Abstract
A growing body of evidence highlights the importance of a mother’s nutrition from preconception through lactation in programming the emerging organ systems and homeostatic pathways of her offspring. The developing immune system may be particularly vulnerable. Indeed, examples of nutrition-mediated immune programming can be found in the literature on intra-uterine growth retardation, maternal micronutrient deficiencies, and infant feeding. Current models of immune ontogeny depict a “layered” expansion of increasingly complex defenses, which may be permanently altered by maternal malnutrition. One programming mechanism involves activation of the maternal hypothalamic-pituitary-adrenal axis in response to nutritional stress. Fetal or neonatal exposure to elevated stress hormones is linked in animal studies to permanent changes in neuroendocrine-immune interactions, with diverse manifestations such as an attenuated inflammatory response or reduced resistance to tumor colonization. Maternal malnutrition may also have a direct influence, as evidenced by nutrient-driven epigenetic changes to developing T regulatory cells and subsequent risk of allergy or asthma. A 3rd programming pathway involves placental or breast milk transfer of maternal immune factors with immunomodulatory functions (e.g. cytokines). Maternal malnutrition can directly affect transfer mechanisms or influence the quality or quantity of transferred factors. The public health implications of nutrition-mediated immune programming are of particular importance in the developing world, where prevalent maternal undernutrition is coupled with persistent infectious challenges. However, early alterations to the immune system, resulting from either nutritional deficiencies or excesses, have broad relevance for immune-mediated diseases, such as asthma, and chronic inflammatory conditions like cardiovascular disease.
Introduction
The “developmental origins of health and disease” paradigm maintains that nutritional or other environmental stimuli during critical periods of growth and development have the potential to permanently “program” the structure and/or function of cell populations, emerging organ systems, or homeostatic pathways (1). Although the immediate sensitivity of the embryo and fetus to external insults was well known, epidemiological studies carried out by Barker et al. (2, 3) in the 1980s first revealed that events in fetal life could influence longer-term disease risk. Work in this field has primarily considered general nutritional conditions reflected by anthropometric measures (e.g. low birth weight) more so than micronutrient nutriture. It has also focused largely on chronic conditions resulting from alterations to the developing brain, kidney, and pancreas (4). A more limited set of studies has addressed plasticity in the immune system, including specific functional changes (5–9), disease susceptibility (10), and risk of mortality from infection (11–17).
Perturbations to the emerging immune system, particularly from nutritional imbalances, both deficiency and excess, can have considerable effects throughout life. Of primary public health concern is excess morbidity and mortality from infectious disease in infants and young children. However, the increased risk may continue throughout childhood, adolescence, and adult life. Moreover, alterations to the immune system’s complex regulatory functions during early life can have consequences for autoimmune or allergic disorders, as well as chronic inflammatory conditions such as metabolic syndrome, cardiovascular disease, diabetes, and cancer (18–20).
Current status of knowledge
Ontogeny of the immune system
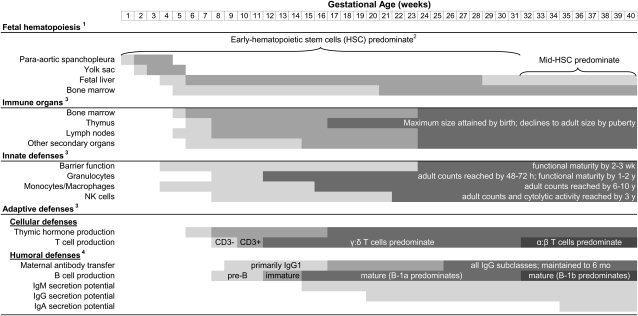
A modern understanding of the origins and development of the immune system is fundamental to studying its nutritional mediation in the fetus. In humans, immune cells and organs rapidly proliferate in the first trimester of pregnancy. Early cells undergo progressive waves of maturation, some unique to the fetal period, as they build the capacity to recognize and adapt defenses to specific pathogens (21). Although the immune system is qualitatively complete at birth, exposures during infancy and early childhood are essential for expansion and priming of adaptive cell populations. These critical periods of development are highly vulnerable to insult, which may permanently alter immune defenses. Ontogenesis of the immune organs, innate and adaptive defenses is discussed below (Fig. 1).
Figure 1.
Developmental timeline of immune cells, organs, and effector functions. Lighter shading indicates stem cell homing to site; darker shading indicates primary site of hematopoiesis at given developmental stage. Timing of early-, middle-, and late-hematopoietic stem cells is not yet well defined in humans. Shading indicates relative maturation of organs (i.e. primordial, populated by progenitors, mature) and cell populations (i.e. cell counts and activity). For maternal antibodies, shading indicates increasing transfer efficiency; adult concentrations of IgM, IgG1, IgG3, and IgG4 are attained by 1–2 y, whereas IgG2 and IgA do not reach adult concentrations until puberty.
Organ development.
Stem cells in the para-aortic splanchopleura, the yolk sac, and the liver sequentially generate lymphomyeloid precursors during the embryonic and early fetal periods (22). Although the liver remains the primary site of fetal hematopoiesis, circulating precursors home to the developing bone marrow as early as gestational wk 5 and this organ progressively assumes responsibility for lymphomyeloid progenitor activity (23). The thymus also emerges in wk 5 from the endoderm of the 3rd pharyngeal pouch (24). Although no lymphocytes are present, thymic epithelial cells differentiate and start secreting hormones at 5–6 wk gestation (25). Colonization by T cell precursors follows (26), with gestational wk 7–14 representing the critical period for thymic growth and development (27).
Secondary lymphoid organs appear during the late embryonic and early fetal periods (28), when liver-derived hematopoietic progenitors home to and cluster with mesenchymal cells at the sites of future organs (29). Lymphotoxin signaling by these clusters enables them to attract and capture additional hematopoietic cells, forming a primitive anlagen (30), and subsequently guides structural development, as in the segregation of B and T cells in the spleen (31). Development of the secondary lymphoid organs is highly ordered and largely complete by the time of birth (30, 32). However, recent work indicates that these organs, and particularly the gut-associated ymphoid tissues, retain considerable plasticity to environmental stimuli throughout life (33).
Lymphopoiesis.
As noted above, precursor cells migrate to the developing immune organs, which support their differentiation and maturation into lymphoid and myeloid immune cell populations (23). A growing body of evidence, largely based on murine models, suggests that these processes are developmentally programmed. Herzenberg (34) has referred to this as the “layered evolution” of adaptive immunity, whereby an early layer of broad defenses is followed by layers of increasingly complex mechanisms that recognize and control specific pathogens. The earliest immune layer is derived from pre-HSC3, unique to the fetal and neonatal period. Pre-HSC are theoretically overtaken by mid-HSC around the time of weaning and finally the HSC of normal adults (35). Other models suggest a distinction only between pre-HSC and adult HSC (36). The concept of layered immune development is supported by the identification of phenotypically distinctive fetal progenitors in mice, which are derived from endothelial cells of the yolk sac and para-aortic splanchopleura (37, 38). Recent research by Mold et al. (21) extends these findings to humans, revealing differences between fetal and adult HSC, as well as the lymphocyte lineages arising at different stages of development.
Innate defenses.
The epidermal and mucosal barriers that comprise the front line of innate defense appear early in gestation but rapidly mature in the 3rd trimester to provide adequate protection within a few weeks of birth (28, 39). Complement proteins are also present in the first trimester and nearly all reach 50–75% of adult concentrations at term (40). Innate effector cells are detectable in emerging immune organs and fetal circulation by the end of the first trimester (41–43). Rapid DNA synthesis and mitotic division enable granulocytes to meet or surpass adult levels within 48–72 h; however, exhaustion of expansion capacity and surface protein expression limit innate effector cell functions during infancy (40, 44, 45). Tissue macrophages, NK cells, and natural killer cytolytic activity do not reach adult levels until later in childhood (46, 47).
Adaptive-cellular defenses.
Lymphoid progenitors are present in the thymus by approximately 8 wk of gestation. Maturing thymocytes produce CD3 mRNA by 10 wk and begin somatic recombination of TCR genes between 11 and 22 wk of gestation (26). Fetal T cells are capable of responding to challenge (48); however, most remain naïve due to reduced in utero antigen exposure (49). Early CD4+ T cells also express CD45RA, limiting their helper activity and thereby suppressing CD8+ cytolytic effector function (50). T lymphocytes that are activated in early life are typified by ant-inflammatory T-helper 2 responses and tolerance induction, whereas inflammatory T-helper 1 responses are developmentally delayed until ∼1 y of age (51). In addition to classic CD4+ and CD8+ effector cells, a subset of CD4+ cells expressing transcription factor FOXP3, i.e. natural TReg cells, are also present in the fetal thymus (52). Some work suggests that TReg production may be at its peak during fetal life (53) and that this subpopulation is largely responsible for tolerance induction in utero (54).
Recent research in humans demonstrates that fetal HSC give rise to T cells that differ both in functional properties and gene expression from adult T cells (21), supporting the hypothesis of layered immune development. Interestingly, fetal HSC-derived T cells exhibited a tolerogenic bias. Similar distinctions are well defined in mice, where early TCR gene rearrangements are highly ordered; bursts of γδ T cells characterized by specific Vγ and Vδ gene segments are produced during fetal and neonatal life (55) but overtaken in infancy by the continuous production of cells with primarily (>95%) αβ TCR (56). Thus, γδ T cells may represent the earliest and most primitive cellular defenses derived from pre-HSC. Although relatively little is known about this population in humans, some extrapolations can be drawn from murine models. Cell populations expressing these specific receptors can be permanently deleted by injecting pregnant dams with anti-γδ TCR antibodies (57). Furthermore, they cannot be replaced in irradiated mice by graft from adult bone marrow. Research in γδ T cell-deficient mice has highlighted the importance of this population in controlling early and rapid multiplication of Listeria microorganisms and malaria parasites (58, 59). Other postulated functions include an innate protective role in the mucosal immune system (56), acting as a bridge between innate and adaptive defenses (60), and downmodulating infection-induced inflammation (61, 62). Natural ligands for human γδ TCR have not been well characterized; however, evidence suggests that γδ T cells differ from αβ T cells primarily by how they are triggered rather than by effector functions (63).
Adaptive-humoral defenses.
Lymphocyte progenitors active during embryogenesis give rise to pre-B cells as early as gestational wk 7 and are actively producing immature B cells in the fetal liver and, to a lesser extent, bone marrow by wk 12 (64). Mature naïve B cells expressing membrane-bound M and D Ig and capable of antibody secretion are present shortly thereafter (65), although only negligible amounts of antibody are produced by the fetus in the absence of intrauterine infection (66). Neonatal humoral responses are generally considered “immature.” Effector functions are limited by low Fc receptor expression and deficiencies in opsonizing complement proteins (51). Moreover, antigen exposure during infancy and early childhood is needed for the B cell priming, production of high affinity antibodies, and memory responses that characterize mature humoral defenses (56). Maturation of certain antibody classes is delayed as well (40); serum IgM reaches adult levels in early childhood, whereas IgA levels not until puberty and adult concentrations of the IgG2 subclass critical for T cell-independent responses to polysaccharides are acquired only by 10–12 y.
B1a cells represent the earliest layer of humoral development. Based on extrapolations from murine models, this population is produced exclusively by a pool of self-replenishing fetal progenitors and cannot be restored by bone marrow stem cells later in life (67, 68). B1b cell progenitors predominantly develop from mid-HSC in the neonatal period, with some limited production by adult bone marrow (69). Although research on the B1 cells has been constrained by lack of exclusive phenotypic markers (70), their functions have been differentiated from conventional B2 cells. B1a cells serve as a first line of defense in the pleural and peritoneal cavities, where they constitutively secrete most of the body’s polyreactive, low-affinity, natural IgM antibodies (71–74). These natural antibodies provide critical protection for infants who have not had prior exposure to common pathogens (75). Recent research is also elucidating the importance of these antibodies in responding to malignant cells (76) and in “housekeeping” after oxidative stress-induced tissue and cellular damage or conventional apoptosis (77–79), suggesting life-long protective roles for B1a cells. Due to their ability to bind self antigens, natural antibodies derived from B1a cells have also been implicated in autoimmune disorders (80); however, their role may in fact be protective (81). Although less is known about B1b cells, some studies suggest their involvement in adaptive responses to T cell-independent antigens, including pneumococcal capsular polysaccharides (82–84).
Transfer of immunity
Although the immune system is qualitatively complete at the time of birth, there are significant delays in the maturation of specific defenses. These delays are necessary to conserve energy and nutrients for other complex organ systems (85). They may further provide a window for the development of tolerance, particularly to food antigens, and protect immature tissues and organs from potentially harmful inflammatory actions. Developmental delays are countered by the transfer of maternal immune factors via the placenta and in breast milk. Thus, maternal immune competence is relevant not only to the health of the pregnancy but also for continued protection of the infant after birth.
Immune transfer mechanisms.
During intrauterine development, the placenta serves as an interface for transport of oxygen, nutrients, and waste and further acts in synthesizing and secreting hormones, growth factors, cytokines, and other bioactive molecules (86, 87). In terms of the immune system, the placenta was initially perceived as a barrier to protect the developing fetal allograft from a maternal immune response (88, 89). Although our understanding of the immune interface between mother and conceptus is still actively evolving (90), it is now well accepted that various immune factors can cross the placenta. To date, antibody transfer from mother to fetus has been the most widely studied; however, there is some evidence of maternal-fetal cell transfer (91–94).
