Abstract
Uterine leiomyomas (fibroids, myomas) are benign tumors that develop from smooth muscle cells. Although the most common gynecologic tumor in premenopausal women, there is still little known of the etiology, the genetics and basic/molecular biology, or the influence of the environment on the development and growth of these tumors. The fact that fibroids occur during the reproductive years and regress after menopause indicates a growth dependent on ovarian hormones. Studies have supported a role of estrogen and progesterone in leiomyoma growth possibly through regulating growth factors and their signaling pathways. Activation of steroid hormone receptors can have a myriad of effects and include upregulation of growth factors and receptor tyrosine kinases (RTKs), which through downstream effector proteins such as mitogen-activated protein kinase p44/42, can mediate transcription, translation, and cell proliferation. Due to their hormonal dependency, fibroids may also be targeted by environmental chemicals whose biological effects are mediated through the estrogen and/or progesterone receptors. This review focuses on the role of growth factors and their receptors (RTKs) in uterine leiomyoma growth and their regulation by ovarian hormones. It also presents data on specific signaling pathways activated in uterine leiomyomas and the “cross talk” between the estrogen receptor α and RTK signaling pathways.
Keywords: Receptor tyrosine kinases (RTKs), hormonal regulation, uterus, leiomyoma, growth factors, fibroids
DIFFERENTIAL EXPRESSION OF ACTIVATED RECEPTOR TYROSINE KINASES IN UTERINE LEIOMYOMAS
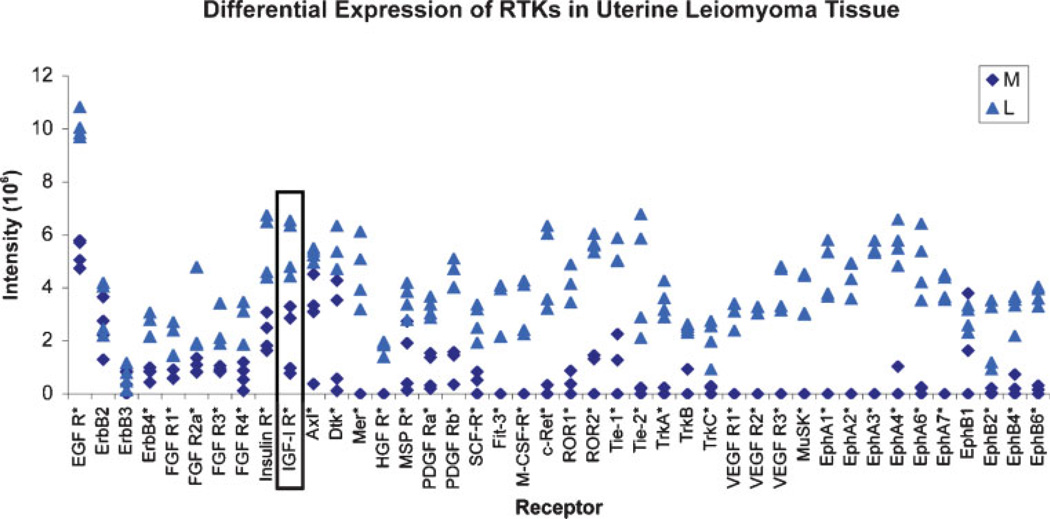
Receptor tyrosine kinase (RTK)-mediated signals play major roles in the regulation of various cellular processes such as control of cell growth and differentiation.1 The essential and diverse roles of RTKs are evident in various developmental abnormalities and tumors that occur due to overexpression of RTK proteins or abnormal stimulation by autocrine growth factor loops contributing to constitutive RTK signaling irregularities.2 Members of large groups of RTK proteins have been classified on the basis of their structural and ligand affinity properties. The RTK family includes several well-known subfamilies; some of them proved highly expressed in leiomyomas (Fig. 1), including the epidermal growth factor receptors (EGFR or ErbB), fibroblast growth factor receptors (FGFR), the insulin and the insulin-like growth factor receptors (IR and IGFR), the platelet-derived growth factor receptors (PDGFRα, β), the vascular endothelial growth factor receptors (VEGFR), and the hepatocyte growth factor receptors (HGFR).1,3 Lesser known RTKs possibly also involved in leiomyoma growth that our laboratory has identified to be overly expressed in fibroids compared with normal myometrium, include EphA1–7, EphB2,4,6, Axl, c-Ret, Tie-1, 2, Ror1, 2, Dtk, and others (Fig. 1).
Figure 1.
Differential expression of phosphorylated growth factor receptor tyrosine kinases (RTKs) in leiomyoma (L) and myometrial (M) tissues (*p<0.02 to 0.03; L vs M).
Uterine leiomyomas express many types of growth factors that may promote leiomyoma growth through local paracrine and/or autocrine mechanisms.4 Several studies have shown that growth factors and their receptor-mediated signaling pathways are important in uterine leiomyoma growth. One such growth factor, epidermal growth factor (EGF), is mitogenic and expressed more in leiomyomas than in myometrial tissue during the luteal phase of the menstrual cycle;5 in addition, its receptor, EGFR, is more sensitive to regulation by sex steroids in leiomyomas than those in the myometrium.6 Leiomyoma cell growth is effectively blocked by TKS050, a new EGFR inhibitor.7 The presence of large quantities of bFGF stored in the extracellular matrix (ECM) of the fibroids and the more intense expression of FGF receptor in leiomyomas than in the myometrium suggest a role for FGF in proliferation of smooth muscle cells in leiomyomas.8,9 The mRNA expression of another potent mitogen, PDGF, has been found in leiomyomas, and PDGF receptor sites per cell are increased in leiomyomas compared with the myometrium.10 PDGF also acts with other growth factors such as transforming growth factor beta (TGF-β), EGF, and the IGFs to enhance proliferation. The mRNA expression and protein levels of IGF-I have been reported to be higher in leiomyomas than in the myometrium. The levels of IGF-I receptor in leiomyomas have also been reported to exceed those of the myometrium,11 which suggest that IGF-I and the IGF-IR signaling pathway may be of major significance in the growth of uterine leiomyomas.
Based on the studies just cited, it appears there may be different families of RTKs and their ligands involved in leiomyoma growth and development. To fully understand the association between upregulation of various growth factor RTKs and leiomyoma development, studies were conducted in our laboratory to assess RTK expression profiles in human uterine leiomyoma and matched myometrial tissues by using a RTK array technique. We found that 39 out of 42 RTKs evaluated were highly expressed in the leiomyomas compared with myometrial samples (Fig. 1).
