surgery is mandatory in severe cases of rectovaginal nodules that result in luminal stenosis and obstructive symptoms.12
Multiple studies suggest that complete excision of endometriotic lesions, including bowel resection when necessary, results in significant improvement in pain, as well as improvement in bowel symptoms and quality of life.9,13–15
Several approaches have been described for surgical treatment of rectovaginal endometriosis, ranging from shaving of superficial rectal lesions,9,16 to laparoscopic full-thickness disc resection (anterior discoid resection [ADR]),3 and segmental bowel resection (low anterior resection [LAR]).17–21
It is unclear whether one procedure is superior to the other. Some studies indicate that ADR is incomplete in 43% of cases,21 but the clinical significance and long-term effects of these findings are unclear. A few long-term studies have been conducted to evaluate the clinical outcomes of these procedures, and no studies have been conducted to compare ADR and LAR. We aim to compare the surgical outcomes and the long-term treatment benefits and complications between the 2 procedures.
MATERIALS AND METHODS
We conducted a retrospective cohort study that included all patients 18 years or older, identified to have had laparoscopic anterior discoid resection (ADR) or low anterior resection (LAR) for treatment of symptomatic rectal endometriosis at Magee-Womens Hospital of the University of Pittsburgh Medical Center from January 1, 2001 through December 31, 2009. Selection of the procedure depended solely on the gynecologic surgeon's discretion at the time of surgery and based on the patient's clinical picture and treatment goals. The Institutional Review Board of the University of Pittsburgh approved the study.
We used multiple search strategies, which included ICD-9 and CPT codes, searching the electronic pathology system using an honest broker contractor, and searching surgeons’ individual case logs for patients who met the above inclusion criteria.
The inpatient and outpatient medical records were reviewed for short- and long-term findings and outcomes. Abstracted information included age, gravidity, parity, symptom duration, presenting symptoms, prior medical and/or surgical treatments, findings on preoperative evaluation such as colonoscopy or endorectal ultrasound (including lesion size and location from the anal verge if the lesion was visualized). Operative details included rectovaginal nodule(s) characteristics (number, size, location, percentage of bowel circumference involved), along with operative time, estimated blood loss, operative complications, and any concomitant procedures. Postoperative data included length of hospitalization, complications of fever, infection, transfusion, reoperation, and 30-day readmission. Pathologic findings included lesion size, depth of invasion, and mucosal involvement. Postoperative complications of anastomotic leakage, bowel stricture, the need for dilation, and the development of a fistula were also examined. Consenting patients were contacted to complete a telephone questionnaire after their chart review has been completed.
The telephone questionnaire included questions from Memorial Sloan-Kettering Cancer Center Bowel Function Instrument (MSKCC-BFI), Constipation Severity Instrument (CSI), Female Sexual Function Index (FSFI), long-term complications, subsequent interventions, satisfaction survey along with the SF-12v2.0 quality of life survey. The surveys were administered to address the patients’ current symptoms, and also patients were asked to try to recall their symptoms prior to the index procedures. The symptoms before and after the procedure were compared. SPSS statistical package was used for statistical analysis (SPSS, Inc., Chicago, Illinois).
Statistical Analysis
Descriptive statistics were used to describe the baseline criteria and operative findings. For independent groups, the Student's t test was used for comparison of normally distributed continuous variables, while the Mann-Whitney U test was used for comparison of continuous variables that are not normally distributed. For dependent groups (Before – After surgery comparison), the Wilcoxon-signed rank test was used. Categorical variables were compared using the chi-square test or Fisher's exact test as appropriate.
RESULTS
Fourteen patients underwent laparoscopic LAR, and 8 patients underwent laparoscopic ADR. The mean follow-up time was 41.27 months (95% CI: 30.46 to 52.08) following the index procedure. A total of 15/22 patients consented to the telephone questionnaire; 6/8 patients from the ADR group and 9/14 patients from the LAR group. Patients who did not administer the telephone questionnaire were excluded from the long-term follow-up portion of the study.
Mean age was significantly higher in patients who required LAR (39 years) than patients who required ADR (32 years), reflecting the possibility of the progressive nature of endometriosis, or potentially, the surgeon's preference of a more aggressive treatment in older patients (Table 1).
Table 1.