An active mechanism for the transfer of maternal antibodies across the placenta was first proposed by Brambell (95) in the 1960s. As hypothesized, a placental Ig receptor, the Brambell receptor or FcRn, has since been localized to cells of the syncytiotrophoblast, with differential binding affinity for the various IgG subclasses (96). Maternal IgG complexes with the FcRn are shuttled across the syncytiotrophoblast and released at the cytotrophoblast. This placental layer is progressively degraded during pregnancy, enabling antibodies to cross and accounting for the observed rise in fetal IgG levels with increasing gestational age (97, 98). An endothelial organelle containing FcγRIIb2 has recently been identified and is thought to transport IgG across the placenta’s endothelial layer to the fetus (99). Although active transport mechanisms are specific for IgG, transfer of both IgA and IgM classes may occur in cases of moderate to severe uterine infections due to placental changes (100, 101). Maternal antibody concentrations in the infant drop precipitously by 3 mo of age, primarily due to hemodilution, and antibodies are fully catabolized by ∼6 mo of age.
Mammals further rely on breast milk to transfer immune factors to their offspring, although the composition of that milk varies widely between species due to qualitative and quantitative differences in pathogen exposure (85). Numerous components of human breast milk are thought to have a primarily non-nutritional role. These include maternal antibodies, maternal leukocytes, cytokines, chemokines, and hormones. Components of breast milk initially considered for their nutritional value may also confer nonspecific protection against pathogens (102). For instance, PUFA released upon digestion of milk fat globules may also act in destabilizing the cell membranes of certain pathogens (103). In humans, sIgA is the primary defensive protein and has been most widely studied.
SIgA is a hallmark of the gut- and other mucosa-associated lymphoid tissues. It performs immune exclusion, preventing bacterial colonization and invasion, and neutralizes certain viruses (104). Maternal plasma cells primed in mucosal sites home to the lactating mammary gland during late pregnancy and lactation under the influences of the mucosal epithelial chemokine CCL28 (105). This enteromammary connection ensures that factors transferred in milk protect infants against pathogens specific to the maternal environment (49). Plasma cells secrete antibodies, which are recognized and taken up by the polymeric Ig receptor on the surface of mammary epithelial cells, into the interstitial space. At the apical cell surface, the external domain or secretory component of the receptor is cleaved with the bound antibody and released into milk (106). The secretory component may also be released free of bound antibody to perform immune exclusion (107). Although sIgA is the predominant antibody in milk, sIgM and sIgG are also present (108).
Passive protection.
The primary function of maternal immune transfer is to protect the developing fetus and breastfeeding infant from early-life infections (49). Protective effects, particularly against gastrointestinal and respiratory tract infections, of both immediate and exclusive breastfeeding have been demonstrated in numerous epidemiologic studies (109–112). Much of this is thought to be mediated by maternal antibodies (49), primed by the mother’s immune system against pathogens in her environment. Maternal vaccination to drive the production of specific antibodies is, in fact, viewed as a potentially important strategy to reduce the burden of neonatal and infant infection (113). Beyond antibodies, other factors with known protective properties are synthesized by mammary epithelial cells and secreted into milk. Lactoferrin and lysozyme, e.g., are capable of degrading certain bacterial cell walls and have unexplained antiviral and antifungal activities (114). Milk oligosaccharides have antimicrobial activity; they act as receptor analogs, mimicking surface carbohydrates on the gut mucosa, thereby confounding bacterial binding and invasion (115). As noted, fatty acids in milk can weaken the cell membranes of pathogens (103). Immune factors often act synergistically to withhold nutrients from, inhibit binding of, or directly lyse pathogens without necessitating activation of a potentially dangerous inflammatory immune response (49).
Immune modulation.
In addition to the passive protection afforded by factors transferred across the placenta or in breast milk, maternal-fetal transfer may modulate the developmental trajectory of the immune system. Cytokines and chemokines, produced and secreted mainly by mammary epithelial cells, are thought to be the primary immunoregulatory factors in milk (116). For example, cytokines such as TGF-β2, IL-10, and, more recently, thymic stromal lymphopoietin are the focus of numerous studies regarding the protective effects of breastfeeding on the development of allergic or atopic diseases (117–120). The actions of maternal antibodies may also extend beyond passive protection. Antibodies directed at the antigen-binding variable regions of B and T cell receptors, i.e. anti-idiotypic antibodies, have long been recognized and are thought to play a role in sustaining and regulating immune cell populations (121). Findings from both animal (122–126) and human studies (127–130) support the notion that transfer of maternal antiidiotypic antibodies can prime fetal and neonatal cells and may be irreplaceable for the selection of B cell repertoires that recognize common pathogens (131).
Although research on other transferred immune factors is more limited, these may also influence the developing immune system. There is some evidence that activated maternal immune cells may have the ability to migrate into infant tissues and interact with fetal or neonatal cells (132, 133). Hormones and growth factors are present at relatively high concentrations in human milk (134, 135). Although they are thought important for mammary tissue growth and secretory function, they may further influence the maturation of the infant gut and associated lymphoid tissues (134, 136, 137). Other nutritional or defensive factors with established immunomodulatory properties include: PUFA (115), nucleotides (138), glycoproteins (139), oligosaccharides (140), and microRNA (141).
Programming pathways
Insults during critical developmental windows may program the structure and/or function of distinct cell populations, organ systems, or homeostatic pathways. Accepting that the immune system develops in layers, with ontogeny of the most basic defenses limited to early life, it is plausible that nutritional insults during this period can permanently alter specific cell populations and/or organogenesis. Early-life nutritional exposures may also have a lasting impact on the development of protective regulatory mechanisms.
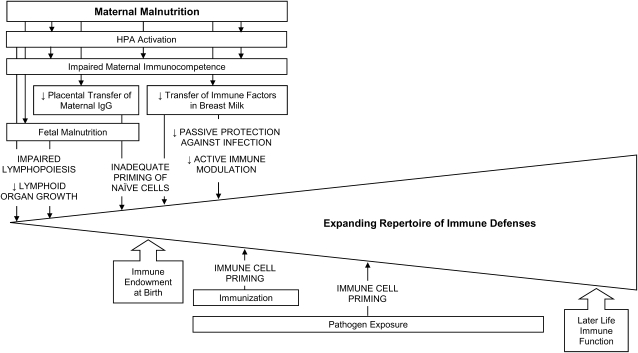
The pathways by which maternal malnutrition may exert an influence on the emerging immune system are discussed below (Fig. 2). These include: 1) alterations to the HPA axis; 2) limiting nutrients available for embryonic and fetal development; and 3) altering the transfer of immunity from mother to child.
Figure 2.
Hypothesized associations between maternal malnutrition and the emerging immune system.
HPA axis.
Malnutrition is perceived by the organism as a threat to homeostasis (i.e. stressor), driving activation of the HPA axis and yielding elevated levels of glucocorticoids (142, 143). This is evident, e.g., in cases of zinc deficiency (144, 145) or protein-energy malnutrition (146). Although fetal exposure to maternal glucocorticoids is usually tightly controlled by placental 11 β-hydroxysteroid dehydrogenase, the activity of this enzyme is reduced by undernutrition (147). Resultant high concentrations of maternal cortisol can directly impact the developing immune system. Early research regarding the potential side effects of corticosteroid administration during pregnancy revealed a reduction in thymic weight (148) as well as morphological changes and a decreased cortical lymphocyte count (149). These observations have since been explained by glucocorticoid activation of an endogenous endonuclease, which results in thymocyte apoptosis (150). Lymphopoiesis, specifically at the pre- and immature stages of B and T cells development, has proven similarly susceptible to endocrine perturbations (151–155). Controlled animal studies suggest that these effects are at least partially reversible (156–158). Indeed, the immediate safety of early-life exposure to corticosteroid drugs in human infants is well accepted (159). However, some concerns have been cited regarding longer term implications for the immune system (160).
Maternal stress hormones also influence the developing and highly sensitive fetal HPA axis (161), with potential consequences for neuroendocrine-immune interactions throughout life. Experimental animal studies of prenatal and neonatal HPA activation have revealed numerous alterations to the immune response later in life. These include differences in the febrile response (162–172), cytokine expression (162, 164, 165, 173–181), antibody production (182), lymphocyte proliferation (183–185), and NK cell activity (186); resistance to tumor colonization (186); intestinal microflora (187); and pain sensation (188). Consequentially, early HPA activation affected longer term risk of both infectious (173) and chronic disease outcomes (175, 186, 189). Some researchers have suggested that these alterations are driven by a potentiated HPA response upon stress later in life, perhaps due to epigenetic changes in the expression of glucocorticoid receptors (190). Support for this hypothesis includes the increased production of corticosteroids or adrenocorticotropin hormone production following acute stress in later life observed in some studies (165–167, 177, 186, 189, 191–194) and, more compellingly, the reversal of observed effects on fever, cytokines, and COX-2 expression by either adrenalectomy or administration of a glucocorticoid receptor-blocking drug (165, 166, 192).
There are some inconsistencies in this literature as to the effects of early-life HPA activation on later life immune responses. For instance, most studies have reported a reduction in the inflammatory response later in life, indicated by lower plasma concentrations of inflammatory cytokines or diminished production of these cytokines upon stimulation (164, 165, 174, 176, 178–180). In contrast, other groups have noted an amplified inflammatory response (173, 175, 181) as well as heightened susceptibility to inflammatory periodontal disease (175). These inconsistencies may be explained in part by differences in animal models (195). The timing of the initial stressor may also be critical; research in rhesus monkeys has demonstrated that exposure to stress hormones during early pregnancy increased the proliferative response of lymphocytes to a foreign antigenic protein, whereas HPA activation in late pregnancy decreased the proliferative response (184). Finally, long-term effects may vary depending on the nature of the early-life stressor (i.e. psychological, nutritional, immune) studied (172). Although differences in study design make it difficult to predict the consequences of early-life exposures, this large body of research underscores the vulnerability of the neuroendocrine-immune axis to developmental programming.
Direct nutritional effects.
Nutritional deficiencies can affect immune ontogeny independently of HPA activation. Murine studies conducted by Beach et al. (196, 197) in the early 1980s indicated that even moderate gestational zinc deprivation can lead to long-term impairments in thymic and spleen size, decreased antibody concentrations, and impaired lymphocyte activity. Although these effects may have been mediated in part by deficiency-related rises in glucocorticoids (144, 198), research from adrenalectomized animals supports an alternate pathway by which deficiency may affect the immune system (145). For example, deficient adrenalectomized mice still exhibited atrophy of the thymic cortex, enlargement of the thymic medulla, and a loss of functional lymphocytes (145). Further support for an alternate pathway is offered by the studies by Beach et al. (199,200); offspring of mice exposed in utero to zinc deficiency also had lower antibody levels and lymphocyte activity than their peers, suggesting an intergenerational epigenetic effect.
Specific molecular mechanisms underlying this type of direct nutritional pathway are an active area of research. Deficiencies during critical periods of tissue growth and differentiation do have the potential to permanently alter structural characteristics. For example, the development of a wide range of tissues and organs is controlled by retinoid receptor signaling (201). This includes the correct patterning of the pharyngeal endoderm (202–204), which comprises the primordial thymus. Restriction of vitamin A by genetic, pharmacologic, or dietary means thus precludes development of the thymus (203, 205–208). Retinoids are also critical for hematopoiesis (209). Although much of the research in this area has focused on the myeloid lineage, investigations of B cell precursors suggest that physiologic levels of retinoic acid may regulate the balance between B lymphocyte growth and apoptosis (210). In more recent work, all-trans retinoic acid treatment of cultured adult B lymphoid progenitors from healthy mice shortened the time required for differentiation into CD19+ cells (211). Cultured fetal progenitors did not respond in a similar manner; however, when mice were exposed to vitamin A deficiency in utero and early life, the researchers noted a stark reduction in both B1a and B1b lymphocyte subsets, which could be corrected by all-trans retinoic acid (212). Although there are currently no published data regarding the actions of retinoids on fetal T lymphocytes, it is possible that the earliest wave of T cells could be similarly affected.
Nutritional factors may also exert an influence on the emerging immune system via epigenetic mechanisms. This has recently been established in studies of allergic, atopic, and autoimmune diseases. One example illustrated that feeding pregnant mice a diet rich in methyl donors had a direct impact on DNA methylation, gene expression, and T lymphocyte development (213). Ultimately, these phenotypic changes to TReg cells increased the severity of allergic airway disease among the offspring of supplemented animals. A similar relationship was reported based on data from a prospective cohort in Norway, in this case showing an association between first trimester folic acid supplementation and increased risk of wheeze in infants and young children (214).
Impaired transfer of immunity.
Maternal immune factors transferred across the placenta or in breast milk can actively modulate immune development. Thus, any impairment to immune transfer may alter the system’s developmental trajectory. Of primary concern is the impact of maternal malnutrition on the quality or quantity of immune factors available for transfer. Interactions between protein-energy or micronutrient deficiencies and immune function are supported by decades of research and are reviewed elsewhere (215–218). The unique immunological state of pregnancy adds yet another dimension. For example, vitamin A deficiency is associated with cytokine dysregulation (219). One human study, conducted among pregnant women in Ghana, suggested that the Type 2 cytokine bias of pregnancy may be exacerbated by vitamin A deficiency (220). In addition to a direct effect on maternal immune factors, malnutrition during pregnancy and lactation may alter the mechanisms of transfer to the fetus or breastfeeding infant.
Placental transfer.
Maternal malnutrition can alter placental development, evidenced by differences in size and morphology, modifications to nutrient transporters, and changes in vascular development and blood flow (221–223). The extent to which these specific alterations may influence maternal transfer of immune factors to the developing fetus is not known. However, both animal and human studies indicate substantial variability in placental transfer, again, with a specific focus on antibodies. Some animal studies have shown differing levels of IgG in neonates, presumably due to altered transfer, with maternal HPA activation, as seen in nutritional stress, during pregnancy (224–226). These effects may depend on the timing and nature of the stressor (225, 227, 228) as well as the sex of the offspring (225).