During the past decade, several studies have identified upregulation of lesser known RTKs in leiomyomas, as we have noted in our RTK array studies. These include Dtk, a RTK reported to be involved in the regulation of hematopoiesis, particularly during embryonic stages of blood cell development, and also possibly involved in tumorigenesis. Upregulation of this receptor may increase the chances of fibroid growth and survival through its hematopoietic effects. If a tumor can somehow increase its blood supply internally through increased hematopoiesis, and externally through increased angiogenesis, it would be more likely to increase in size faster, and prevent necrosis of nutrient and oxygen starved regions.12 Another RTK not often reported in uterine leiomyomas is stem cell factor receptor (SCF-R). SCF-R is thought to play an essential role in erythroid cell development. Stem cell factor (SCF) and erythropoietin (Epo) synergistically activate mitogen-activated protein kinases (MAPKs) that correlate cell growth. This receptor may play a role synergistically with some of the factors that are responsible for increased hematopoiesis, by increasing survival of those erythroid progenitor cells developed in the tumor through upregulation of hematopoietic factors.8 Fit-3 is a hematopoietic growth factor and may act in concert with some of the other hematopoietic growth factors to increase tumor blood supply.
Macrophage colony-stimulating factor (M-CSF) is a growth factor of the mononuclear phagocytic lineage that participates in immunological and inflammatory reactions, bone metabolism, and pregnancy. M-CSF/M-CSF-R could potentially participate in the physiology of infectious, inflammatory, and neoplastic diseases.13 Another RTK is RET, which is the receptor for glial-derived neurotrophic factor growth factors. Like other RTKs, once activated, RET recruits a variety of signaling molecules that mediate a myriad of biological responses.14 Ror1 is related to muscle-specific kinase (MuSK), a muscle-specific tyrosine kinase. The Ror family of proteins are expressed in many tissue types during development and are thought to play a role in cell migration and in orientation of cell polarity.15 Mice lacking one of the two Ror RTK gene products display defects in bone and heart formation. MuSK seems to be involved at the neuromuscular junction with mediation of the synapse-inducing role of motor neuron-derived agrin (Agrin is a motor neuron-derived factor that directs formation of the postsynaptic apparatus of the neuromuscular junction).16
EphA, a RTK expressed in leiomyomas is involved in developmental and pathological angiogenesis. Particularly A-class Eph RTKs have recently emerged as critical regulators of tumor angiogenesis. Overexpression of Eph RTKs has been observed in several types of cancers in mouse models and humans. Expression of the ligand, ephrin-A1, has been predominantly detected in tumor cells, while the majority of EphA2 RTK protein is localized to tumor-associated endothelium, suggesting that ephrin-A1 might serve as a proangiogenic signal to attract EphA2-positive endothelial cells. Ephrin-A1 expression is seen in normal human endometrial epithelial cells and may contribute to normal endometrial angiogenesis.17 There are also interactions between VEGF and EphA, and because VEGF contributes to angiogenesis, this interaction might promote tumor growth and expand the blood supply during proliferation.3 The upregulation of these different families of phosphorylated growth factor RTK proteins in tumors and in leiomyoma samples found in the preceding studies and in our RTK array thus indicates that multiple growth factors are important in the pathogenesis and growth of fibroids.
Another RTK overexpressed in leiomyomas is the Ax1 receptor. This is the RTK for the ligand Gas6, the protein product of growth arrest-specific gene 6. Ax1 has functions in developmental processes and plays roles in the function of the hematopoietic and nervous systems and in tumorigenesis. Gas6 is a growth-potentiating factor for thrombin-induced proliferation of vascular smooth muscle cells. Levels of Ax1 receptor and Gas6 are reportedly higher in uterine leiomyoma tissues compared with normal myometrial tissues. The signal transduction of Gas6 and Ax1 might be important factors involved in the development and growth of uterine leiomyomas.18
RECEPTOR TYROSINE KINASE SIGNALING IN UTERINE LEIOMYOMAS
Growth factors recognize and activate their cognate receptors and stimulate receptor dimerization, tyrosine kinase activation, and auto-phosphorylation. The autophosphorylated RTKs recruit and activate a receptor-specific complement of intracellular signaling pathways that relay information to the nucleus and other intracellular compartments.19 Upon ligand binding, the kinase is activated and autophosphorylates itself on tyrosine residues located within the cytoplasmic tail, creating docking sites for proteins containing phosphotyrosine-binding domains and forming the starting point for a variety of different signaling cascades that regulate cell physiology. In particular, Ras-Erk/MAP kinase and phosphatidylinositide-3 kinase (PI3K)-AKT pathways represent two critical signaling cascades induced on the activation of RTKs by trophic factors.3,20
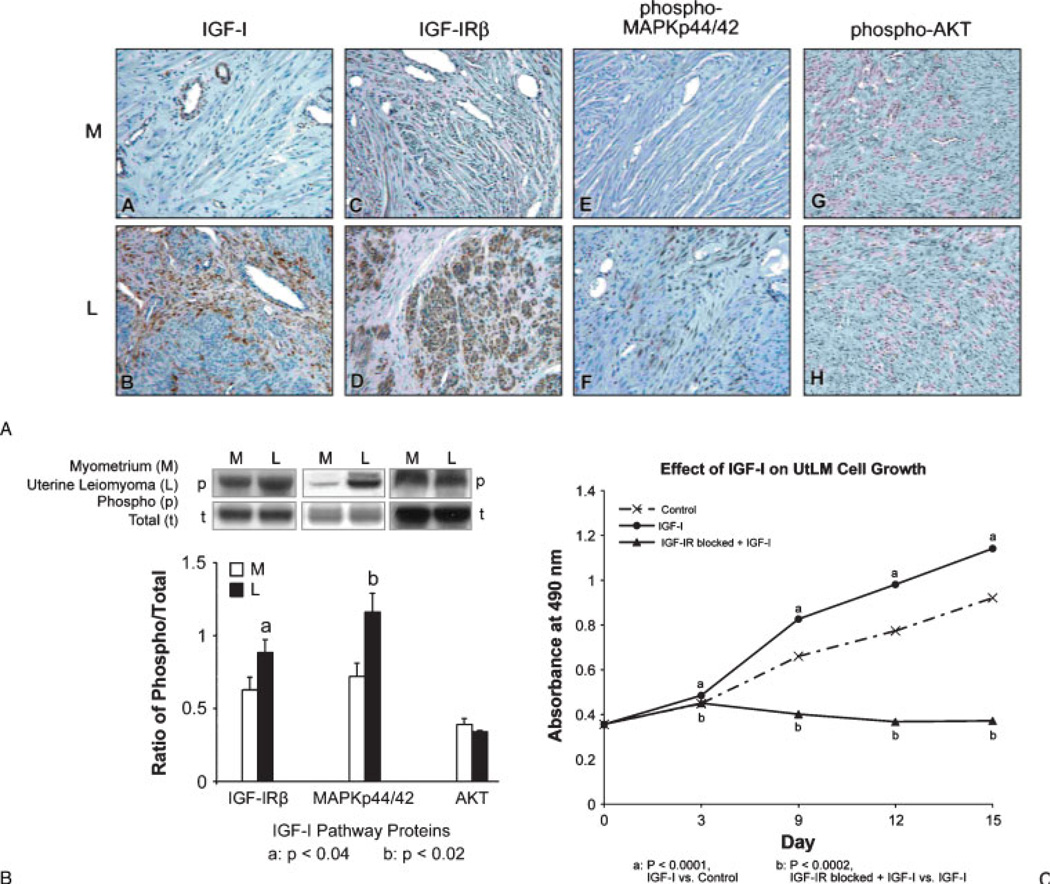