Demographics and Operative Findings
| Variable | ADRa | LARa | P Value |
|---|---|---|---|
| n=8 (Mean±SD; Median) | n=14 (Mean±SD; Median) | ||
| Age | 32.38 ±4.241; 32 | 38.93± 8.297; 39 | .04 |
| Symptom Duration | 7.313± 5.8122; 6.5 | 7.562 ± 6.7255; 4 | .913 |
| Location | . | 112.78 ± 60.731; 100 | . |
| OR Time | 3.94 ± 0.971; 4.25 | 7.1 ± 2.76; 6 | .001 |
| Estimated Blood Loss | 134.38 ± 64; 125 | 276.92 ± 187.767; 200 | .035 |
| Size of Lesion | 28.75 ± 6.944; 30 | 35.17 ± 11.011; 35 | .118 |
| Depth of Invasion | 15.5 ± 3.536; 15.5 | 15.57 ± 2.76; 15 | . |
| Length of Stay | 3.5 ± 0.756; 3 | 5.07± 1.072; 5 | .002 |
ADR = laparoscopic anterior discoid resection; LAR = low anterior resection.
Patients undergoing laparoscopic ADR had significantly less operative time (3.94 vs. 7.1 hours, P=.001), blood loss (134.38 vs. 276.92 mL, P=.035), and hospital stay (3.5 vs. 5.07 days, P=.002) (Table 2). Patients who required LAR had a significantly higher rate of mucosal involvement (61.5% vs. 0%) (Table 1).
Table 2.
Preoperative Patient Characteristics and Operative Procedures
| Preoperative Findings | ADR | LAR | P Value |
|---|---|---|---|
| n=8 [Frequency (%)] | n=14 [Frequency (%)] | ||
| Dyschezia | 5 (62.5%) | 8 (57.1%) | 1.000 |
| Hematochezia | 3 (37.5%) | 7 (50%) | .675 |
| Constipation | 5 (62.5%) | 11 (78.6%) | .624 |
| Dyspareunia | 5 (62.5%) | 10 (76.9%) | .631 |
| Prior hormonal treatment | 2 (25%) | 7 (50%) | .380 |
| Prior surgical treatment | 6 (75%) | 13 (92.9%) | .527 |
| Colonoscopy | 2 (25%) | 6 (42.9%) | .649 |
| Operative Details | |||
| Hysterectomy | 2 (25%) | 4 (28.6%) | 1.000 |
| BSO | 1 (12.5%) | 5 (35.7%) | .351 |
| Endometriosis excision | 7 (87.5%) | 14 (100%) | .364 |
| Concomitant procedures | 6 (75%) | 14 (100%) | .121 |
| Operative complications | 1 (12.5%) | 3 (21.4%) | 1.000 |
| Number of lesions >1 | 1 (12.5%) | 7 (50%) | .167 |
| Mucosal involvement | 0 (0%) | 8 (61.5%) | .015 |
| Postoperative GnRh agonist | 0 (0%) | 4 (30.8%) | .249 |
ADR = laparoscopic anterior discoid resection; BSO = bilateral salpingo-oophorectomy; LAR = low anterior resection.
No statistically significant difference was found in the size of the lesion (28.75mm vs. 35.17mm, P=.118), depth of invasion (15.5mm vs. 15.57mm), location, or operative complications. Fifty percent of the LAR group had ≥2 lesions as opposed to 12.5% of the ADR group (P=.015) (Tables 1 and 2).
Neither group had intraoperative complications secondary to visceral injury. Three patients in the LAR group (21.4%) had anastomotic strictures, 2 of them required dilation (Table 3).
Table 3.
Short-term Complications
| Outcomes | ADRa (n=6) | LARa (n=9) | P Value | |
|---|---|---|---|---|
| Readmission | Frequency (%) | 0 (0%) | 1 (11.1%) | 1.000 |
| Stricture | Frequency (%) | 0 (0%) | 3 (30%) | .250 |
| Dilation | Frequency (%) | 0 (0%) | 2 (20%) | .500 |
| Fistula | Frequency (%) | 0 (0%) | 0 (0%) | . |
| Persistent Constipation | Frequency (%) | 4 (66.7%) | 7 (77.8%) | 1.000 |
| Persistent Pain | Frequency (%) | 3 (50%) | 3 (30%) | .607 |
ADR = laparoscopic anterior discoid resection; BSO = bilateral salpingo-oophorectomy; LAR = low anterior resection.
Concomitant hysterectomy and/or bilateral salpingo-oophorectomy (BSO) did not seem to influence questionnaire scores in either group; however, due to the small number of patients who underwent hysterectomy and/or BSO (8), it is difficult to draw meaningful conclusions from the lack of statistical significance of these comparisons.