In healthy human pregnancies, antibody transfer is highly efficient and the concentration of maternal IgG in cord blood exceeds that of IgG in the mother’s serum (229). However, several studies have reported transfer efficiency ratios of <1. Because the majority of maternal IgG is transferred in the last 4–6 wk of pregnancy, preterm delivery is a well-known cause of IgG deficiency in early infancy (98). Placental anomalies associated with the preterm delivery may compound this problem by limiting transfer efficiency, as has been reported in cases of placental malaria (230–233) and HIV infection (233–235). Maternal hypergammaglobulinemia, frequently observed in areas with a high burden of infections, also results in a transfer efficiency ratio < 1 (232, 236, 237), because high levels of maternal antibody exceed the number of placental FcRn (95). Few studies have investigated nutritional influences on placental IgG transfer. Cavalcante et al. (238) reported a 14% reduction in antibody transfer among women with low compared to normal weight-for-height in early pregnancy. Total and pathogen-specific IgG appear to be limited also in small-for-gestational age infants (239–241). Transfer of IgG1 and IgG2 are particularly affected (242). Other work illustrates a role for micronutrients; antenatal zinc supplementation increased cord blood concentrations of all IgG subclasses (243) and, although observational, maternal anemia has been associated with reduced transfer efficiency (239).
Breast milk transfer.
Several studies have also explored variability in breast milk immune factors. Again, animal studies showed an impact of maternal HPA activation on milk sIgA concentrations (244, 245). Human breast milk composition is determined by stage of lactation (246, 247), maternal age, parity (247, 248), and infant’s sex (249). Maternal systemic infection, indicated by elevated serum levels of C-reactive protein, does not appear to alter overall milk volume or immune protein concentrations (250, 251). However, both clinical and subclinical mastitis have an effect on various immune factors (252–254), reflecting their joint actions in protecting both mother and infant. Seasonal variations in breast milk immune factors have also been reported, independent of their impact on maternal and/or infant infection (255, 256). Recent work has pointed to variability in breast milk immune factors by maternal country of birth (comparing immigrants from developing countries to indigenous population) and parity (257, 258).
Some research groups have investigated the role of maternal nutritional status on breast milk immune factors. To our knowledge, only one study has considered nutrition-related variability in breast milk immune factors in the context of a randomized trial. It found that postpartum vitamin A or β-carotene supplementation had no effect on sIgA, lactoferrin, lysozyme, or IL-8 at 3 mo postpartum (252); however, concentrations of these factors were within normal ranges in all treatment groups. With a few exceptions (259, 260), observational studies have not detected any association between maternal nutritional status and breast milk immune factors (246, 261–263). However, there has been little standardization with regard to: 1) nutritional assessment and classification; 2) breast milk collection, processing, and storage; 3) laboratory techniques; and 4) sampling and statistical analyses.
Evidence of nutritionally mediated immune programming
Evidence from human studies, both experimental and epidemiological, suggests that early-life nutritional exposures may influence the developing immune system, with consequences extending even into adulthood.
Intra-uterine growth retardation.
Although intra-uterine growth retardation has multiple etiologies, maternal malnutrition is a major determinant of poor fetal growth. Substantial epidemiological evidence links LBW, a rough indicator of intra-uterine growth retardation, with increased risk of infectious morbidity and mortality later in life. Often-cited studies using data from the United States have highlighted LBW and even moderately LBW as strong determinants of infant mortality and morbidity during infancy and childhood (264–266). However, LBW in developed countries is predominantly due to preterm delivery rather than intra-uterine growth retardation. In Scandinavia, researchers have noted more frequent hospitalization for infectious morbidity throughout childhood and mortality through 15 y of age among children born LBW, even after controlling for gestational age (267, 268). Data from the Dutch Famine also suggest a link between early- and mid-gestation exposure to famine, resulting in lower birth weight and excess mortality up to the age of 18 y (15).
Work in Brazil confirms the association between LBW and both neonatal and post-neonatal mortality (269). Preterm infants had a 2.5-fold higher mortality risk than small-for-gestational age infants during the neonatal period, although these differences were less pronounced afterwards. Further analysis also revealed that infants with both low birth length and low ponderal index were at greatest risk of hospitalization and mortality in the first year of life regardless of gestational age (270). Using datasets from multiple countries, Ashworth (271) found increased risk of neonatal and postneonatal morality among infants born LBW due to intra-uterine growth retardation. Even though RR for small–for-gestational age infants were less consistent across studies than for preterm infants, their mortality risk was elevated even beyond infancy. Similar findings emerged from analysis of long-standing demographic surveillance in the Gambia. Moore et al. (12) reported increased infectious mortality among adults born during the hungry season, characterized by an elevated incidence of nutritionally mediated LBW. This seasonal pattern was not apparent in adult mortality trends from Bangladesh (13), Senegal (16), or Burkina Faso (17). Neither was mortality after the age of 18 y affected by in utero exposure to the Dutch Famine (15). Yet the authors of this latter work stressed the small number of deaths in this cohort, to date, and their limited power to detect survival differences.
Findings from the case-control study by Moore et al. (12) led to the exploration of immune function in Gambian primary school children. Participants were challenged with human diploid cell rabies and 23 valent pneumococcal capsular polysaccharide vaccines, and both a delayed-type hypersensitivity skin reaction and mucosal antibodies were measured (5). This study found no differences between children born in the hungry compared to harvest seasons. Addressing these same questions in an older Pakistani population for whom birth weights were available, researchers reported a less robust response to the Vi polysaccharide typhoid vaccine among those born at a LBW (7). A typhoid vaccine challenge yielded similar results among LBW infants in the Philippines in their teenage years at the time of the study (8). This lack of effect among Gambian individuals compared to study participants in both Pakistan and Bangladesh may be due to their younger age, i.e. differences may not become apparent until later in life (272).
Although differential response to predominantly T cell-independent vaccine responses would suggest otherwise, subsequent work has focused on altered thymic development. In the Gambian children, researchers noted significant seasonal variation in thymic size and function; hungry season babies had a lower thymic index and impaired thymic function, indicated by lower serum TCR excision circles (6). Similar work in Bangladesh confirmed this seasonal pattern and further demonstrated a direct association between thymic size and infant weight (273). In the Philippines, researchers noted that serum thymopoietin concentrations were indeed lower among those who had been born small for gestational age compared to their appropriate for gestational age peers (9). If these differences in the smaller thymus were not due to nutrient restriction in early pregnancy, it has been suggested that they could be mediated by seasonal variations in breast milk IL-7 (274). Subsequent explorations of T lymphocyte kinetics in these populations have yielded conflicting results (275, 276).
Maternal micronutrient deficiencies.
The impact of maternal micronutrient deficiencies on the emerging immune system is a relatively new area of inquiry. Trials of single or multiple micronutrient deficiencies conducted in developing countries have focused primarily on outcomes of infectious morbidity and mortality. In rural Nepal, maternal supplementation with either vitamin A or β-carotene failed to improve fetal or infant survival (277). However, after restricting the analysis to infants whose mothers reported night blindness during pregnancy, supplementation substantially reduced, but did not eliminate, the increased risk of early infant mortality (278). Subsequent work in the same population demonstrated that maternal folic acid supplementation, delivered alone or with iron, reduced early infant mortality among those delivered preterm (279). Similar findings were recently reported from China, where early neonatal deaths were reduced by 54% in the iron-folic acid arm of the trial compared to folic acid alone (280). Trials of antenatal zinc supplementation have also documented significant reductions in infectious morbidity among infants (281–283).
To date, only the antenatal zinc supplementation trials have directly assessed any aspects of infant immune function. The trial conducted in Bangladesh reported fewer anergic delayed-type hypersensitivity responses to purified protein derivative among LBW infants born to supplemented mothers (284). No similar effect was apparent among normal birth weight infants. The authors found no impact on immune response to Haemophilus influenzae type b vaccine, although they noted that high natural infection rates may have masked any beneficial effect. Data from a separate trial in Indonesia showed an impact of antenatal zinc supplementation on ex vivo cytokine response among infants (282). Taken together, the findings of maternal supplementation trials with regard to infant mortality, morbidity, and immune function are suggestive of immune programming. However, any effects may be modified by the severity of maternal deficiency, size at birth, and/or gestational age at delivery.
In addition to studies in developing countries, there is an increasing focus on the importance of maternal micronutrient status in the literature on allergic and atopic diseases (285). Much of this work has concentrated on vitamin E, which is important for promoting a tolerogenic response upon initial allergen exposure (286). Although no randomized trials are yet available, evidence from prospective cohort studies links low maternal vitamin E intake during pregnancy with increased peripheral blood mononuclear cell responsiveness, wheezing/asthma, and markers of airway inflammation (287–290). Vitamin D also plays a role in tolerance induction and the promotion of a regulatory phenotype (291). The impact of vitamin D status during pregnancy on development of immune-mediated diseases is thus a growing area of investigation (292). For example, investigators from a multi-country prospective cohort study reported that vitamin D supplement use during pregnancy increased tolerogenic antigen-presenting cells in cord blood (293). Additional research is needed to expand these findings, both to clarify mechanisms of action and to test prophylactic efficacy in randomized trials (285).
Infant feeding.
Infants exposed to human milk are characterized by an antiinflammatory and tolerance-inducing gut environment (49) as well as immunophenotypic differences in lymphocyte populations (294). Improved mucosal (295) and systemic immune function (296) have also been reported after exposure to breast milk, although the latter may be modified by gestational age (297). Furthermore, breast-fed infants have a larger thymic size than formula-fed infants (298, 299), which correlates well both with thymic function (300) and infant survival (301).
To assess changes in immune function related to breast milk exposure, many groups have relied on vaccine response as an accepted indicator of immune function (302). There have been reports of improved antibody response to the Hemophilus influenzae B conjugate (303, 304), oral polio (305, 306), diphtheria (305), and tetanus toxoid vaccines (305). However, most studies have found little difference in antibody responses between breast- and formula-fed infants for common vaccine antigens (307–312). Findings regarding cellular responses are similarly inconclusive. Although Pabst et al. (313,314) noted an increased T cell proliferative response to BCG vaccination in breastfed infants (313), they also reported lower responses for both measles hemagglutinin (314) and a recall tetanus toxoid challenge (313).
The longer term effects of breastfeeding are also widely debated (315). Several studies have reported a reduced incidence of allergic diseases (316–318), presumably through an early influence on regulatory mechanisms. Similar work has emerged for other diseases of the immune system including rheumatoid arthritis (319, 320), Type 1 diabetes mellitus (321, 322), Crohn’s disease (323, 324), and certain lymphomas (325). However, these findings are countered by a number of studies showing little to no effect (326–334). Whether assessing the response to vaccination or long-term effects on immune-mediated disease, the observational evidence thus far is open to doubt. An impact of infant feeding on the developing immune system is biologically plausible and likely to remain an active area of research (335). However, an experimental design, such as long-term follow-up of participants from the Promotion of Breastfeeding Intervention Trial (336), is needed to further guide this debate.
Conclusions
The emerging immune system is vulnerable to insult, particularly during embryonic and early fetal life. Even once the system is qualitatively complete, the fetus and infant requires continued input from its mother in the form of immune factors transferred across the placenta and in breast milk. Maternal nutritional status may affect both of these pathways. Initial work on nutritional programming of the immune system employed a vaccine challenge as a broad indicator of the body’s ability to respond to infection (5, 7, 8). Although the implications of an altered vaccine response would be easily translatable, the lack of consistency across these studies has been difficult to explain (272). Rather than considering overall immune function, it might be more revealing to focus on those aspects of the immune system that are most plausibly affected by early-life nutritional insults.
Early lymphopoiesis
As discussed above, distinct waves of progenitor cells produce diverse lymphocyte populations with progressively complex functions. The earliest wave of progenitors, a long-lived and self-sustaining population generated only by fetal stem cells, gives rise to the innate-like B1a cells and specialized T cells (67). Little research exists into the influence of early-life nutrition on lymphopoiesis. As noted above, researchers have reported reduced lymphocyte counts and altered cell function in animals exposed to prenatal/neonatal stressors or glucocorticoids (158, 178, 182, 184, 185). These findings may reflect glucocorticoid-mediated changes in the number or function of lymphocyte precursors (151–154). Altered lymphocyte counts or function have also been reported in the animal literature regarding in utero exposure to nutrient deficiencies (337–339). One recent study indicated that fetal exposure to vitamin A deficiency can affect early progenitors, resulting in smaller B1a and B1b lymphocyte populations (212). Given that the earliest wave of progenitors is produced solely by fetal stem cells, any insults during this critical period could have a permanent effect. Future research should closely monitor lymphopoietic trajectories, specifically the earliest progenitors and their innate-like progeny, and investigate any longer term functional consequences that may result from B1a and early T cell deficits.
Immune organ development
Primary and secondary lymphoid organs (e.g. gut-associated lymphoid tissue) undergo rapid expansion during the late fetal and early neonatal periods, during which time they may be uniquely sensitive to nutritional insults. The thymus has been most widely studied to date. Indeed, researchers have long recognized the sensitivity of the thymus to nutritional status (340, 341). Protein-energy malnutrition (342–344) and various single nutrient deficiencies (345–348) are associated with thymic atrophy, attributed both to thymocyte depletion by apoptosis (342, 343, 349) and decreased cell proliferation (350). Morphological changes and decreased hormone production have also been reported for thymic epithelial cells (351). These alterations are mediated at least in part by concomitant hormonal changes (152, 352) and are thought to be reversible. Based on ultrasound images, the thymus recovers in size within 2 mo of nutrition rehabilitation (353, 354). Recovery has not been well studied at the cellular level.