Some studies have shown that ovarian hormones, such as estradiol (E2), can induce PDGF secretion and trigger rapid protein tyrosine phosphorylation of a subset of intracellular proteins, and activate the MAP-kinase pathway in leiomyoma cells.21 Others have shown that leiomyoma growth and fibrosis formation may be regulated through both TGF-β/Smad and MAPK-mediated signaling pathways.22 In addition, it has been reported that phosphorylation of EGF-R and PDGF-R/MAPK signaling pathways mediated by NADPH oxidase-derived ROS leads to leiomyoma cell proliferation.23 Although many of the RTKs have been identified and have been thought to play a role in fibroid growth or in the production of ECM proteins, in our studies we have found that IGF-IR is a major RTK protein that appears to be pivotal to the adequate function of other growth factors and plays a critical role in the mitogenic action of many growth factors. In our RTK array studies, we have confirmed the overexpressed levels of phosphorylated IGF-IR in leiomyoma tissue (Fig. 1) and have also found that the downstream effectors, Src homology/collagen (Shc), growth factor receptor-bound protein 2 (Grb2), and MAPKp44/42, are also increased and involved in IGF-IR signaling in these tumors during the proliferative phase, whereas IRS-I, PI3K, and AKT are not4 (Figs. 2A, B); our results are consistent with the findings of no difference in IR tyrosine kinase activity and IRS-1 expression between normal myometrium and leiomyomas in another study.24 In vitro studies in our laboratory have also shown that IGF-I (100 ng/mL) stimulates the proliferation of uterine leiomyoma cells (UtLM) (Fig. 2C) and that phosphorylated IGF-IRβ, Shc, and MAPKp44/42 are also overexpressed in IGF-I-treated UtLM cells, similar to the tissue findings. Further, neutralizing antibody against the IGF-IRβ blocked these effects. These data indicate that activation of the IGF-IR/MAPK pathway in fibroids is important in uterine leiomyoma growth.4
Figure 2.
(A) Insulinlike growth factor (IGF)-I (A, B), IGF-IRβ (C, D), phospho-MAPKp44/42 (E, F) were significantly overexpressed in leiomyomas (L) compared with myometrial (M) tissue by immunohistochemistry. There was no difference in staining between myometrial and tumor tissues for phospho-AKT (G, H). (B) Western blot analysis of IGFI/IGF-IR pathway activation in leiomyoma (L) and myometrial (M) tissues. (C) Proliferative effects of IGF-I (100 ng/mL) on uterine leiomyoma (UtLM) cells. The proliferative effect of IGF-I was inhibited by a neutralizing antibody to IGF-IRβ (A–C: Modified and reprinted, with permission, from Yu L, Saile K, Swartz CD, et al. Differential expression of receptor tyrosine kinases (RTKs) and IGF-1 pathway activation in human uterine leiomyomas. Mol Med 2008;14(5–6):264–275.)
REGULATION OF RECEPTOR TYROSINE KINASES BY ESTROGEN AND PROGESTERONE
Uterine leiomyomas grow during the reproductive years but regress after menopause, indicating that these tumors are regulated by hormones.25 Both of the ovarian hormones, estrogen and progesterone, are believed to play an important role in the growth of uterine leiomyomas; 25 however, increasing evidence indicates that there are other modulators of leiomyoma growth in addition to the sex steroids.26 Studies have shown that circulating steroid hormone levels are similar for women with and without leiomyomas, indicating that specific local tissue-regulated factors may be involved in the pathogenesis of these tumors.27 The heterogeneity of leiomyoma growth within a given uterus, despite identical exposure of these tumors to similar circulating sex steroid concentrations, suggests the involvement of local cytokines and/or growth factors.28
Estrogen elicits its physiological effects by binding to estrogen receptors (ERs)α and β. However, nontraditional ERα inactivation may also occur by growth factor signaling pathways via phosphorylation of the ERα at specific serines sites.29 Studies have shown that in human breast cancer cells, ERα is mainly phosphorylated on Ser118 and to a lesser extent on Ser104 and Ser106, in the presence or absence of estradiol binding. 29,30 Phosphorylation of ERα influences its activity and may cause ERα-mediated transcription. Prior to our recent publication31 there were no studies that had reported the role of phosphorylation of ERα at Ser118 in human or rodent uterine leiomyomas.
Progesterone (P4) has also been shown to have both an inhibitory and stimulatory effect on leiomyoma growth.5 Evidence indicates that this hormone can induce leiomyoma cell proliferation and upregulate EGF.32 The downregulation of IGF-I mRNA and protein expression by progesterone has been shown in uterine leiomyoma cells in vitro.33 The mechanisms involved in the “cross talk” between the steroid hormone receptor pathways and their interactions with growth factor receptor pathways remain to be elucidated for uterine leiomyomas.
A few studies have suggested that hormones and growth factor signaling pathways mediate the growth of leiomyomas.26,34 In vitro, it has been shown that interactions occur between the MAPK and estrogen pathways via the ER.21 Constitutively activated MAPK (phospho-p44/42 MAPK) is highly expressed in leiomyoma and myometrial tissues; furthermore, this protein is significantly increased in leiomyomas compared with normal myometrial tissue.4,35 One study has suggested there is an increase in MAPK activity following estradiol treatment of leiomyoma cells and that an indirect interaction occurs between the ER and growth factor pathways due to increased secretion of PDGF and activation of the MAPK pathway.21 With the exception of our laboratory findings, currently no other studies have addressed the mechanisms by which phosphorylation of ERα can occur via growth factor pathways in fibroids. However, the exact molecular mechanism(s) by which phosphorylation occurs remains to be investigated.