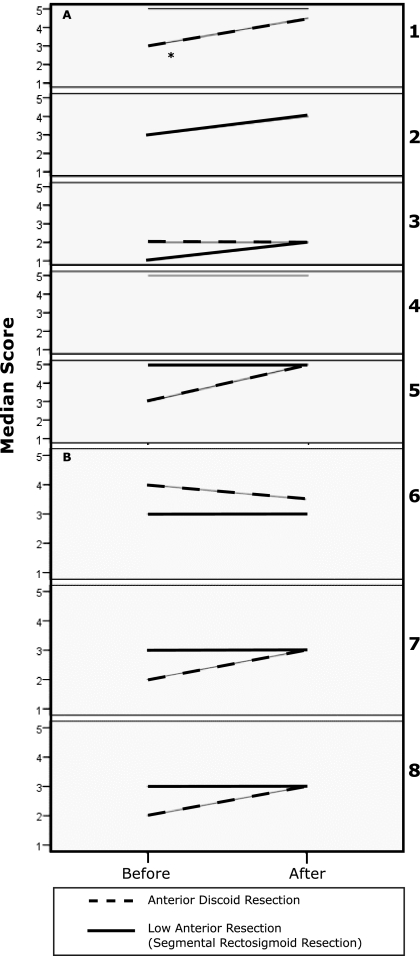
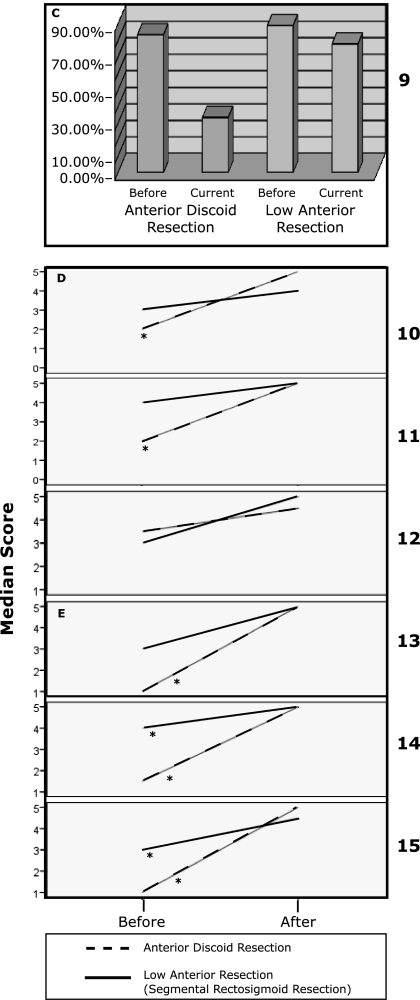
The ADR group had significant improvement in diarrhea and dyschezia on the Memorial Sloan-Kettering Cancer Center Bowel Function Instrument (Figure 1A) and the Constipation Severity Instrument (Figure 1B and 1D), respectively. The improvement in diarrhea and dyschezia was not statistically significant in the LAR group. Both the ADR and the LAR groups had significant improvement in dyspareunia on the Female Sexual Function Index (FSI) (Figure 1E).
Figure 1.
A. Q1–Q5 Memorial Sloan-Kettering Cancer Center Bowel Function Instrument.* Indicates a statistically significant difference.
1. Have you had diarrhea (no form, watery stool)?
2. Have you had loose stool?
3. Have you been able to control the passage of gas?
4. Have you had soilage (leakage of stool) of your garments during the day?
5. Have you had to alter your activities because of your bowel function?
B. Q6–Q8 Constipation Severity Instrument.
6. How often do you experience straining or difficulty in having a bowel movement?
7. How severe is this for you?
8. How much does this bother you?.
C. Q9. Constipation Severity Instrument.
9. Do you experience any rectal or anal pain while having bowel movements?
D. Q10–Q12 Constipation Severity Instrument.
10. During the last month, on average, how severe was the pain in your rectum/anus while having a bowel movement?
11. How much suffering do you experience because of rectal/anal pain?
12. During the past month, due to your bowel habits, how often have you had rectal bleeding during/after a bowel movement?
E. Q13–Q15 Female Sexual Function Index (FSI)
13. Over the past 4 weeks, how often did you experience discomfort or pain during vaginal intercourse?
14. Over the past 4 weeks, how often did you experience discomfort or pain following vaginal intercourse?
15. Over the past 4 weeks, how would you rate your level (degree) of discomfort or pain during or following vaginal intercourse?.