The thymus emerges early in pregnancy, undergoing a critical period of development from 7 to 14 wk of gestation and reaches its maximum size relative to body weight by the time of birth (24, 27). The period of “thymic education” in mid-pregnancy, at which point CD4+CD8+ thymocytes undergo both positive and negative selection, is also considered a period of susceptibility (355). Given the organ’s sensitivity to malnutrition, exposures during these critical periods may be particularly important. Indeed, animal studies support a link between protein-energy and/or micronutrient deficiencies during pregnancy and thymic size/function among offspring (196, 356–359). There is some evidence that these in utero effects on the thymus are not fully reversible (196). As discussed above, epidemiologic studies also support the notion that early-life nutritional exposures can have a lasting impact on thymic size and function (6, 9, 274, 276). Additional research on nutritional programming of immune organ development is clearly warranted, with future studies including both primary and secondary lymphoid organs.
Regulatory mechanisms
Exposures during pregnancy and early life may further influence the development of protective regulatory mechanisms, i.e. how the immune system maintains a balance between mounting an effective immune response against pathogens and preventing inflammatory damage to host tissues. As previously described, there are several examples from the animal literature regarding the lasting impact of in utero or neonatal exposure to stressors on inflammatory processes later in life (162, 164, 165, 173–181). These changes have largely been attributed to programming of the fetal HPA axis, which can permanently alter interactions between the neuroendocrine and immune systems (161, 190). Early-life influences on immunoregulatory mechanisms are also evident from the literature on allergic, atopic, and autoimmune disorders (360, 361). Adequate maternal intake of vitamins E and D in particular appear important for emerging regulatory mechanisms and protection against airway inflammation (285, 287–290, 293). Recent reports also indicate the sensitivity of TReg cell populations to epigenetic programming (213, 362).
The highly complex nature of immune regulation makes it difficult to predict how early nutritional exposures may influence regulatory development. In the HPA programming literature, long-term effects have varied widely depending on the timing of the stressor (184), the nature of the stressor (172), and the choice of animal model (195). The allergy/atopy literature also stresses the complex and often confusing interplay between “a host’s immune response, characteristics of the invading microorganisms, the level and variety of the environmental microbial exposure and a genetic background (363).” Although prior research offers some basis for future studies, there is also ample justification for exploratory work in this field as scientists continue to unravel the mechanisms of immune regulation.
Summary
Maternal malnutrition, including both nutritional deficiencies and excesses, is highly prevalent and can have lasting consequences for the health of offspring. Here we have highlighted several plausible pathways by which in utero nutrient deprivation may influence the nascent immune system. Indeed, considerable evidence exists in the literature of associations between early-life nutritional exposures and immune alterations extending throughout childhood and, in some cases, adult life. Perturbations to the emerging immune system resulting from early nutritional imbalances may have immediate consequences for susceptibility to infection during infancy as well as implications for later-life risk of immune-mediated and/or inflammatory disease.
Acknowledgments
I thank Dr. Keith P. West Jr. from the Johns Hopkins Bloomberg School of Public Health for his feedback on the manuscript. The sole author had responsibility for all parts of the manuscript.
Footnotes
Supported in part by the Procter and Gamble Company, Cincinnati, OH; the U.S. Agency for International Development, Washington, DC, under the Global Research Activity (GHS-A-00-03-00019-00) with Johns Hopkins University; and The Bill and Melinda Gates Foundation (grant no. 614), Seattle, WA.
Author disclosures: A. C. Palmer, no conflicts of interest.
Abbreviations used: HPA, hypothalamic-pituitary-adrenal; HSC, hematopoietic stem cell; LBW, low birth weight; sIgA, secretory IgA; TCR, T cell receptor; TReg, regulatory T cell.
Literature Cited
- 1.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261:412–7 [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80 [DOI] [PubMed] [Google Scholar]
- 4.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 2010;427:333–47 [DOI] [PubMed] [Google Scholar]
- 5.Moore SE, Collinson AC, Prentice AM. Immune function in rural Gambian children is not related to season of birth, birth size, or maternal supplementation status. Am J Clin Nutr. 2001;74:840–7 [DOI] [PubMed] [Google Scholar]
- 6.Collinson AC, Moore SE, Cole TJ, Prentice AM. Birth season and environmental influences on patterns of thymic growth in rural Gambian infants. Acta Paediatr. 2003;92:1014–20 [PubMed] [Google Scholar]
- 7.Moore SE, Jalil F, Ashraf R, Chen Szu S, Prentice AM, Hanson LA. Birth weight predicts response to vaccination in adults born in an urban slum in Lahore, Pakistan. Am J Clin Nutr. 2004;80:453–9 [DOI] [PubMed] [Google Scholar]
- 8.McDade TW, Beck MA, Kuzawa C, Adair LS. Prenatal undernutrition, postnatal environments, and antibody response to vaccination in adolescence. Am J Clin Nutr. 2001;74:543–8 [DOI] [PubMed] [Google Scholar]
- 9.McDade TW, Beck MA, Kuzawa CW, Adair LS. Prenatal undernutrition and postnatal growth are associated with adolescent thymic function. J Nutr. 2001;131:1225–31 [DOI] [PubMed] [Google Scholar]
- 10.Villamor E, Iliadou A, Cnattingius S. Evidence for an effect of fetal growth on the risk of tuberculosis. J Infect Dis. 2010;201:409–13 [DOI] [PubMed] [Google Scholar]
- 11.Moore SE, Cole TJ, Poskitt EM, Sonko BJ, Whitehead RG, McGregor IA, Prentice AM. Season of birth predicts mortality in rural Gambia. Nature. 1997;388:434. [DOI] [PubMed] [Google Scholar]
- 12.Moore SE, Cole TJ, Collinson AC, Poskitt EM, McGregor IA, Prentice AM. Prenatal or early postnatal events predict infectious deaths in young adulthood in rural Africa. Int J Epidemiol. 1999;28:1088–95 [DOI] [PubMed] [Google Scholar]
- 13.Moore SE, Fulford AJ, Streatfield PK, Persson LA, Prentice AM. Comparative analysis of patterns of survival by season of birth in rural Bangladeshi and Gambian populations. Int J Epidemiol. 2004;33:137–43 [DOI] [PubMed] [Google Scholar]
- 14.Jaffar S, Leach A, Greenwood A, Greenwood B. Season of birth is not associated with delayed childhood mortality in Upper River Division, The Gambia. Trop Med Int Health. 2000;5:628–32 [DOI] [PubMed] [Google Scholar]
- 15.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Adult survival after prenatal exposure to the Dutch famine 1944–45. Paediatr Perinat Epidemiol. 2001;15:220–5 [DOI] [PubMed] [Google Scholar]
- 16.Simondon KB, Elguero E, Marra A, Diallo A, Aaby P, Simondon F. Season of birth is not associated with risk of early adult death in rural Senegal. Int J Epidemiol. 2004;33:130–6 [DOI] [PubMed] [Google Scholar]
- 17.Kynast-Wolf G, Hammer GP, Muller O, Kouyate B, Becher H. Season of death and birth predict patterns of mortality in Burkina Faso. Int J Epidemiol. 2006;35:427–35 [DOI] [PubMed] [Google Scholar]
- 18.Bellinger DL, Lubahn C, Lorton D. Maternal and early life stress effects on immune function: relevance to immunotoxicology. J Immunotoxicol. 2008;5:419–44 [DOI] [PubMed] [Google Scholar]
- 19.Bjorksten B. Disease outcomes as a consequence of environmental influences on the development of the immune system. Curr Opin Allergy Clin Immunol. 2009;9:185–9 [DOI] [PubMed] [Google Scholar]
- 20.Ferguson LR. Chronic inflammation and mutagenesis. Mutat Res. 2010;690:3–11 [DOI] [PubMed] [Google Scholar]
- 21.Mold JE, Venkatasubrahmanyam S, Burt TD, Michaelsson J, Rivera JM, Galkina SA, Weinberg K, Stoddart CA, McCune JM. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010;330:1695–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mikkola HK, Orkin SH. The journey of developing hematopoietic stem cells. Development. 2006;133:3733–44 [DOI] [PubMed] [Google Scholar]
- 23.Moore MA. Commentary: the role of cell migration in the ontogeny of the lymphoid system. Stem Cells Dev. 2004;13:1–21 [DOI] [PubMed] [Google Scholar]
- 24.Tortora GJ, Grabowski SR. Principles of anatomy and physiology. 10th ed Hoboken (NJ): John Wiley & Sons, Inc; 2003 [Google Scholar]
- 25.Khlystova ZS, Kalinina II, Shmeleva SP, Ryabchikov OP. Development of endocrine and lymphocytopoietic functions of the thymus in human embryogenesis. Bull Exp Biol Med. 2000;130:1001–4 [PubMed] [Google Scholar]
- 26.Haynes BF, Martin ME, Kay HH, Kurtzberg J. Early events in human T cell ontogeny. Phenotypic characterization and immunohistologic localization of T cell precursors in early human fetal tissues. J Exp Med. 1988;168:1061–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adinolfi M. Ontogeny of human natural and acquired immunity. Curr Top Microbiol Immunol. 1997;222:67–102 [DOI] [PubMed] [Google Scholar]
- 28.Teitelbaum JE, Allan Walker W. The development of mucosal immunity. Eur J Gastroenterol Hepatol. 2005;17:1273–8 [DOI] [PubMed] [Google Scholar]
- 29.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–74 [DOI] [PubMed] [Google Scholar]
- 30.Mebius RE. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303 [DOI] [PubMed] [Google Scholar]
- 31.Randall TD, Carragher DM, Rangel-Moreno J. Development of secondary lymphoid organs. Annu Rev Immunol. 2008;26:627–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer J, MacDonald TT, Finn T, Isaacson PG. The development of gut associated lymphoid tissue in the terminal ileum of fetal human intestine. Clin Exp Immunol. 1986;64:536–43 [PMC free article] [PubMed] [Google Scholar]
- 33.Ruddle NH, Akirav EM. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953. [DOI] [PubMed] [Google Scholar]
- 35.Tung JW, Herzenberg LA. Unraveling B-1 progenitors. Curr Opin Immunol. 2007;19:150–5 [DOI] [PubMed] [Google Scholar]
- 36.Hardy RR. B-1 B cell development. J Immunol. 2006;177:2749–54 [DOI] [PubMed] [Google Scholar]
- 37.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301 [DOI] [PubMed] [Google Scholar]
- 38.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA. 2011;108:1468–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartlidge P. The epidermal barrier. Semin Neonatol. 2000;5:273–80 [DOI] [PubMed] [Google Scholar]
- 40.Burgio GR, Ugazio AG, Notarangelo LD. Immunology of the neonate. Curr Opin Immunol. 1989;2:770–7 [DOI] [PubMed] [Google Scholar]
- 41.Forestier F, Daffos F, Galacteros F, Bardakjian J, Rainaut M, Beuzard Y. Hematological values of 163 normal fetuses between 18 and 30 weeks of gestation. Pediatr Res. 1986;20:342–6 [DOI] [PubMed] [Google Scholar]
- 42.Uksila J, Lassila O, Hirvonen T, Toivanen P. Development of natural killer cell function in the human fetus. J Immunol. 1983;130:153–6 [PubMed] [Google Scholar]
- 43.Kelemen E, Janossa M. Macrophages are the first differentiated blood cells formed in human embryonic liver. Exp Hematol. 1980;8:996–1000 [PubMed] [Google Scholar]
- 44.Anderson DC, Hughes BJ, Smith CW. Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest. 1981;68:863–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shigeoka AO, Santos JI, Hill HR. Functional analysis of neutrophil granulocytes from healthy, infected, and stressed neonates. J Pediatr. 1979;95:454–60 [DOI] [PubMed] [Google Scholar]
- 46.Klein RB, Fischer TJ, Gard SE, Biberstein M, Rich KC, Stiehm ER. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977;60:467–72 [PubMed] [Google Scholar]
- 47.Harrison CJ, Waner JL. Natural killer cell activity in infants and children excreting cytomegalovirus. J Infect Dis. 1985;151:301–7 [DOI] [PubMed] [Google Scholar]
- 48.Whitelaw A, Parkin J. Development of immunity. Br Med Bull. 1988;44:1037–51 [DOI] [PubMed] [Google Scholar]
- 49.Kelly D, Coutts AG. Early nutrition and the development of immune function in the neonate. Proc Nutr Soc. 2000;59:177–85 [DOI] [PubMed] [Google Scholar]
- 50.Clement LT, Vink PE, Bradley GE. Novel immunoregulatory functions of phenotypically distinct subpopulations of CD4+ cells in the human neonate. J Immunol. 1990;145:102–8 [PubMed] [Google Scholar]
- 51.Garvy BA. Host defense against pulmonary infection in neonates. Clin Appl Immunol Rev. 2004;4:205–23 [Google Scholar]