ESTROGEN RECEPTOR α, RECEPTOR TYROSINE KINASE, AND MITOGEN-ACTIVATED PROTEIN KINASE “CROSS TALK” IN UTERINE LEIOMYOMAS
It is generally accepted that estrogen exerts its effects in estrogen-responsive tissues by binding to the ER and modulating the transcription of target genes including growth factors.22 Nevertheless, genomic effects of estrogen that are attributable to transcriptional activation following ligand receptor binding can be supplemented or augmented by cytoplasmic signaling pathways, such as the MAPK pathway transducted from growth factor receptors or plasma membrane localized ERs.22 Therefore, estrogen signaling might be coupled to growth factor signaling through feedback mechanisms in which the regulation of growth factors by estrogen could be enhanced by the activation of growth factor receptors. E2 treatment of leiomyoma cells produces rapid and transient activation of the MAP-kinase pathway, including the rapid protein tyrosine phosphorylation of proteins, such as GAP, PI3K, and PLC. Moreover, PDGF, alone or in association with other growth factors, is a major growth factor involved in the proliferation response of leiomyoma cells to E2 stimulation, which suggests that growth factor secretion is an initial and integral part of the signaling events mediated by the ER, perhaps unrelated to E2 transcriptional modulation.21 The expression of IGF-I mRNA is increased in leiomyomas, and ERα mRNA is positively correlated with IGF-I mRNA, which implies that estrogen upregulates the gene encoding IGF-I through ERα in leiomyoma;34,37 ERβ may also be involved in the IGF-I signaling pathway in leiomyoma, although to a lesser extent.37 Interruption of the estrogen signaling pathway using dominant-negative estrogen receptors (DNER) results in modulation of both estrogen- and progesterone-regulated genes including cyclin D1, Cox2, PCNA, VEGF, EGF, COMT, and MMP1 that control leiomyoma cell proliferation, ECM formation, and estrogen metabolism.38
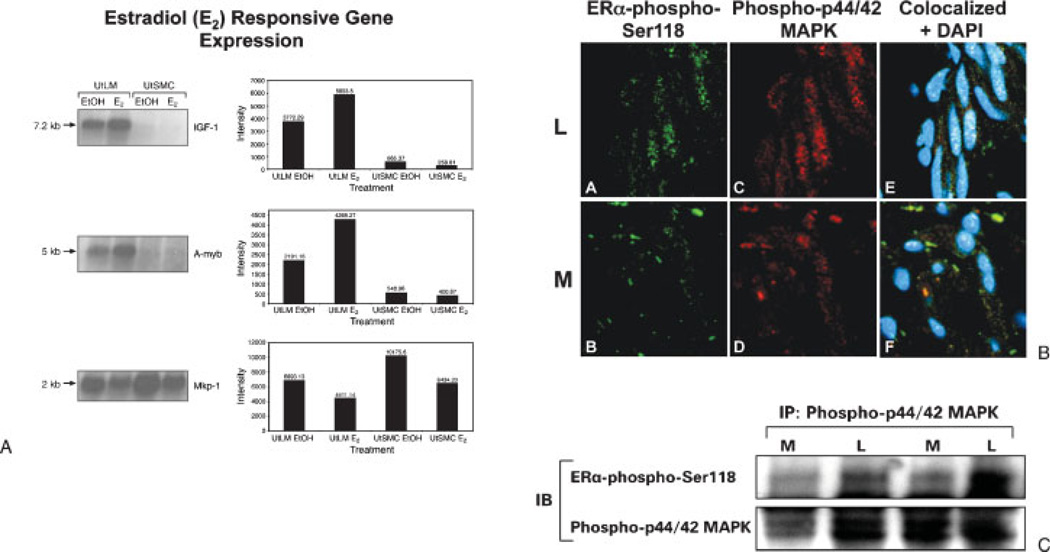
As shown by our laboratory4,39,40 and others,11,41 IGF-IR is one of the growth factor receptors that is highly expressed in human uterine leiomyoma tissues relative to the expression in myometrium. Estrogen has been shown to regulate IGF-I gene expression in the rodent42 and nonhuman primate uterus,43 and IGF-I gene expression is most abundant in the uterus in the proliferative phase in women when estrogen levels are higher.44 Previously we have conducted in vitro studies using microarray analysis where we found that IGF-I and other novel genes potentially involved in the IGF-IR-MAPK signaling pathways were differentially expressed in uterine leiomyoma cells compared with uterine myometrial cells following estradiol (E2) treatment. Our results showed that E2 upregulated the gene expression in leiomyoma cells of IGF-I and A-myb, a transcription factor that promotes cell cycle progression; however, there was downregulation of map kinase phosphatase (MKP-1), a dual specificity phosphatase that dephosphorylates MAPK and prolongs its activity (Fig. 3A).
Figure 3.
(A) Changes in insulinlike growth factor (IGF)-I, A-myb, and MKP-1 mRNA levels in uterine leiomyoma (UtLM) and uterine smooth muscle cells (UtSMC) 24 hours following treatment with 17β-E2 by Northern blot analyses (Reprinted with permission from Swartz CD, Afshari CA, Yu L, Hall KE, Dixon D. Estrogen-induced changes in IGF-1, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol Hum Reprod 2005;11(6):441–450.) (B) Colocalization of estrogen receptor (ER)α-phospho-Ser118 and phospho-p44/42 MAPK in myometrium and uterine leiomyoma tissue. The nuclei are positively stained for ERα-phospho-Ser118 (green fluorescence), phospho-p44/42 MAPK (red fluorescence), and both ERα-phospho-Ser118 and phospho-p44/42 MAPK (white/ yellow fluorescence). DAPI was used to stain the nuclei. (C) Immunoprecipitation of ERα-phospho-Ser118 and phospho-p44/42 MAPK in myometrial (M) and uterine leiomyoma (L) tissue lysates from the proliferative phase. (B, C: Modified and reprinted, with permission, from Hermon TL, Moore AB, Yu L, et al. Estrogen receptor alpha (ERalpha) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch 2008;453(6):557–569.)
In an attempt to identify whether RTK pathways might be involved in estrogen-regulated uterine leiomyoma growth, studies were done in our laboratory to determine if significantly increased levels of ERα-phospho- Ser118 and phospho-MAPK observed in the tumors from the proliferative phase were colocalized and if there was interaction between these two proteins. We found that colocalization of ERα-phospho-Ser118 and phospho-MAPK was more apparent in the nuclei of leiomyoma compared with myometrial cells in tissue samples using confocal microscopy, and that there was increased immunoprecipitation of ERα-phospho-Ser118 and phospho-MAPK in leiomyomas compared with myometrial tissue (Figs. 3B, C) during the proliferative phase. Based on our findings, we concluded there is interaction between the ERα and the IGF-I growth factor signaling pathways.