The ADR group had consistently higher increments of improvement than the changes seen in the LAR group in diarrhea, constipation, performance of daily activities, dyschezia, rectal pain, and dyspareunia. Although the improvements in constipation, performance of daily activities, and rectal pain did not reach statistical significance, the consistent patterns of improvement raise the possibility of better outcomes in the ADR group than in the LAR group (Figure 1). Overall satisfaction rate with the procedure was 93.3% and was not statistically significantly different between the 2 groups.
SF-12 scores were comparable between the 2 groups and within the expected population norms for age and sex.
DISCUSSION
Several approaches to the laparoscopic treatment of rectovaginal endometriosis have been described. The 2 main procedures commonly used are ADR and LAR. It is unclear from the literature when to use either procedure, and there are no available objective criteria to indicate the use of one procedure rather than the other. Furthermore, there are no long-term studies comparing the safety, efficacy, and long-term outcomes of the 2 main procedures.
This study is, to our knowledge, the first in the literature to compare the patient characteristics, operative and pathologic findings, along with short- and long-term outcomes between ADR and LAR.
As expected, it is clear that ADR is superior to LAR in terms of operative time, estimated blood loss, and length of hospitalization. Both procedures had a high patient satisfaction rate and significant improvement in dyspareunia. No cases of spontaneous dehiscence, leakage, and subsequent reoperation were noted in this study. No cases were converted to laparotomy. The rate of strictures and need for subsequent dilation were predictably higher in the LAR group.
It was surprising, though, to see a significant improvement in diarrhea and dyschezia in the ADR group, but not in the LAR group. It was also revealing to see the consistently higher increments of improvement in diarrhea, constipation, performance of daily activities, dyschezia, rectal pain, and dyspareunia in the ADR group compared with those in the LAR group. It is possible that the lack of statistical significance is due to the limited number of patients in this study.
Debate has been raised about the completeness of endometriosis excision when anterior discoid resection is used. Remorgida et al21 reported positive margins for residual endometriosis in 43.8% of patients who underwent ADR for rectovaginal endometriosis. It is not clear, however, if the risk of recurrence and reoperation is higher based on these results. Our study demonstrated that subjects who underwent ADR had no mucosal involvement, while the mucosa was involved in 61.5% of patients who underwent LAR. Additionally, half of the patients who underwent LAR had 2 or more lesions, which is significantly higher than that in the ADR group. Clearly clinical decision-making is biased both by the surgeon's impression of the extent of the disease and the ability to perform ADR without compromising the rectal lumen with the primary closure of the defect.
Bailey et al13 reported follow-up data on 130 women after aggressive surgical management of colorectal endometriosis. Sixty months after surgery, 86% of the patients had complete or nearly complete relief of symptoms and no recurrence.
Kavallaris et al14 followed 50 patients after laparoscopic bowel resection with minilaparotomy. Seventy-two percent of patients were symptom free at a mean of 32 months after surgery. Four percent of patients had recurrent disease that was resected.
A prospective study by Thomassin et al22 including 27 women undergoing colorectal resection for endometriosis reported that at 22-month follow-up, significant relief of nonmenstrual pelvic pain, dysmenorrhea, dyspareunia, dyschezia, and diarrhea, and improved overall quality of life were noted. Our cohort of patients was followed for a mean of 41 months with excellent improvement in symptoms and 93% satisfaction.
Among the well-documented surgical complications of bowel resection and reanastomosis are anastomotic dehiscence and leakage in 3% to 7% of cases and up to 20% in low rectal anastomosis.23 Transient or permanent colostomy may be required in cases of dehiscence and leakage.19 Perineal abscesses and rectovaginal fistulae have also been reported. Jerby et al18 observed one rectovaginal fistula among 7 women, whereas, Urbach et al24 reported one rectovaginal fistula among 29 women after resection by laparotomy. Transient bowel strictures are common. Transient neurogenic bladder leading to urinary retention and, in severe cases, permanent areflexic bladder have also been reported, but the latter is a rare complication.15,19,25 Our cohort of patients had minimal postoperative complications, with only 2 patients requiring rectal dilation for the treatment of distal stricture in the LAR group.
De novo digestive symptoms can develop, particularly after rectal ampulla resection. These may include constipation, difficult defecation, a sense of incomplete emptying, or diarrhea.26,27 The effect of such long-term subjective complications on the patient's quality of life is controversial when the benefits of aggressive treatment of rectovaginal endometriosis are considered.