- 52.Cupedo T, Nagasawa M, Weijer K, Blom B, Spits H. Development and activation of regulatory T cells in the human fetus. Eur J Immunol. 2005;35:383–90 [DOI] [PubMed] [Google Scholar]
- 53.Tuovinen H, Laurinolli TT, Rossi LH, Pekkarinen PT, Mattila I, Arstila TP. Thymic production of human FOXP3(+) regulatory T cells is stable but does not correlate with peripheral FOXP3 expression. Immunol Lett. 2008;117:146–53 [DOI] [PubMed] [Google Scholar]
- 54.Michaelsson J, Mold JE, McCune JM, Nixon DF. Regulation of T cell responses in the developing human fetus. J Immunol. 2006;176:5741–8 [DOI] [PubMed] [Google Scholar]
- 55.Bonneville M, O'Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–78 [DOI] [PubMed] [Google Scholar]
- 56.Janeway C, Travers PJ, Walport M, Shlomchik MJ. Immunobiology: the immune system in health and disease. 6th ed New York: Garland Science Publishing; 2005 [Google Scholar]
- 57.Xiong N, Raulet DH. Development and selection of gammadelta T cells. Immunol Rev. 2007;215:15–31 [DOI] [PubMed] [Google Scholar]
- 58.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026 [DOI] [PubMed] [Google Scholar]
- 59.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–85 [DOI] [PubMed] [Google Scholar]
- 60.Moser B, Brandes M. Gammadelta T cells: an alternative type of professional APC. Trends Immunol. 2006;27:112–8 [DOI] [PubMed] [Google Scholar]
- 61.Carding SR, Allan W, Kyes S, Hayday A, Bottomly K, Doherty PC. Late dominance of the inflammatory process in murine influenza by gamma/delta + T cells. J Exp Med. 1990;172:1225–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6 [DOI] [PubMed] [Google Scholar]
- 63.Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev. 2007;215:46–58 [DOI] [PubMed] [Google Scholar]
- 64.Gathings WE, Lawton AR, Cooper MD. Immunofluorescent studies of the development of pre-B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977;7:804–10 [DOI] [PubMed] [Google Scholar]
- 65.Hofman FM, Danilovs J, Husmann L, Taylor CR. Ontogeny of B cell markers in the human fetal liver. J Immunol. 1984;133:1197–201 [PubMed] [Google Scholar]
- 66.Stoll BJ, Lee FK, Hale E, Schwartz D, Holmes R, Ashby R, Czerkinsky C, Nahmias AJ. Immunoglobulin secretion by the normal and the infected newborn infant. J Pediatr. 1993;122:780. [DOI] [PubMed] [Google Scholar]
- 67.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007;7:213–9 [DOI] [PubMed] [Google Scholar]
- 68.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci USA. 2011;108:2879–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci USA. 1992;89:3320–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hayakawa K, Asano M, Shinton SA, Gui M, Wen LJ, Dashoff J, Hardy RR. Positive selection of anti-thy-1 autoreactive B-1 cells and natural serum autoantibody production independent from bone marrow B cell development. J Exp Med. 2003;197:87–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Avrameas S. Natural autoantibodies: from ‘horror autotoxicus’ to ‘gnothi seauton’. Immunol Today. 1991;12:154–9 [DOI] [PubMed] [Google Scholar]
- 72.Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol. 1995;7:812–8 [DOI] [PubMed] [Google Scholar]
- 73.Casali P, Schettino EW. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–79 [DOI] [PubMed] [Google Scholar]
- 74.Holodick NE, Tumang JR, Rothstein TL. Immunoglobulin secretion by B1 cells: differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. Eur J Immunol. 2010;40:3007–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–62 [DOI] [PubMed] [Google Scholar]
- 76.Vollmers HP, Brandlein S. Natural antibodies and cancer. New Biotechnol. 2009;25:294–8 [DOI] [PubMed] [Google Scholar]
- 77.Binder CJ, Silverman GJ. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol. 2005;26:385–404 [DOI] [PubMed] [Google Scholar]
- 78.Chou MY, Hartvigsen K, Hansen LF, Fogelstrand L, Shaw PX, Boullier A, Binder CJ, Witztum JL. Oxidation-specific epitopes are important targets of innate immunity. J Intern Med. 2008;263:479–88 [DOI] [PubMed] [Google Scholar]
- 79.Binder CJ, Chou MY, Fogelstrand L, Hartvigsen K, Shaw PX, Boullier A, Witztum JL. Natural antibodies in murine atherosclerosis. Curr Drug Targets. 2008;9:190–5 [DOI] [PubMed] [Google Scholar]
- 80.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun Rev. 2006;5:403–8 [DOI] [PubMed] [Google Scholar]
- 81.Schwartz-Albiez R, Monteiro RC, Rodriguez M, Binder CJ, Shoenfeld Y. Natural antibodies, intravenous immunoglobulin and their role in autoimmunity, cancer and inflammation. Clin Exp Immunol. 2009;158 Suppl 1:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alugupalli KR. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr Top Microbiol Immunol. 2008;319:105–30 [DOI] [PubMed] [Google Scholar]
- 83.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18 [DOI] [PubMed] [Google Scholar]
- 84.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21:379–90 [DOI] [PubMed] [Google Scholar]
- 85.Goldman AS, Chheda S, Garofalo R. Evolution of immunologic functions of the mammary gland and the postnatal development of immunity. Pediatr Res. 1998;43:155–62 [DOI] [PubMed] [Google Scholar]
- 86.Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16:608–48 [DOI] [PubMed] [Google Scholar]
- 87.Schneider H. The role of the placenta in nutrition of the human fetus. Am J Obstet Gynecol. 1991;164:967–73 [DOI] [PubMed] [Google Scholar]
- 88.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–38 [Google Scholar]
- 89.Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–6 [DOI] [PubMed] [Google Scholar]
- 90.Abrahams VM. Thirty years of reproductive immunology: an introduction. Am J Reprod Immunol. 2010;63:411–2 [DOI] [PubMed] [Google Scholar]
- 91.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sargent IL. Maternal and fetal immune responses during pregnancy. Exp Clin Immunogenet. 1993;10:85–102 [PubMed] [Google Scholar]
- 93.Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci USA. 1996;93:705–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bonney EA, Matzinger P. The maternal immune system's interaction with circulating fetal cells. J Immunol. 1997;158:40–7 [PubMed] [Google Scholar]
- 95.Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2:1087–93 [DOI] [PubMed] [Google Scholar]
- 96.Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol. 1996;157:3317–22 [PubMed] [Google Scholar]
- 97.Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–9 [DOI] [PubMed] [Google Scholar]
- 98.Malek A, Sager R, Kuhn P, Nicolaides KH, Schneider H. Evolution of maternofetal transport of immunoglobulins during human pregnancy. Am J Reprod Immunol. 1996;36:248–55 [DOI] [PubMed] [Google Scholar]
- 99.Takizawa T, Anderson CL, Robinson JM. A novel Fc gamma R-defined, IgG-containing organelle in placental endothelium. J Immunol. 2005;175:2331–9 [DOI] [PubMed] [Google Scholar]
- 100.Ben-Hur H, Gurevich P, Elhayany A, Avinoach I, Schneider DF, Zusman I. Transport of maternal immunoglobulins through the human placental barrier in normal pregnancy and during inflammation. Int J Mol Med. 2005;16:401–7 [PubMed] [Google Scholar]
- 101.Zusman I, Gurevich P, Ben-Hur H. Two secretory immune systems (mucosal and barrier) in human intrauterine development, normal and pathological. Int J Mol Med. 2005;16:127–33 [PubMed] [Google Scholar]
- 102.Peterson JA, Patton S, Hamosh M. Glycoproteins of the human milk fat globule in the protection of the breast-fed infant against infections. Biol Neonate. 1998;74:143–62 [DOI] [PubMed] [Google Scholar]
- 103.Hamosh M, Peterson JA, Henderson TR, Scallan CD, Kiwan R, Ceriani RL, Armand M, Mehta NR, Hamosh P. Protective function of human milk: the milk fat globule. Semin Perinatol. 1999;23:242–9 [DOI] [PubMed] [Google Scholar]
- 104.Brandtzaeg P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine. 2003;21:3382–8 [DOI] [PubMed] [Google Scholar]
- 105.Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200:805–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Johansen FE, Braathen R, Brandtzaeg P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J Immunol. 2001;167:5185–92 [DOI] [PubMed] [Google Scholar]
- 107.Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–24 [DOI] [PubMed] [Google Scholar]
- 108.Van de Perre P. Transfer of antibody via mother's milk. Vaccine. 2003;21:3374–6 [DOI] [PubMed] [Google Scholar]
- 109.Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000;355:451–5 [PubMed] [Google Scholar]
- 110.Duijts L, Ramadhani MK, Moll HA. Breastfeeding protects against infectious diseases during infancy in industrialized countries. A systematic review. Matern Child Nutr. 2009;5:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Edmond KM, Zandoh C, Quigley MA, Amenga-Etego S, Owusu-Agyei S, Kirkwood BR. Delayed breastfeeding initiation increases risk of neonatal mortality. Pediatrics. 2006;117:e380–6 [DOI] [PubMed] [Google Scholar]
- 112.Edmond KM, Kirkwood BR, Amenga-Etego S, Owusu-Agyei S, Hurt LS. Effect of early infant feeding practices on infection-specific neonatal mortality: an investigation of the causal links with observational data from rural Ghana. Am J Clin Nutr. 2007;86:1126–31 [DOI] [PubMed] [Google Scholar]
- 113.Greenwood B. Maternal immunisation in developing countries. Vaccine. 2003;21:3436–41 [DOI] [PubMed] [Google Scholar]
- 114.Lonnerdal B. Nutritional and physiologic significance of human milk proteins. Am J Clin Nutr. 2003;77:S1537–43 [DOI] [PubMed] [Google Scholar]
- 115.Field CJ, Clandinin MT, Van Aerde JE. Polyunsaturated fatty acids and T-cell function: implications for the neonate. Lipids. 2001;36:1025–32 [DOI] [PubMed] [Google Scholar]
- 116.Garofalo R. Cytokines in human milk. J Pediatr. 2010;156:S36–40 [DOI] [PubMed] [Google Scholar]
- 117.Peroni DG, Pescollderungg L, Piacentini GL, Rigotti E, Maselli M, Watschinger K, Piazza M, Pigozzi R, Boner AL. Immune regulatory cytokines in the milk of lactating women from farming and urban environments. Pediatr Allergy Immunol. 2010;21:977–82 [DOI] [PubMed] [Google Scholar]
- 118.Penttila IA. Milk-derived transforming growth factor-beta and the infant immune response. J Pediatr. 2010;156:S21–5 [DOI] [PubMed] [Google Scholar]
- 119.Rigotti E, Piacentini GL, Ress M, Pigozzi R, Boner AL, Peroni DG. Transforming growth factor-beta and interleukin-10 in breast milk and development of atopic diseases in infants. Clin Exp Allergy. 2006;36:614–8 [DOI] [PubMed] [Google Scholar]
- 120.Macfarlane TV, Seager AL, Moller M, Morgan G, Thornton CA. Thymic stromal lymphopoietin is present in human breast milk. Pediatr Allergy Immunol. 2010;21:e454–6 [DOI] [PubMed] [Google Scholar]
- 121.Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris). 1974;125C:373–89 [PubMed] [Google Scholar]
- 122.Borghesi C, Nicoletti C. Autologous anti-idiotypic antibody response is regulated by the level of circulating complementary idiotype. Immunology. 1996;89:172–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hiernaux J, Bona C, Baker PJ. Neonatal treatment with low doses of anti-idiotypic antibody leads to the expression of a silent clone. J Exp Med. 1981;153:1004–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kearney JF, Pollok BA, Stohrer R. Analysis of idiotypic heterogeneity in the anti-alpha 1–3 dextran and anti-phosphorylcholine responses using monoclonal anti-idiotype antibodies. Ann N Y Acad Sci. 1983;418:151–70 [DOI] [PubMed] [Google Scholar]
- 125.Rubinstein LJ, Yeh M, Bona CA. Idiotype-anti-idiotype network. II. Activation of silent clones by treatment at birth with idiotypes is associated with the expansion of idiotype-specific helper T cells. J Exp Med. 1982;156:506–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stein KE, Soderstrom T. Neonatal administration of idiotype or antiidiotype primes for protection against Escherichia coli K13 infection in mice. J Exp Med. 1984;160:1001–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mellander L, Carlsson B, Hanson LA. Appearance of secretory IgM and IgA antibodies to Escherichia coli in saliva during early infancy and childhood. J Pediatr. 1984;104:564–8 [DOI] [PubMed] [Google Scholar]
- 128.Mellander L, Carlsson B, Hanson LA. Secretory IgA and IgM antibodies to E. coli O and poliovirus type I antigens occur in amniotic fluid, meconium and saliva from newborns. A neonatal immune response without antigenic exposure: a result of anti-idiotypic induction? Clin Exp Immunol. 1986;63:555–61 [PMC free article] [PubMed] [Google Scholar]
- 129.Hahn-Zoric M, Carlsson B, Bjorkander J, Osterhaus AD, Mellander L, Hanson LA. Presence of non-maternal antibodies in newborns of mothers with antibody deficiencies. Pediatr Res. 1992;32:150–4 [DOI] [PubMed] [Google Scholar]