ENVIRONMENTAL ESTROGENS, INSULIN-LIKE GROWTH FACTOR I RECEPTOR, MITOGEN-ACTIVATED PROTEIN KINASE, AND ESTROGEN RECEPTOR α INTERACTIONS IN UTERINE LEIOMYOMAS
Environmental estrogens, such as natural plant compounds (phytoestrogens) and industrial by-products (industrial estrogens) have been found to increase the incidence of uterine leiomyomas and uterine adenocarcinoma in rodents later in life.45,46 Direct evidence for a role of environmental estrogens in the pathogenesis of fibroids is limited; however, both in vivo and in vitro rodent models have shown that the enhanced sensitivity of uterine leiomyomas to environmental estrogens is possibly modulated via the ER.47 Genistein, an estrogenic soy-derived compound belonging to the isoflavone class of phytoestrogens, is commonly consumed in the diet, and there is some concern as to the beneficial and/or adverse physiological effects of this compound. Plasma concentrations of genistein have been reported to reach 4 µM (~1 µg/mL) in Japanese populations consuming a soy-rich diet.48 At cellular and molecular levels, genistein at concentrations ~1 µM (~0.27 µg/mL) stimulates the growth of human breast cancer cells.49 Similar effects of genistein (≤1 µg/mL) have also been observed in human UtLM cells in our laboratory.40
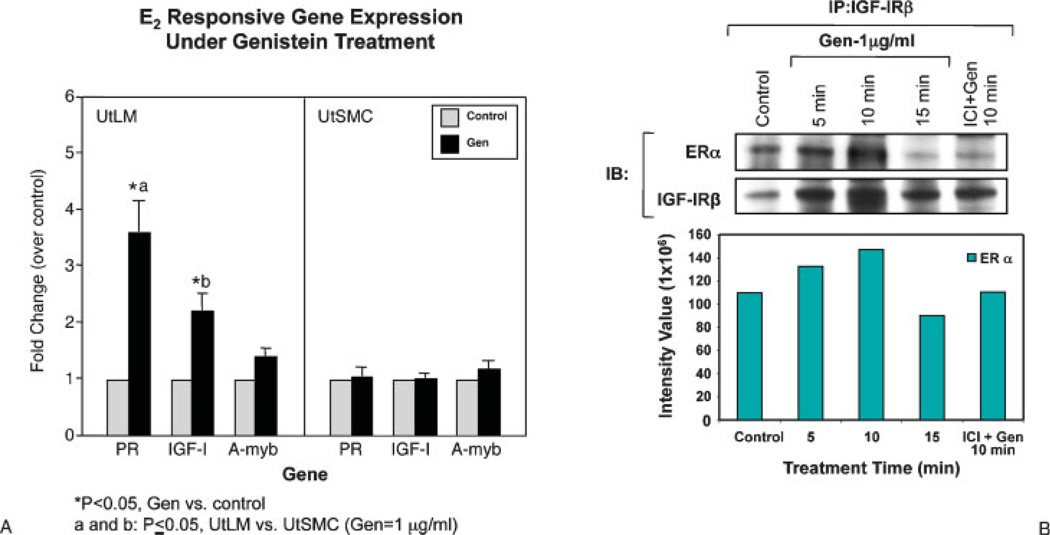
In our studies, we have found that increased proliferation in response to a low dose of genistein treatment observed in UtLM cells compared with uterine smooth muscle cells (UtSMC) may be due to direct activation of gene transcription through the classical estrogen-responsive promoter element in UtLM cells and to significantly increased mRNA expression of early estrogen-responsive genes, such as progesterone receptor and IGF-I in UtLM, but not UtSMC (Fig. 4A). MAPK, Src homology/collagen (Shc), and ERα were also transiently activated, and interactions between ERα and IGF-I receptor (IGF-IR) were rapidly induced by genistein in UtLM cells (Fig. 4B). Further, the ER antagonist ICI 182,780 and the MAPK/ERK kinase (MEK) inhibitor PD98059 inhibited early phosphorylation and receptor association events, and they abrogated the stimulatory growth effects of genistein in UtLM cells.
Figure 4.
(A) Real-time polymerase chain reaction measurement of selected estrogen-responsive genes expressed in human uterine leiomyoma (UtLM) cells and human uterine smooth muscle cells (UtSMC) treated with 0.3% dimethylsulfoxide (control) or 1 µg/mL of genistein (Gen) for 24 hours. (B) Gen-induced interactions between estrogen receptor (ER)α and insulin-like growth factor I receptor (IGF-IR) in human uterine LM cells were determined by using immunoprecipitation (IP) and immunoblotting (IB). The interaction between ERα and IGF-IR was inhibited by the estrogen antagonist ICI 182,780 (A, B: Reprinted, with permission, from Di X, Yu L, Moore AB, et al. A low concentration of genistein induces estrogen receptor-alpha and insulin-like factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod 2008;23(8):1873–1883.)