One of the inherent drawbacks of retrospective studies, such as this study, is the lack of adequate documentation and quantification of baseline criteria and symptoms. In this study, we attempted to assess the patients’ baseline (preoperative) symptoms by asking the patients to recall to the best of their ability and answer the survey questions about their symptoms before the surgery. The same survey was then administered to address current symptoms. A comparison was conducted between the preoperative and current (postoperative) scores. Although this approach may provide valuable information to overcome the retrospective nature of the study, there is a potential of recall bias in reporting the historic, preoperative symptoms.
CONCLUSION
ADR compared with LAR is associated with a shorter operative time, lower blood loss, shorter hospital stay, and a lower rate of anastomotic strictures in the treatment of rectal endometriosis. Other outcomes and satisfaction rates are comparable between the 2 procedures. When feasible, anterior discoid resection should be the treatment of choice for rectovaginal endometriosis. However, this may not be feasible in cases of multifocal rectal involvement, large rectal nodules, recurrent disease, and when luminal stenosis secondary to advanced fibrosis has ensued.
Acknowledgments
The authors would like to acknowledge CTSI/ Li Wang, MS, and Claire Bunker, PhD, for statistical support.
Contributor Information
Nash S. Moawad, Minimally Invasive Gynecologic Surgery, Department of Obstetrics, Gynecology and Reproductive Sciences, Magee-Womens Hospital of UPMC, Pittsburgh, Pennsylvania, USA..
Richard Guido, Minimally Invasive Gynecologic Surgery, Department of Obstetrics, Gynecology and Reproductive Sciences, Magee-Womens Hospital of UPMC, Pittsburgh, Pennsylvania, USA..
Ramesh Ramanathan, Minimally Invasive Surgery, Department of General Surgery, Magee-Womens Hospital of UPMC, Pittsburgh, Pennsylvania, USA..
Suketu Mansuria, Minimally Invasive Gynecologic Surgery, Department of Obstetrics, Gynecology and Reproductive Sciences, Magee-Womens Hospital of UPMC, Pittsburgh, Pennsylvania, USA..
Ted Lee, Minimally Invasive Gynecologic Surgery, Department of Obstetrics, Gynecology and Reproductive Sciences, Magee-Womens Hospital of UPMC, Pittsburgh, Pennsylvania, USA..
References:
- 1. Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987; 69: 727–730 [PubMed] [Google Scholar]
- 2. Redwine DB. Variations in tubal configuration in endometriosis? Fertil Steril. 2006; 85: 267; author reply 267 [DOI] [PubMed] [Google Scholar]
- 3. Nezhat C, Nezhat F, Pennington E, Nezhat CH, Ambroze W. Laparoscopic disk excision and primary repair of the anterior rectal wall for the treatment of full-thickness bowel endometriosis. Surg Endosc. 1994; 8: 682–685 [DOI] [PubMed] [Google Scholar]
- 4. Ponka JL, Brush BE, Hodgkinson CP. Colorectal endometriosis. Dis Colon Rectum. 1973; 16: 490–499 [DOI] [PubMed] [Google Scholar]
- 5. Prystowsky JB, Stryker SJ, Ujiki GT, Poticha SM. Gastrointestinal endometriosis. Incidence and indications for resection. Arch Surg. 1988; 123: 855–858 [DOI] [PubMed] [Google Scholar]
- 6. Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991; 55: 759–765 [DOI] [PubMed] [Google Scholar]
- 7. Fauconnier A, Chapron C, Dubuisson JB, Vieira M, Dousset B, Breart G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril. 2002; 78: 719–726 [DOI] [PubMed] [Google Scholar]
- 8. Fauconnier A, Chapron C. Endometriosis and pelvic pain: epidemiological evidence of the relationship and implications. Hum Reprod Update. 2005; 11: 595–606 [DOI] [PubMed] [Google Scholar]
- 9. Keckstein J, Wiesinger H. Deep endometriosis, including intestinal involvement–the interdisciplinary approach. Minim Invasive Ther Allied Technol. 2005; 14: 160–166 [DOI] [PubMed] [Google Scholar]