- 130.Hahn-Zoric M, Carlsson B, Jeansson S, Ekre HP, Osterhaus AD, Roberton D, Hanson LA. Anti-idiotypic antibodies to poliovirus antibodies in commercial immunoglobulin preparations, human serum, and milk. Pediatr Res. 1993;33:475–80 [DOI] [PubMed] [Google Scholar]
- 131.Elliott M, Kearney JF. Idiotypic regulation of development of the B-cell repertoire. Ann N Y Acad Sci. 1992;651:336–45 [DOI] [PubMed] [Google Scholar]
- 132.Eglinton BA, Roberton DM, Cummins AG. Phenotype of T cells, their soluble receptor levels, and cytokine profile of human breast milk. Immunol Cell Biol. 1994;72:306–13 [DOI] [PubMed] [Google Scholar]
- 133.Michie CA. The long term effects of breastfeeding: a role for the cells in breast milk? J Trop Pediatr. 1998;44:2–3 [DOI] [PubMed] [Google Scholar]
- 134.Grosvenor CE, Picciano MF, Baumrucker CR. Hormones and growth factors in milk. Endocr Rev. 1993;14:710–28 [DOI] [PubMed] [Google Scholar]
- 135.Kobata R, Tsukahara H, Ohshima Y, Ohta N, Tokuriki S, Tamura S, Mayumi M. High levels of growth factors in human breast milk. Early Hum Dev. 2008;84:67–9 [DOI] [PubMed] [Google Scholar]
- 136.Hirai C, Ichiba H, Saito M, Shintaku H, Yamano T, Kusuda S. Trophic effect of multiple growth factors in amniotic fluid or human milk on cultured human fetal small intestinal cells. J Pediatr Gastroenterol Nutr. 2002;34:524–8 [DOI] [PubMed] [Google Scholar]
- 137.Takeda T, Sakata M, Minekawa R, Yamamoto T, Hayashi M, Tasaka K, Murata Y. Human milk induces fetal small intestinal cell proliferation involvement of a different tyrosine kinase signaling pathway from epidermal growth factor receptor. J Endocrinol. 2004;181:449–57 [DOI] [PubMed] [Google Scholar]
- 138.Aggett P, Leach JL, Rueda R, MacLean WC., Jr Innovation in infant formula development: a reassessment of ribonucleotides in 2002. Nutrition. 2003;19:375–84 [DOI] [PubMed] [Google Scholar]
- 139.Zhou YJ, Gao J, Yang HM, Yuan XL, Chen TX, He ZJ. The role of the lactadherin in promoting intestinal DCs development in vivo and vitro. Clin Dev Immunol. 2010;2010:357541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E, Urbanek R, Szepfalusi Z. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol. 2010;21:1179–88 [DOI] [PubMed] [Google Scholar]
- 141.Kosaka N, Izumi H, Sekine K, Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence. 2010;1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fowden AL, Giussani DA, Forhead AJ. Endocrine and metabolic programming during intrauterine development. Early Hum Dev. 2005;81:723–34 [DOI] [PubMed] [Google Scholar]
- 143.Foss B, Dyrstad SM. Stress in obesity: cause or consequence? Med Hypotheses. 2011;77:7–10 [DOI] [PubMed] [Google Scholar]
- 144.DePasquale-Jardieu P, Fraker PJ. The role of corticosterone in the loss in immune function in the zinc-deficient A/J mouse. J Nutr. 1979;109:1847–55 [DOI] [PubMed] [Google Scholar]
- 145.DePasquale-Jardieu P, Fraker PJ. Further characterization of the role of corticosterone in the loss of humoral immunity in zinc-deficient A/J mice as determined by adrenalectomy. J Immunol. 1980;124:2650–5 [PubMed] [Google Scholar]
- 146.Soliman AT, ElZalabany MM, Salama M, Ansari BM. Serum leptin concentrations during severe protein-energy malnutrition: correlation with growth parameters and endocrine function. Metabolism. 2000;49:819–25 [DOI] [PubMed] [Google Scholar]
- 147.Jansson T, Powell TL. Role of the placenta in fetal programming: underlying mechanisms and potential interventional approaches. Clin Sci (Lond). 2007;113:1–13 [DOI] [PubMed] [Google Scholar]
- 148.Johnson JW, Mitzner W, London WT, Palmer AE, Scott R. Betamethasone and the rhesus fetus: multisystemic effects. Am J Obstet Gynecol. 1979;133:677–84 [DOI] [PubMed] [Google Scholar]
- 149.Sawyer R, Hendrickx A, Osburn B, Terrell T, Anderson J. Abnormal morphology of the fetal monkey (Macaca mulatta) thymus exposed to a corticosteroid. J Med Primatol. 1977;6:145–50 [DOI] [PubMed] [Google Scholar]
- 150.Cohen JJ, Duke RC. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984;132:38–42 [PubMed] [Google Scholar]
- 151.Garvy BA, Telford WG, King LE, Fraker PJ. Glucocorticoids and irradiation-induced apoptosis in normal murine bone marrow B-lineage lymphocytes as determined by flow cytometry. Immunology. 1993;79:270–7 [PMC free article] [PubMed] [Google Scholar]
- 152.Fraker PJ, Osati-Ashtiani F, Wagner MA, King LE. Possible roles for glucocorticoids and apoptosis in the suppression of lymphopoiesis during zinc deficiency: a review. J Am Coll Nutr. 1995;14:11–7 [DOI] [PubMed] [Google Scholar]
- 153.Laakko T, Fraker P. Rapid changes in the lymphopoietic and granulopoietic compartments of the marrow caused by stress levels of corticosterone. Immunology. 2002;105:111–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fraker PJ, King LE. Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr. 2004;24:277–98 [DOI] [PubMed] [Google Scholar]
- 155.Claycombe K, King LE, Fraker PJ. A role for leptin in sustaining lymphopoiesis and myelopoiesis. Proc Natl Acad Sci USA. 2008;105:2017–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hendrickx AG, Sawyer RH, Terrell TG, Osburn BI, Henrickson RV, Steffek AJ. Teratogenic effects of triamcinolone on the skeletal and lymphoid systems in nonhuman primates. Fed Proc. 1975;34:1661–7 [PubMed] [Google Scholar]
- 157.Ioachim HL. The cortisone-induced wasting disease of newborn rats: histopathological and autoradiographic studies. J Pathol. 1971;104:201–5 [DOI] [PubMed] [Google Scholar]
- 158.Murthy KK, Moya FR. Effect of betamethasone on maternal, fetal and neonatal rat cellular immunity. Early Hum Dev. 1994;36:1–11 [DOI] [PubMed] [Google Scholar]
- 159.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–8 [DOI] [PubMed] [Google Scholar]
- 160.Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav Rev. 2005;29:227–35 [DOI] [PubMed] [Google Scholar]
- 161.Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–80 [DOI] [PubMed] [Google Scholar]
- 162.Bilbo SD, Wieseler JL, Barrientos RM, Tsang V, Watkins LR, Maier SF. Neonatal bacterial infection alters fever to live and simulated infections in adulthood. Psychoneuroendocrinology. 2010;35:369–81 [DOI] [PubMed] [Google Scholar]
- 163.Boisse L, Mouihate A, Ellis S, Pittman QJ. Long-term alterations in neuroimmune responses after neonatal exposure to lipopolysaccharide. J Neurosci. 2004;24:4928–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reyes TM, Coe CL. Prenatal manipulations reduce the proinflammatory response to a cytokine challenge in juvenile monkeys. Brain Res. 1997;769:29–35 [DOI] [PubMed] [Google Scholar]
- 165.Ellis S, Mouihate A, Pittman QJ. Early life immune challenge alters innate immune responses to lipopolysaccharide: implications for host defense as adults. FASEB J. 2005;19:1519–21 [DOI] [PubMed] [Google Scholar]
- 166.Ellis S, Mouihate A, Pittman QJ. Neonatal programming of the rat neuroimmune response: stimulus specific changes elicited by bacterial and viral mimetics. J Physiol. 2006;571:695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hashimoto M, Watanabe T, Fujioka T, Tan N, Yamashita H, Nakamura S. Modulating effects of prenatal stress on hyperthermia induced in adult rat offspring by restraint or LPS-induced stress. Physiol Behav. 2001;73:125–32 [DOI] [PubMed] [Google Scholar]
- 168.Spencer SJ, Field E, Pittman QJ. Neonatal programming by neuroimmune challenge: effects on responses and tolerance to septic doses of lipopolysaccharide in adult male and female rats. J Neuroendocrinol. 2010;22:272–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Spencer SJ, Auer RN, Pittman QJ. Rat neonatal immune challenge alters adult responses to cerebral ischaemia. J Cereb Blood Flow Metab. 2006;26:456–67 [DOI] [PubMed] [Google Scholar]
- 170.Spencer SJ, Martin S, Mouihate A, Pittman QJ. Early-life immune challenge: defining a critical window for effects on adult responses to immune challenge. Neuropsychopharmacology. 2006;31:1910–8 [DOI] [PubMed] [Google Scholar]
- 171.Spencer SJ, Boisse L, Mouihate A, Pittman QJ. Long term alterations in neuroimmune responses of female rats after neonatal exposure to lipopolysaccharide. Brain Behav Immun. 2006;20:325–30 [DOI] [PubMed] [Google Scholar]
- 172.Walker FR, Hodyl NA, Krivanek KM, Hodgson DM. Early life host-bacteria relations and development: long-term individual differences in neuroimmune function following neonatal endotoxin challenge. Physiol Behav. 2006;87:126–34 [DOI] [PubMed] [Google Scholar]
- 173.Avitsur R, Hunzeker J, Sheridan JF. Role of early stress in the individual differences in host response to viral infection. Brain Behav Immun. 2006;20:339–48 [DOI] [PubMed] [Google Scholar]
- 174.Beloosesky R, Maravi N, Weiner Z, Khatib N, Awad N, Boles J, Ross MG, Itskovitz-Eldor J. Maternal lipopolysaccharide-induced inflammation during pregnancy programs impaired offspring innate immune responses. Am J Obstet Gynecol. 2010;203:185e1–4 [DOI] [PubMed] [Google Scholar]
- 175.Breivik T, Stephan M, Brabant GE, Straub RH, Pabst R, von Horsten S. Postnatal lipopolysaccharide-induced illness predisposes to periodontal disease in adulthood. Brain Behav Immun. 2002;16:421–38 [DOI] [PubMed] [Google Scholar]
- 176.Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87:675–81 [DOI] [PubMed] [Google Scholar]
- 177.Fisher RE, Karrow NA, Quinton M, Finegan EJ, Miller SP, Atkinson JL, Boermans HJ. Endotoxin exposure during late pregnancy alters ovine offspring febrile and hypothalamic-pituitary-adrenal axis responsiveness later in life. Stress. 2010;13:334–42 [DOI] [PubMed] [Google Scholar]
- 178.Hodyl NA, Krivanek KM, Lawrence E, Clifton VL, Hodgson DM. Prenatal exposure to a pro-inflammatory stimulus causes delays in the development of the innate immune response to LPS in the offspring. J Neuroimmunol. 2007;190:61–71 [DOI] [PubMed] [Google Scholar]
- 179.Hodyl NA, Krivanek KM, Clifton VL, Hodgson DM. Innate immune dysfunction in the neonatal rat following prenatal endotoxin exposure. J Neuroimmunol. 2008;204:126–30 [DOI] [PubMed] [Google Scholar]
- 180.Kentner AC, McLeod SA, Field EF, Pittman QJ. Sex-dependent effects of neonatal inflammation on adult inflammatory markers and behavior. Endocrinology. 2010;151:2689–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Walker FR, Hodyl NA, Hodgson DM. Neonatal bacterial endotoxin challenge interacts with stress in the adult male rat to modify KLH specific antibody production but not KLH stimulated ex vivo cytokine release. J Neuroimmunol. 2009;207:57–65 [DOI] [PubMed] [Google Scholar]
- 182.Eishi Y, Hirokawa K, Hatakeyama S. Long-lasting impairment of immune and endocrine systems of offspring induced by injection of dexamethasone into pregnant mice. Clin Immunol Immunopathol. 1983;26:335–49 [DOI] [PubMed] [Google Scholar]
- 183.Coe CL, Lubach GR. Prenatal influences on neuroimmune set points in infancy. Ann N Y Acad Sci. 2000;917:468–77 [DOI] [PubMed] [Google Scholar]
- 184.Coe CL, Lubach GR, Karaszewski JW. Prenatal stress and immune recognition of self and nonself in the primate neonate. Biol Neonate. 1999;76:301–10 [DOI] [PubMed] [Google Scholar]
- 185.Coe CL, Lubach GR, Karaszewski JW, Ershler WB. Prenatal endocrine activation alters postnatal cellular immunity in infant monkeys. Brain Behav Immun. 1996;10:221–34 [DOI] [PubMed] [Google Scholar]
- 186.Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr Res. 2001;50:750–5 [DOI] [PubMed] [Google Scholar]
- 187.Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38:414–21 [DOI] [PubMed] [Google Scholar]
- 188.Hodyl NA, Walker FR, Krivanek KM, Clifton VL, Hodgson DM. Prenatal endotoxin exposure alters behavioural pain responses to lipopolysaccharide in adult offspring. Physiol Behav. 2010;100:143–7 [DOI] [PubMed] [Google Scholar]
- 189.Shanks N, Windle RJ, Perks PA, Harbuz MS, Jessop DS, Ingram CD, Lightman SL. Early-life exposure to endotoxin alters hypothalamic-pituitary-adrenal function and predisposition to inflammation. Proc Natl Acad Sci USA. 2000;97:5645–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shanks N, Lightman SL. The maternal-neonatal neuro-immune interface: are there long-term implications for inflammatory or stress-related disease? J Clin Invest. 2001;108:1567–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nilsson C, Jennische E, Ho HP, Eriksson E, Bjorntorp P, Holmang A. Postnatal endotoxin exposure results in increased insulin sensitivity and altered activity of neuroendocrine axes in adult female rats. Eur J Endocrinol. 2002;146:251–60 [DOI] [PubMed] [Google Scholar]