SUMMARY
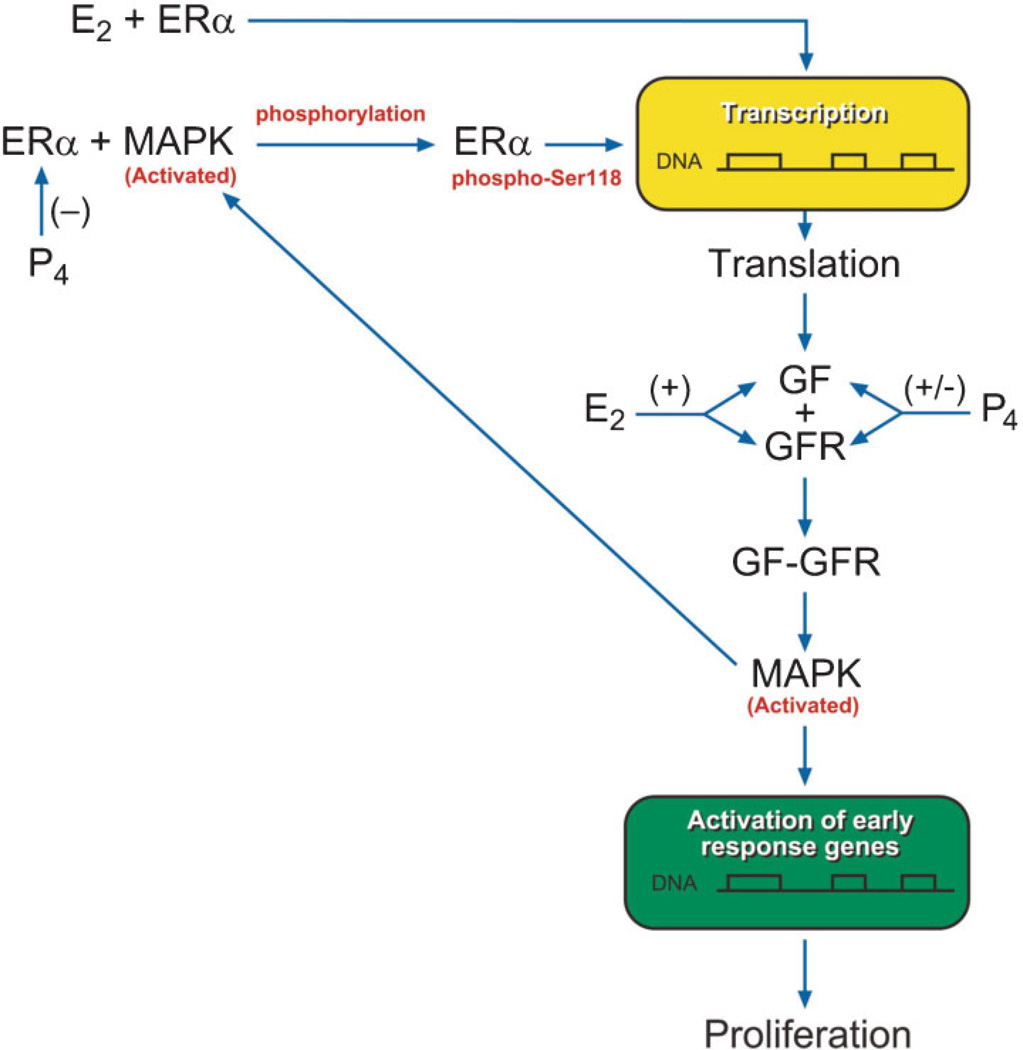
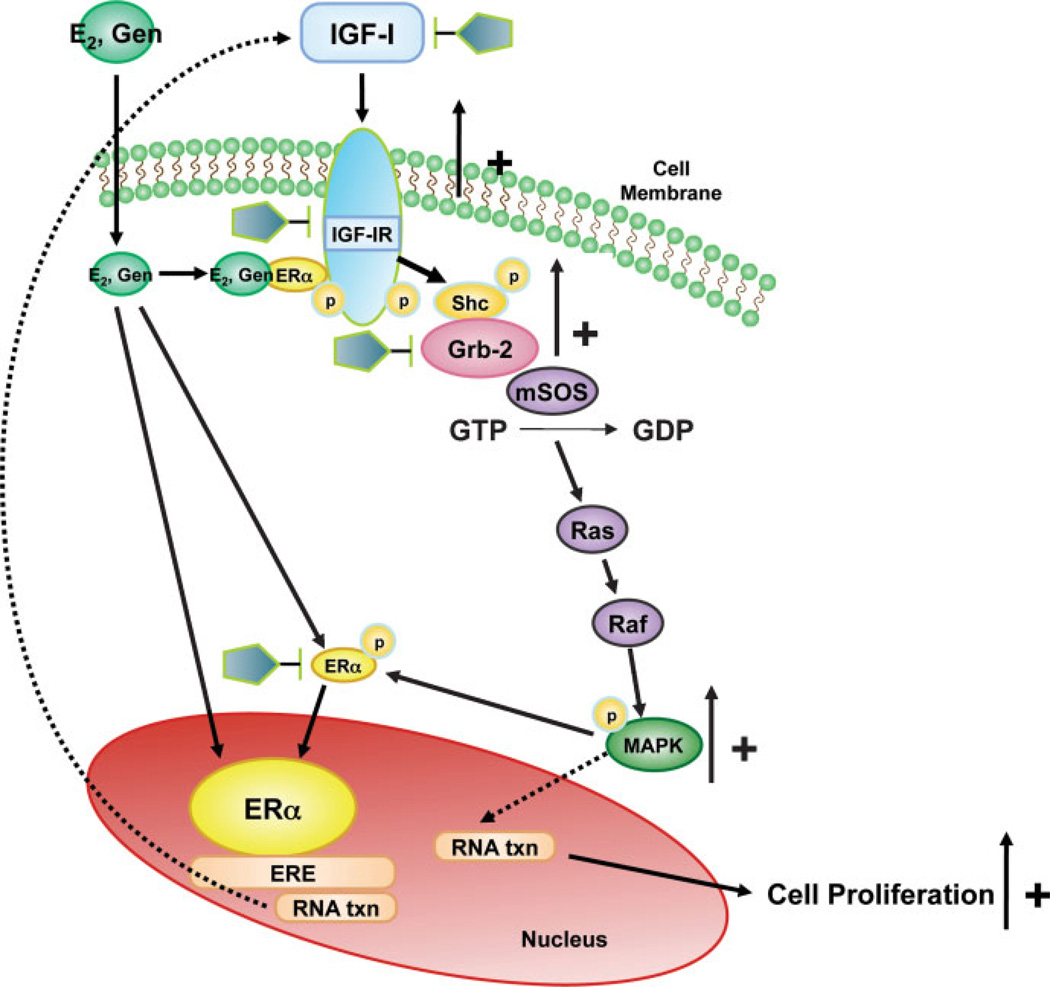
The collective data on RTKs show their importance in uterine leiomyoma regulation and growth. Specific RTKs such as IGF-IR overexpression appears to be associated with increased activation of the downstream effector phospho-MAPK predominantly by a Shc/Grb-2 mediated pathway in uterine leiomyoma tissue, and this is mimicked in vitro. Additionally, studies demonstrate that there is “cross talk” between the ERα and RTK pathways. Specifically, it has been shown that ERα-phospho-Ser118 and phospho-MAPK protein expression levels are significantly increased independently and highly coexpressed in uterine leiomyomas from women in the proliferative phase of the menstrual cycle compared with myometrial tissue samples and compared with tumors from the secretory phase. It has been suggested that a possible mechanism for the interaction between the ERα and growth factor signaling pathways may be through the upregulation of growth factor signaling pathways by E2 and subsequent activation of the downstream effector MAPK resulting in phosphorylation of ERα at serine 118 as shown in Fig. 5. These studies are novel in that pathways involved in phosphorylation of ERα in uterine leiomyomas had not been previously reported. There is further evidence that RTK signaling pathways play an important role in the growth of UtLM cells in response to estrogens and that interactions between the RTKs and ERα and their signaling pathways may also be involved in uterine leiomyoma growth induced by environmental estrogens. These findings underscore the importance of the RTK signaling pathways in uterine leiomyoma growth and in regulating the effects of exogenous estrogens. As shown in Fig. 6, we propose that genistein binds to ERα and induces interactions between ERα and IGF-IR, leading to early phosphorylation of Shc (P-Shc) and MAPK, which may then in turn lead to activation of ERα at Ser sites resulting in enhanced ER transcriptional activity and upregulation of IGF-I expression, thus resulting in a positive feedback regulatory mechanism and/or autocrine mechanism that stimulates UtLM cell proliferation. This would provide a novel explanation for the hyperresponsiveness of UtLM cells to a low dose of genistein, compared with UtSMC as we have previously reported.41 By delineating molecular signaling mechanisms and identifying novel proteins activated in fibroids that are responsible for their growth, it is possible that unique molecules can be targeted for the treatment of uterine fibroids.