- 10. Vercellini P, Crosignani PG, Somigliana E, Berlanda N, Barbara G, Fedele L. Medical treatment for rectovaginal endometriosis: what is the evidence? Hum Reprod. 2009; 24: 2504–2514 [DOI] [PubMed] [Google Scholar]
- 11. Chapron C, Chopin N, Borghese B, Malartic C, Decuypere F, Foulot H. Surgical management of deeply infiltrating endometriosis: an update. Ann N Y Acad Sci. 2004; 1034: 326–37 [DOI] [PubMed] [Google Scholar]
- 12. Singh KK, Lessells AM, Adam DJ, et al. Presentation of endometriosis to general surgeons: a 10-year experience. Br J Surg. 1995; 82: 1349–1351 [DOI] [PubMed] [Google Scholar]
- 13. Bailey HR, Ott MT, Hartendorp P. Aggressive surgical management for advanced colorectal endometriosis. Dis Colon Rectum. 1994; 37: 747–753 [DOI] [PubMed] [Google Scholar]
- 14. Kavallaris A, Chalvatzas N, Hornemann A, Banz C, Diedrich K, Agic A. 94 months follow-up after laparoscopic assisted vaginal resection of septum rectovaginale and rectosigmoid in women with deep infiltrating endometriosis. Arch Gynecol Obstet. [DOI] [PubMed] [Google Scholar]
- 15. Fedele L, Bianchi S, Zanconato G, Bettoni G, Gotsch F. Long-term follow-up after conservative surgery for rectovaginal endometriosis. Am J Obstet Gynecol. 2004; 190: 1020–1024 [DOI] [PubMed] [Google Scholar]
- 16. Redwine DB. Surgical Management of Endometriosis. New York: Martin Dunitz, 2004 [Google Scholar]
- 17. Redwine DB, Sharpe DR. Laparoscopic segmental resection of the sigmoid colon for endometriosis. J Laparoendosc Surg. 1991; 1: 217–220 [DOI] [PubMed] [Google Scholar]
- 18. Jerby BL, Kessler H, Falcone T, Milsom JW. Laparoscopic management of colorectal endometriosis. Surg Endosc. 1999; 13: 1125–1128 [DOI] [PubMed] [Google Scholar]
- 19. Darai E, Thomassin I, Barranger E, et al. Feasibility and clinical outcome of laparoscopic colorectal resection for endometriosis. Am J Obstet Gynecol. 2005; 192: 394–400 [DOI] [PubMed] [Google Scholar]
- 20. Duepree HJ, Senagore AJ, Delaney CP, Marcello PW, Brady KM, Falcone T. Laparoscopic resection of deep pelvic endometriosis with rectosigmoid involvement. J Am Coll Surg. 2002; 195: 754–758 [DOI] [PubMed] [Google Scholar]
- 21. Remorgida V, Ragni N, Ferrero S, Anserini P, Torelli P, Fulcheri E. How complete is full thickness disc resection of bowel endometriotic lesions? A prospective surgical and histological study. Hum Reprod. 2005; 20: 2317–2320 [DOI] [PubMed] [Google Scholar]
- 22. Thomassin I, Bazot M, Detchev R, Barranger E, Cortez A, Darai E. Symptoms before and after surgical removal of colorectal endometriosis that are assessed by magnetic resonance imaging and rectal endoscopic sonography. Am J Obstet Gynecol. 2004; 190: 1264–1271 [DOI] [PubMed] [Google Scholar]
- 23. Fingerhut A, Elhadad A, Hay JM, Lacaine F, Flamant Y. Infraperitoneal colorectal anastomosis: hand-sewn versus circular staples. A controlled clinical trial. French Associations for Surgical Research Surgery. 1994; 116: 484–490 [PubMed] [Google Scholar]
- 24. Urbach DR, Reedijk M, Richard CS, Lie KI, Ross TM. Bowel resection for intestinal endometriosis. Dis Colon Rectum. 1998; 41: 1158–1164 [DOI] [PubMed] [Google Scholar]
- 25. Seracchioli R, Poggioli G, Pierangeli F, et al. Surgical outcome and long-term follow up after laparoscopic rectosigmoid resection in women with deep infiltrating endometriosis. BJOG. 2007; 114: 889–895 [DOI] [PubMed] [Google Scholar]
- 26. Zanolla R, Torelli T, Campo B, Ordesi G. Micturitional dysfunction after anterior resection for rectal cancer. Rehabilitative treatment. Dis Colon Rectum. 1988; 31: 707–709 [DOI] [PubMed] [Google Scholar]
- 27. Dehni N, Tiret E, Singland JD, et al. Long-term functional outcome after low anterior resection: comparison of low colorectal anastomosis and colonic J-pouch-anal anastomosis. Dis Colon Rectum. 1998; 41: 817–822; discussion 822–823 [DOI] [PubMed] [Google Scholar]