- 192.Soriano RN, Branco LG. Reduced stress fever is accompanied by increased glucocorticoids and reduced PGE(2) in adult rats exposed to endotoxin as neonates. J Neuroimmunol. 2010;225:77–81 [DOI] [PubMed] [Google Scholar]
- 193.Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Walker FR, Knott B, Hodgson DM. Neonatal endotoxin exposure modifies the acoustic startle response and circulating levels of corticosterone in the adult rat but only following acute stress. J Psychiatr Res. 2008;42:1094–103 [DOI] [PubMed] [Google Scholar]
- 195.Hodyl NA, Walker FR, Krivanek KM, Clifton V, Hodgson DM. Modelling prenatal bacterial infection: functional consequences of altered hypothalamic pituitary adrenal axis development. Behav Brain Res. 2007;178:108–14 [DOI] [PubMed] [Google Scholar]
- 196.Beach RS, Gershwin ME, Hurley LS. Reversibility of development retardation following murine fetal zinc deprivation. J Nutr. 1982;112:1169–81 [DOI] [PubMed] [Google Scholar]
- 197.Beach RS, Gershwin ME, Hurley LS. Persistent immunological consequences of gestation zinc deprivation. Am J Clin Nutr. 1983;38:579–90 [DOI] [PubMed] [Google Scholar]
- 198.Quarterman J, Humphries WR. Effect of zinc deficiency and zinc supplementation on adrenals, plasma steroids and thymus in rats. Life Sci. 1979;24:177–83 [DOI] [PubMed] [Google Scholar]
- 199.Beach RS, Gershwin ME, Hurley LS. Gestational zinc deprivation in mice: persistence of immunodeficiency for three generations. Science. 1982;218:469–71 [DOI] [PubMed] [Google Scholar]
- 200.Keen CL, Gershwin ME. Zinc deficiency and immune function. Annu Rev Nutr. 1990;10:415–31 [DOI] [PubMed] [Google Scholar]
- 201.Morriss-Kay GM, Ward SJ. Retinoids and mammalian development. Int Rev Cytol. 1999;188:73–131 [DOI] [PubMed] [Google Scholar]
- 202.Niederreither K, Vermot J, Messaddeq N, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is essential for heart morphogenesis in the mouse. Development. 2001;128:1019–31 [DOI] [PubMed] [Google Scholar]
- 203.Wendling O, Dennefeld C, Chambon P, Mark M. Retinoid signaling is essential for patterning the endoderm of the third and fourth pharyngeal arches. Development. 2000;127:1553–62 [DOI] [PubMed] [Google Scholar]
- 204.Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–94 [DOI] [PubMed] [Google Scholar]
- 205.Niederreither K, Vermot J, Le Roux I, Schuhbaur B, Chambon P, Dolle P. The regional pattern of retinoic acid synthesis by RALDH2 is essential for the development of posterior pharyngeal arches and the enteric nervous system. Development. 2003;130:2525–34 [DOI] [PubMed] [Google Scholar]
- 206.Vermot J, Niederreither K, Garnier JM, Chambon P, Dolle P. Decreased embryonic retinoic acid synthesis results in a DiGeorge syndrome phenotype in newborn mice. Proc Natl Acad Sci USA. 2003;100:1763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ryckebusch L, Bertrand N, Mesbah K, Bajolle F, Niederreither K, Kelly RG, Zaffran S. Decreased levels of embryonic retinoic acid synthesis accelerate recovery from arterial growth delay in a mouse model of DiGeorge syndrome. Circ Res. 2010;106:686–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994;120:2749–71 [DOI] [PubMed] [Google Scholar]
- 209.Evans T. Regulation of hematopoiesis by retinoid signaling. Exp Hematol. 2005;33:1055–61 [DOI] [PubMed] [Google Scholar]
- 210.Fahlman C, Jacobsen SE, Smeland EB, Lomo J, Naess CE, Funderud S, Blomhoff HK. All-trans- and 9-cis-retinoic acid inhibit growth of normal human and murine B cell precursors. J Immunol. 1995;155:58–65 [PubMed] [Google Scholar]
- 211.Chen X, Esplin B, Garrett K, Welner R, Webb C, Kincade P. Retinoids accelerate B lineage lymphoid differentiation. J Immunol. 2008;180:138–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen X, Welner R, Kincade P. A possible contribution of retinoids to regulation of fetal B lymphopoiesis. Eur J Immunol. 2009;39:2515–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, Bailey N, Potts EN, Whitehead G, Brass DM, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–9 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 214.Haberg SE, London SJ, Stigum H, Nafstad P, Nystad W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch Dis Child. 2009;94:180–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Erickson KL, Medina EA, Hubbard NE. Micronutrients and innate immunity. J Infect Dis. 2000;182 Suppl 1:S5–10 [DOI] [PubMed] [Google Scholar]
- 216.Field CJ, Johnson IR, Schley PD. Nutrients and their role in host resistance to infection. J Leukoc Biol. 2002;71:16–32 [PubMed] [Google Scholar]
- 217.Cunningham-Rundles S, McNeeley DF, Moon A. Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol. 2005;115:1119–28, quiz 29 [DOI] [PubMed] [Google Scholar]
- 218.Raqib R, Cravioto A. Nutrition, immunology, and genetics: future perspectives. Nutr Rev. 2009;67 Suppl 2:S227–36 [DOI] [PubMed] [Google Scholar]
- 219.Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92 [DOI] [PubMed] [Google Scholar]
- 220.Cox SE, Arthur P, Kirkwood BR, Yeboah-Antwi K, Riley EM. Vitamin A supplementation increases ratios of proinflammatory to anti-inflammatory cytokine responses in pregnancy and lactation. Clin Exp Immunol. 2006;144:392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M. Programming placental nutrient transport capacity. J Physiol. 2006;572:5–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Borowicz PP, Arnold DR, Johnson ML, Grazul-Bilska AT, Redmer DA, Reynolds LP. Placental growth throughout the last two thirds of pregnancy in sheep: vascular development and angiogenic factor expression. Biol Reprod. 2007;76:259–67 [DOI] [PubMed] [Google Scholar]
- 223.Reynolds LP, Borowicz PP, Caton JS, Vonnahme KA, Luther JS, Buchanan DS, Hafez SA, Grazul-Bilska AT, Redmer DA. Uteroplacental vascular development and placental function: an update. Int J Dev Biol. 2010;54:355–66 [DOI] [PubMed] [Google Scholar]
- 224.Sobrian SK, Vaughn VT, Bloch EF, Burton LE. Influence of prenatal maternal stress on the immunocompetence of the offspring. Pharmacol Biochem Behav. 1992;43:537–47 [DOI] [PubMed] [Google Scholar]
- 225.Coe CL, Crispen HR. Social stress in pregnant squirrel monkeys (Saimiri boliviensis peruviensis) differentially affects placental transfer of maternal antibody to male and female infants. Health Psychol. 2000;19:554–9 [PubMed] [Google Scholar]
- 226.Tuchscherer M, Kanitz E, Otten W, Tuchscherer A. Effects of prenatal stress on cellular and humoral immune responses in neonatal pigs. Vet Immunol Immunopathol. 2002;86:195–203 [DOI] [PubMed] [Google Scholar]
- 227.Yorty JL, Bonneau RH. Transplacental transfer and subsequent neonate utilization of herpes simplex virus-specific immunity are resilient to acute maternal stress. J Virol. 2003;77:6613–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yorty JL, Bonneau RH. Prenatal transfer of low amounts of herpes simplex virus (HSV)-specific antibody protects newborn mice against HSV infection during acute maternal stress. Brain Behav Immun. 2004;18:15–23 [DOI] [PubMed] [Google Scholar]
- 229.Kohler PF, Farr RS. Elevation of cord over maternal IgG immunoglobulin: evidence for an active placental IgG transport. Nature. 1966;210:1070–1 [DOI] [PubMed] [Google Scholar]
- 230.Brair ME, Brabin BJ, Milligan P, Maxwell S, Hart CA. Reduced transfer of tetanus antibodies with placental malaria. Lancet. 1994;343:208–9 [DOI] [PubMed] [Google Scholar]
- 231.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, Milligan PJ, Wesumperuma L, Broadhead RL, Brabin BJ, Johnson PM, Hart CA. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79:F202–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Okoko BJ, Wesumperuma LH, Ota MO, Pinder M, Banya W, Gomez SF, McAdam KP, Hart AC. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J Infect Dis. 2001;184:627–32 [DOI] [PubMed] [Google Scholar]
- 233.Cumberland P, Shulman CE, Maple PA, Bulmer JN, Dorman EK, Kawuondo K, Marsh K, Cutts FT. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis. 2007;196:550–7 [DOI] [PubMed] [Google Scholar]
- 234.de Moraes-Pinto MI, Almeida AC, Kenj G, Filgueiras TE, Tobias W, Santos AM, Carneiro-Sampaio MM, Farhat CK, Milligan PJ, Johnson PM, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173:1077–84 [DOI] [PubMed] [Google Scholar]
- 235.Farquhar C, Nduati R, Haigwood N, Sutton W, Mbori-Ngacha D, Richardson B, John-Stewart G. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40:494–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Hood N, Chan MC, Maxwell SM, Familusi JB, Hart CA. Placental transfer of tetanus toxoid antibodies in Nigerian mothers. Ann Trop Paediatr. 1994;14:179–82 [DOI] [PubMed] [Google Scholar]
- 237.Hartter HK, Oyedele OI, Dietz K, Kreis S, Hoffman JP, Muller CP. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J. 2000;19:635–41 [DOI] [PubMed] [Google Scholar]
- 238.Cavalcante RS, Kopelman BI, Costa-Carvalho BT. Placental transfer of Haemophilus influenzae type b antibodies in malnourished pregnant women. Braz J Infect Dis. 2008;12:47–51 [DOI] [PubMed] [Google Scholar]
- 239.Wesumperuma HL, Perera AJ, Pharoah PO, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in Sri Lanka. Ann Trop Med Parasitol. 1999;93:169–77 [DOI] [PubMed] [Google Scholar]
- 240.Okoko BJ, Wesumperuma LH, Hart AC. Materno-foetal transfer of H. influenzae and pneumococcal antibodies is influenced by prematurity and low birth weight: implications for conjugate vaccine trials. Vaccine. 2001;20:647–50 [DOI] [PubMed] [Google Scholar]
- 241.Okoko JB, Wesumperuma HL, Hart CA. The influence of prematurity and low birthweight on transplacental antibody transfer in a rural West African population. Trop Med Int Health. 2001;6:529–34 [DOI] [PubMed] [Google Scholar]
- 242.Okoko BJ, Wesumperuma HL, Fern J, Yamuah LK, Hart CA. The transplacental transfer of IgG subclasses: influence of prematurity and low birthweight in the Gambian population. Ann Trop Paediatr. 2002;22:325–32 [DOI] [PubMed] [Google Scholar]
- 243.Osendarp SJ, West CE, Black RE. The need for maternal zinc supplementation in developing countries: an unresolved issue. J Nutr. 2003;133:S817–27 [DOI] [PubMed] [Google Scholar]
- 244.Machado-Neto R, Graves CN, Curtis SE. Immunoglobulins in piglets from sows heat-stressed prepartum. J Anim Sci. 1987;65:445–55 [DOI] [PubMed] [Google Scholar]
- 245.Nardone A, Lacetera N, Bernabucci U, Ronchi B. Composition of colostrum from dairy heifers exposed to high air temperatures during late pregnancy and the early postpartum period. J Dairy Sci. 1997;80:838–44 [DOI] [PubMed] [Google Scholar]
- 246.Prentice A, Prentice AM, Cole TJ, Paul AA, Whitehead RG. Breast-milk antimicrobial factors of rural Gambian mothers. I. Influence of stage of lactation and maternal plane of nutrition. Acta Paediatr Scand. 1984;73:796–802 [DOI] [PubMed] [Google Scholar]
- 247.Prentice A, Prentice AM, Cole TJ, Whitehead RG. Determinants of variations in breast milk protective factor concentrations of rural Gambian mothers. Arch Dis Child. 1983;58:518–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Lewis-Jones DI, Lewis-Jones MS, Connolly RC, Lloyd DC, West CR. The influence of parity, age and maturity of pregnancy on antimicrobial proteins in human milk. Acta Paediatr Scand. 1985;74:655–9 [DOI] [PubMed] [Google Scholar]
- 249.Powe CE, Knott CD, Conklin-Brittain N. Infant sex predicts breast milk energy content. Am J Hum Biol. 2010;22:50–4 [DOI] [PubMed] [Google Scholar]
- 250.Lonnerdal B, Zavaleta N, Kusunoki L, Lanata CF, Peerson JM, Brown KH. Effect of postpartum maternal infection on proteins and trace elements in colostrum and early milk. Acta Paediatr. 1996;85:537–42 [DOI] [PubMed] [Google Scholar]
- 251.Zavaleta N, Lanata C, Butron B, Peerson JM, Brown KH, Lonnerdal B. Effect of acute maternal infection on quantity and composition of breast milk. Am J Clin Nutr. 1995;62:559–63 [DOI] [PubMed] [Google Scholar]
- 252.Filteau SM, Rice AL, Ball JJ, Chakraborty J, Stoltzfus R, de Francisco A, Willumsen JF. Breast milk immune factors in Bangladeshi women supplemented postpartum with retinol or {beta}-carotene. Am J Clin Nutr. 1999;69:953–8 [DOI] [PubMed] [Google Scholar]
- 253.Filteau SM, Lietz G, Mulokozi G, Bilotta S, Henry CJ, Tomkins AM. Milk cytokines and subclinical breast inflammation in Tanzanian women: effects of dietary red palm oil or sunflower oil supplementation. Immunology. 1999;97:595–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Semba RD, Kumwenda N, Taha TE, Hoover DR, Lan Y, Eisinger W, Mtimavalye L, Broadhead R, Miotti PG, Van Der Hoeven L, et al. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol. 1999;6:671–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Prentice A, Watkinson M, Prentice AM, Cole TJ, Whitehead RG. Breast-milk antimicrobial factors of rural Gambian mothers. II. Influence of season and prevalence of infection. Acta Paediatr Scand. 1984;73:803–9 [DOI] [PubMed] [Google Scholar]