Figure 5.
Proposed pathway for the interaction between estrogen receptor (ER)α and growth factor (GF) and growth factor receptor (GFR) signaling pathways and regulation by estradiol (E2) and progesterone (P4). MAPK, mitogen-activated protein kinase. Reprinted, with permission, from Hermon TL, Moore AB, Yu L, et al. Estrogen receptor alpha (ERalpha) phospho-serine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch 2008;453(6):557–569.)
Figure 6.
Proposed pathway for the interaction between estrogen receptor (ER)α and growth factor (GF) signaling pathways and regulation by estradiol (E2) and genistein (Gen). We propose that Gen binds to ERα and induces interactions between ERα and insulin-like growth factor I receptor (IGF-IR), leading to early phosphorylation of Shc (P-Shc) and mitogen-activated protein kinase (MAPK), with subsequent activation of ER at Ser 118 sites resulting in enhanced ER transcriptional activity and upregulation of IGF-I expression resulting in a positive feedback regulatory mechanism and/or autocrine mechanism that stimulates UtLM cell proliferation. Also IGF-I can bind to IGF-IR, and in turn activate the MAPK pathway through a Shc/Grb-2 pathway, which can result in proliferation and also phosphorylation of ERα at serine 118. The pentagons represent possible novel sites that can be targeted for the treatment of symptomatic fibroids. GDP, guanosine diphosphate; GTP, guanosine triphosphate.
REFERENCES
- 1.Li E, Hristova K. Role of receptor tyrosine kinase transmembrane domains in cell signaling and human pathologies. Biochemistry. 2006;45(20):6241–6251. doi: 10.1021/bi060609y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennasroune A, Gardin A, Aunis D, Crémel G, Hubert P. Tyrosine kinase receptors as attractive targets of cancer therapy. Crit Rev Oncol Hematol. 2004;50(1):23–38. doi: 10.1016/j.critrevonc.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2(10):795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Saile K, Swartz CD, et al. Differential expression of receptor tyrosine kinases (RTKs) and IGF-I pathway activation in human uterine leiomyomas. Mol Med. 2008;14(5–6):264–275. doi: 10.2119/2007-00101.Yu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Ohara N, Wang Z, et al. A novel selective progesterone receptor modulator asoprisnil (J867) downregulates the expression of EGF, IGF-I, TGFbeta3 and their receptors in cultured uterine leiomyoma cells. Hum Reprod. 2006;21(7):1869–1877. doi: 10.1093/humrep/del035. [DOI] [PubMed] [Google Scholar]
- 6.Flake GP, Andersen J, Dixon D. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect. 2003;111(8):1037–1054. doi: 10.1289/ehp.5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shushan A, Ben-Bassat H, Mishani E, Laufer N, Klein BY, Rojansky N. Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil Steril. 2007;87(1):127–135. doi: 10.1016/j.fertnstert.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 8.Mangrulkar RS, Ono M, Ishikawa M, Takashima S, Klagsbrun M, Nowak RA. Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol Reprod. 1995;53(3):636–646. doi: 10.1095/biolreprod53.3.636. [DOI] [PubMed] [Google Scholar]
- 9.Wolańska M, Bańkowski E. Fibroblast growth factors (FGF) in human myometrium and uterine leiomyomas in various stages of tumour growth. Biochimie. 2006;88(2):141–146. doi: 10.1016/j.biochi.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Liang M, Wang H, Zhang Y, Lu S, Wang Z. Expression and functional analysis of platelet-derived growth factor in uterine leiomyomata. Cancer Biol Ther. 2006;5(1):28–33. doi: 10.4161/cbt.5.1.2234. [DOI] [PubMed] [Google Scholar]
- 11.Van der Ven LT, Roholl PJ, Gloudemans T, et al. Expression of insulin-like growth factors (IGFs), their receptors and IGF binding protein-3 in normal, benign and malignant smooth muscle tissues. Br J Cancer. 1997;75(11):1631–1640. doi: 10.1038/bjc.1997.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crosier PS, Freeman SA, Orlic D, Bodine DM, Crosier KE. The Dtk receptor tyrosine kinase, which binds protein S, is expressed during hematopoiesis. Exp Hematol. 1996;24(2):318–323. [PubMed] [Google Scholar]
- 13.Motoyoshi K. Function, molecular structure and gene expression of macrophage colony-stimulating factor [in Japanese] Nippon Rinsho. 1992;50(8):1861–1866. [PubMed] [Google Scholar]
- 14.Pützer BM, Drosten M. The RET proto-oncogene: a potential target for molecular cancer therapy. Trends Mol Med. 2004;10(7):351–357. doi: 10.1016/j.molmed.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Forrester WC. The Ror receptor tyrosine kinase family. Cell Mol Life Sci. 2002;59(1):83–96. doi: 10.1007/s00018-002-8407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoch W. Molecular dissection of neuromuscular junction formation. Trends Neurosci. 2003;26(7):335–337. doi: 10.1016/S0166-2236(03)00131-0. [DOI] [PubMed] [Google Scholar]
- 17.Brantley-Sieders DM, Chen J. Eph receptor tyrosine kinases in angiogenesis: from development to disease. Angiogenesis. 2004;7(1):17–28. doi: 10.1023/B:AGEN.0000037340.33788.87. [DOI] [PubMed] [Google Scholar]
- 18.Sun WS, Fujimoto J, Tamaya T. Clinical implications of coexpression of growth arrest-specific gene 6 and receptor tyrosine kinases Axl and Sky in human uterine leiomyoma. Mol Hum Reprod. 2003;9(11):701–707. doi: 10.1093/molehr/gag082. [DOI] [PubMed] [Google Scholar]
- 19.Schlessinger J, Lemmon MA. Nuclear signaling by receptor tyrosine kinases: the first robin of spring. Cell. 2006;127(1):45–48. doi: 10.1016/j.cell.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 20.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–3121. doi: 10.1038/sj.onc.1210394. [DOI] [PubMed] [Google Scholar]
- 21.Barbarisi A, Petillo O, Di Lieto A, et al. 17-beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol. 2001;186(3):414–424. doi: 10.1002/1097-4652(2000)9999:999<000::AID-JCP1040>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 22.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mesquita FS, Dyer SN, Heinrich DA, et al. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod. 2010;82(2):341–351. doi: 10.1095/biolreprod.108.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orcy RB, Brum I, da Silva RS, Kucharski LC, Corleta HE, Capp E. Insulin receptor tyrosine kinase activity and substrate 1 (IRS-1) expression in human myometrium and leiomyoma. Eur J Obstet Gynecol Reprod Biol. 2005;123(1):107–110. doi: 10.1016/j.ejogrb.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 25.Cook JD, Walker CL. Treatment strategies for uterine leiomyoma: the role of hormonal modulation. Semin Reprod Med. 2004;22(2):105–111. doi: 10.1055/s-2004-828616. [DOI] [PubMed] [Google Scholar]
- 26.Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78(1):1–12. doi: 10.1016/s0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- 27.Buttram VC, Jr, Reiter RC. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981;36(4):433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- 28.Dixon D, Flake GP, Moore AB, et al. Cell proliferation and apoptosis in human uterine leiomyomas and myometria. Virchows Arch. 2002;441(1):53–62. doi: 10.1007/s00428-001-0568-7. [DOI] [PubMed] [Google Scholar]
- 29.Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68(1):1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 30.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. EMBO J. 1996;15(9):2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 31.Hermon TL, Moore AB, Yu L, Kissling GE, Castora FJ, Dixon D. Estrogen receptor alpha (ERalpha) phosphoserine-118 is highly expressed in human uterine leiomyomas compared to matched myometrium. Virchows Arch. 2008;453(6):557–569. doi: 10.1007/s00428-008-0679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimomura Y, Matsuo H, Samoto T, Maruo T. Upregulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83(6):2192–2198. doi: 10.1210/jcem.83.6.4879. [DOI] [PubMed] [Google Scholar]
- 33.Yamada T, Nakago S, Kurachi O, et al. Progesterone downregulates insulin-like growth factor-I expression in cultured human uterine leiomyoma cells. Hum Reprod. 2004;19(4):815–821. doi: 10.1093/humrep/deh146. [DOI] [PubMed] [Google Scholar]
- 34.Swartz CD, Afshari CA, Yu L, Hall KE, Dixon D. Estrogen-induced changes in IGF-I, Myb family and MAP kinase pathway genes in human uterine leiomyoma and normal uterine smooth muscle cell lines. Mol Hum Reprod. 2005;11(6):441–450. doi: 10.1093/molehr/gah174. [DOI] [PubMed] [Google Scholar]
- 35.Chegini N, Luo X, Ding L, Ripley D. The expression of Smads and transforming growth factor beta receptors in leiomyoma and myometrium and the effect of gonadotropin releasing hormone analogue therapy. Mol Cell Endocrinol. 2003;209(1–2):9–16. doi: 10.1016/j.mce.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 36.Kato S, Endoh H, Masuhiro Y, et al. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270(5241):1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 37.Zhao Y, Zhang W, Wang S. The expression of estrogen receptor isoforms alpha, beta and insulin-like growth factor-I in uterine leiomyoma. Gynecol Endocrinol. 2008;24(10):549–554. doi: 10.1080/09513590802340522. [DOI] [PubMed] [Google Scholar]
- 38.Hassan MH, Salama SA, Arafa HM, Hamada FM, Al-Hendy A. Adenovirus-mediated delivery of a dominant-negative estrogen receptor gene in uterine leiomyoma cells abrogates estrogen- and progesterone-regulated gene expression. J Clin Endocrinol Metab. 2007;92(10):3949–3957. doi: 10.1210/jc.2007-0823. [DOI] [PubMed] [Google Scholar]
- 39.Dixon D, He H, Haseman JK. Immunohistochemical localization of growth factors and their receptors in uterine leiomyomas and matched myometrium. Environ Health Perspect. 2000;108 Suppl 5:795–802. doi: 10.1289/ehp.00108s5795. [DOI] [PubMed] [Google Scholar]
- 40.Di X, Yu L, Moore AB, et al. A low concentration of genistein induces estrogen receptor-alpha and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod. 2008;23(8):1873–1883. doi: 10.1093/humrep/den087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Ven LT, Gloudemans T, Roholl PJ, et al. Growth advantage of human leiomyoma cells compared to normal smooth-muscle cells due to enhanced sensitivity toward insulin-like growth factor I. Int J Cancer. 1994;59(3):427–434. doi: 10.1002/ijc.2910590323. [DOI] [PubMed] [Google Scholar]
- 42.Murphy LJ, Ghahary A. Uterine insulin-like growth factor-1: regulation of expression and its role in estrogen-induced uterine proliferation. Endocr Rev. 1990;11(3):443–453. doi: 10.1210/edrv-11-3-443. [DOI] [PubMed] [Google Scholar]
- 43.Adesanya OO, Zhou J, Bondy CA. Sex steroid regulation of insulin-like growth factor system gene expression and proliferation in primate myometrium. J Clin Endocrinol Metab. 1996;81(5):1967–1974. doi: 10.1210/jcem.81.5.8626866. [DOI] [PubMed] [Google Scholar]
- 44.Giudice LC, Irwin JC, Dsupin BA, et al. Insulin-like growth factor (IGF), IGF binding protein (IGFBP), and IGF receptor gene expression and IGFBP synthesis in human uterine leiomyomata. Hum Reprod. 1993;8(11):1796–1806. doi: 10.1093/oxfordjournals.humrep.a137937. [DOI] [PubMed] [Google Scholar]
- 45.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199(2):142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 46.Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61(11):4325–4328. [PubMed] [Google Scholar]
- 47.Hunter DS, Hodges LC, Eagon PK, et al. Influence of exogenous estrogen receptor ligands on uterine leiomyoma: evidence from an in vitro/in vivo animal model for uterine fibroids. Environ Health Perspect. 2000;108 suppl 5:829–834. doi: 10.1289/ehp.00108s5829. [DOI] [PubMed] [Google Scholar]
- 48.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342(8881):1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 49.Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17(2):271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]