- 256.Weaver LT, Arthur HM, Bunn JE, Thomas JE. Human milk IgA concentrations during the first year of lactation. Arch Dis Child. 1998;78:235–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Amoudruz P, Holmlund U, Schollin J, Sverremark-Ekstrom E, Montgomery SM. Maternal country of birth and previous pregnancies are associated with breast milk characteristics. Pediatr Allergy Immunol. 2009;20:19–29 [DOI] [PubMed] [Google Scholar]
- 258.Holmlund U, Amoudruz P, Johansson MA, Haileselassie Y, Ongoiba A, Kayentao K, Traore B, Doumbo S, Schollin J, Doumbo O, et al. Maternal country of origin, breast milk characteristics and potential influences on immunity in offspring. Clin Exp Immunol. 2010;162:500–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Miranda R, Saravia NG, Ackerman R, Murphy N, Berman S, McMurray DN. Effect of maternal nutritional status on immunological substances in human colostrum and milk. Am J Clin Nutr. 1983;37:632–40 [DOI] [PubMed] [Google Scholar]
- 260.Chang SJ. Antimicrobial proteins of maternal and cord sera and human milk in relation to maternal nutritional status. Am J Clin Nutr. 1990;51:183–7 [DOI] [PubMed] [Google Scholar]
- 261.Garg M, Thirupuram S, Saha K. Colostrum composition, maternal diet and nutrition in north India. J Trop Pediatr. 1988;34:79–87 [DOI] [PubMed] [Google Scholar]
- 262.Reddy V, Bhaskaram C, Raghuramulu N, Jagadeesan V. Antimicrobial factors in human milk. Acta Paediatr Scand. 1977;66:229–32 [DOI] [PubMed] [Google Scholar]
- 263.Hennart PF, Brasseur DJ, Delogne-Desnoeck JB, Dramaix MM, Robyn CE. Lysozyme, lactoferrin, and secretory immunoglobulin A content in breast milk: influence of duration of lactation, nutrition status, prolactin status, and parity of mother. Am J Clin Nutr. 1991;53:32–9 [DOI] [PubMed] [Google Scholar]
- 264.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90 [DOI] [PubMed] [Google Scholar]
- 265.Read JS, Clemens JD, Klebanoff MA. Moderate low birth weight and infectious disease mortality during infancy and childhood. Am J Epidemiol. 1994;140:721–33 [DOI] [PubMed] [Google Scholar]
- 266.Becerra JE, Atrash HK, Perez N, Saliceti JA. Low birthweight and infant mortality in Puerto Rico. Am J Public Health. 1993;83:1572–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Samuelsen SO, Magnus P, Bakketeig LS. Birth weight and mortality in childhood in Norway. Am J Epidemiol. 1998;148:983–91 [DOI] [PubMed] [Google Scholar]
- 268.Hviid A, Melbye M. The impact of birth weight on infectious disease hospitalization in childhood. Am J Epidemiol. 2007;165:756–61 [DOI] [PubMed] [Google Scholar]
- 269.Victora CG, Barros FC, Vaughan JP, Martines JC, Beria JU. Birthweight, socio-economic status and growth of Brazilian infants. Ann Hum Biol. 1987;14:49–57 [DOI] [PubMed] [Google Scholar]
- 270.Morris SS, Victora CG, Barros FC, Halpern R, Menezes AM, Cesar JA, Horta BL, Tomasi E. Length and ponderal index at birth: associations with mortality, hospitalizations, development and post-natal growth in Brazilian infants. Int J Epidemiol. 1998;27:242–7 [DOI] [PubMed] [Google Scholar]
- 271.Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. Eur J Clin Nutr. 1998;52 Suppl 1:S34–41; discussion S-2 [PubMed] [Google Scholar]
- 272.Moore SE, Collinson AC, Tamba N'gom P, Aspinall R, Prentice AM. Early immunological development and mortality from infectious disease in later life. Proc Nutr Soc. 2006;65:311–8 [DOI] [PubMed] [Google Scholar]
- 273.Moore SE, Prentice AM, Wagatsuma Y, Fulford AJ, Collinson AC, Raqib R, Vahter M, Persson LA, Arifeen SE. Early-life nutritional and environmental determinants of thymic size in infants born in rural Bangladesh. Acta Paediatr. 2009;98:1168–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ngom PT, Collinson AC, Pido-Lopez J, Henson SM, Prentice AM, Aspinall R. Improved thymic function in exclusively breastfed infants is associated with higher interleukin 7 concentrations in their mothers' breast milk. Am J Clin Nutr. 2004;80:722–8 [DOI] [PubMed] [Google Scholar]
- 275.Ghattas H, Wallace DL, Solon JA, Henson SM, Zhang Y, Ngom PT, Aspinall R, Morgan G, Griffin GE, Prentice AM, et al. Long-term effects of perinatal nutrition on T lymphocyte kinetics in young Gambian men. Am J Clin Nutr. 2007;85:480–7 [DOI] [PubMed] [Google Scholar]
- 276.Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M, Moore SE, Fuchs G. Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. Am J Clin Nutr. 2007;85:845–52 [DOI] [PubMed] [Google Scholar]
- 277.Katz J, West KP, Jr, Khatry SK, Pradhan EK, LeClerq SC, Christian P, Wu LS, Adhikari RK, Shrestha SR, Sommer A. Maternal low-dose vitamin A or beta-carotene supplementation has no effect on fetal loss and early infant mortality: a randomized cluster trial in Nepal. Am J Clin Nutr. 2000;71:1570–6 [DOI] [PubMed] [Google Scholar]
- 278.Christian P, West KP, Jr, Khatry SK, LeClerq SC, Kimbrough-Pradhan E, Katz J, Shrestha SR. Maternal night blindness increases risk of mortality in the first 6 months of life among infants in Nepal. J Nutr. 2001;131:1510–2 [DOI] [PubMed] [Google Scholar]
- 279.Christian P, West KP, Khatry SK, Leclerq SC, Pradhan EK, Katz J, Shrestha SR, Sommer A. Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster-randomized trial in Nepal. Am J Clin Nutr. 2003;78:1194–202 [DOI] [PubMed] [Google Scholar]
- 280.Zeng L, Dibley MJ, Cheng Y, Dang S, Chang S, Kong L, Yan H. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337:a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Osendarp SJ, van Raaij JM, Darmstadt GL, Baqui AH, Hautvast JG, Fuchs GJ. Zinc supplementation during pregnancy and effects on growth and morbidity in low birthweight infants: a randomised placebo controlled trial. Lancet. 2001;357:1080–5 [DOI] [PubMed] [Google Scholar]
- 282.Wieringa FT, Dijkhuizen MA. Muhilal, Van der Meer JW. Maternal micronutrient supplementation with zinc and beta-carotene affects morbidity and immune function of infants during the first 6 months of life. Eur J Clin Nutr. 2010;64:1072–9 [DOI] [PubMed] [Google Scholar]
- 283.Iannotti LL, Zavaleta N, Leon Z, Huasquiche C, Shankar AH, Caulfield LE. Maternal zinc supplementation reduces diarrheal morbidity in Peruvian infants. J Pediatr. 2010;156:960–4, 4 e1–2 [DOI] [PubMed] [Google Scholar]
- 284.Osendarp SJ, Fuchs GJ, van Raaij JM, Mahmud H, Tofail F, Black RE, Prabhakar H, Santosham M. The effect of zinc supplementation during pregnancy on immune response to Hib and BCG vaccines in Bangladesh. J Trop Pediatr. 2006;52:316–23 [DOI] [PubMed] [Google Scholar]
- 285.Devereux G. Session 1: allergic disease: nutrition as a potential determinant of asthma. Proc Nutr Soc. 2010;69:1–10 [DOI] [PubMed] [Google Scholar]
- 286.Tan PH, Sagoo P, Chan C, Yates JB, Campbell J, Beutelspacher SC, Foxwell BM, Lombardi G, George AJ. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J Immunol. 2005;174:7633–44 [DOI] [PubMed] [Google Scholar]
- 287.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32:43–50 [DOI] [PubMed] [Google Scholar]
- 288.Litonjua AA, Tantisira KG, Finn PW. Maternal antioxidant intake during pregnancy and cord blood lymphoproliferative responses. Am J Respir Crit Care Med. 2004;169:A501(abstr) [Google Scholar]
- 289.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA, Jr, Weiss ST, Gillman MW, Gold DR. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84:903–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Devereux G, Turner SW, Craig LC, McNeill G, Martindale S, Harbour PJ, Helms PJ, Seaton A. Low maternal vitamin E intake during pregnancy is associated with asthma in 5-year-old children. Am J Respir Crit Care Med. 2006;174:499–507 [DOI] [PubMed] [Google Scholar]
- 291.Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep. 2011;11:29–36 [DOI] [PubMed] [Google Scholar]
- 292.Lucas RM, Ponsonby AL, Pasco JA, Morley R. Future health implications of prenatal and early-life vitamin D status. Nutr Rev. 2008;66:710–20 [DOI] [PubMed] [Google Scholar]
- 293.Rochat MK, Ege MJ, Plabst D, Steinle J, Bitter S, Braun-Fahrlander C, Dalphin JC, Riedler J, Roponen M, Hirvonen MR, et al. Maternal vitamin D intake during pregnancy increases gene expression of ILT3 and ILT4 in cord blood. Clin Exp Allergy. 2010;40:786–94 [DOI] [PubMed] [Google Scholar]
- 294.Hawkes JS, Neumann MA, Gibson RA. The effect of breast feeding on lymphocyte subpopulations in healthy term infants at 6 months of age. Pediatr Res. 1999;45:648–51 [DOI] [PubMed] [Google Scholar]
- 295.Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–18 [DOI] [PubMed] [Google Scholar]
- 296.Weaver LT, Wadd N, Taylor CE, Greenwell J, Toms GL. The ontogeny of serum IgA in the newborn. Pediatr Allergy Immunol. 1991;2:72–5 [Google Scholar]
- 297.Brandtzaeg P. Local immunity and breast-feeding. : Calder PC, Field CJ, Gill HS, Nutrition and immune function. Wallingford (UK): CABI Publishing; 2002 [Google Scholar]
- 298.Hasselbalch H, Jeppesen DL, Engelmann MD, Michaelsen KF, Nielsen MB. Decreased thymus size in formula-fed infants compared with breastfed infants. Acta Paediatr. 1996;85:1029–32 [DOI] [PubMed] [Google Scholar]
- 299.Hasselbalch H, Engelmann MD, Ersboll AK, Jeppesen DL, Fleischer-Michaelsen K. Breast-feeding influences thymic size in late infancy. Eur J Pediatr. 1999;158:964–7 [DOI] [PubMed] [Google Scholar]
- 300.Jeppesen DL. The size of the thymus: an important immunological diagnostic tool? Acta Paediatr. 2003;92:994–6 [PubMed] [Google Scholar]
- 301.Aaby P, Marx C, Trautner S, Rudaa D, Hasselbalch H, Jensen H, Lisse I. Thymus size at birth is associated with infant mortality: a community study from Guinea-Bissau. Acta Paediatr. 2002;91:698–703 [DOI] [PubMed] [Google Scholar]
- 302.Albers R, Antoine JM, Bourdet-Sicard R, Calder PC, Gleeson M, Lesourd B, Samartin S, Sanderson IR, Van Loo J, Vas Dias FW, et al. Markers to measure immunomodulation in human nutrition intervention studies. Br J Nutr. 2005;94:452–81 [DOI] [PubMed] [Google Scholar]
- 303.Pabst HF, Spady DW. Effect of breast-feeding on antibody response to conjugate vaccine. Lancet. 1990;336:269–70 [DOI] [PubMed] [Google Scholar]
- 304.Greenberg DP, Vadheim CM, Partridge S, Chang SJ, Chiu CY, Ward JI. Immunogenicity of Haemophilus influenzae type b tetanus toxoid conjugate vaccine in young infants. The Kaiser-UCLA Vaccine Study Group. J Infect Dis. 1994;170:76–81 [DOI] [PubMed] [Google Scholar]
- 305.Hahn-Zoric M, Fulconis F, Minoli I, Moro G, Carlsson B, Bottiger M, Raiha N, Hanson LA. Antibody responses to parenteral and oral vaccines are impaired by conventional and low protein formulas as compared to breast-feeding. Acta Paediatr Scand. 1990;79:1137–42 [DOI] [PubMed] [Google Scholar]
- 306.Pickering LK, Granoff DM, Erickson JR, Masor ML, Cordle CT, Schaller JP, Winship TR, Paule CL, Hilty MD. Modulation of the immune system by human milk and infant formula containing nucleotides. Pediatrics. 1998;101:242–9 [DOI] [PubMed] [Google Scholar]
- 307.Stephens S, Kennedy CR, Lakhani PK, Brenner MK. In-vivo immune responses of breast- and bottle-fed infants to tetanus toxoid antigen and to normal gut flora. Acta Paediatr Scand. 1984;73:426–32 [DOI] [PubMed] [Google Scholar]
- 308.Scheifele D, Bjornson GJ, Guasparini R, Friesen B, Meekison W. Breastfeeding and antibody responses to routine vaccination in infants. Lancet. 1992;340:1406. [DOI] [PubMed] [Google Scholar]
- 309.Karron RA, Steinhoff MC, Subbarao EK, Wilson MH, Macleod K, Clements ML, Fries LF, Murphy BR. Safety and immunogenicity of a cold-adapted influenza A (H1N1) reassortant virus vaccine administered to infants less than six months of age. Pediatr Infect Dis J. 1995;14:10–6 [DOI] [PubMed] [Google Scholar]
- 310.Zoppi G, Gasparini R, Mantovanelli F, Gobio-Casali L, Astolfi R, Crovari P. Diet and antibody response to vaccinations in healthy infants. Lancet. 1983;2:11–4 [DOI] [PubMed] [Google Scholar]
- 311.Decker MD, Edwards KM, Bradley R, Palmer P. Comparative trial in infants of four conjugate Haemophilus influenzae type b vaccines. J Pediatr. 1992;120:184–9 [DOI] [PubMed] [Google Scholar]
- 312.Watemberg N, Dagan R, Arbelli Y, Belmaker I, Morag A, Hessel L, Fritzell B, Bajard A, Peyron L. Safety and immunogenicity of Haemophilus type b-tetanus protein conjugate vaccine, mixed in the same syringe with diphtheria-tetanus-pertussis vaccine in young infants. Pediatr Infect Dis J. 1991;10:758–63 [DOI] [PubMed] [Google Scholar]
- 313.Pabst HF, Godel J, Grace M, Cho H, Spady DW. Effect of breast-feeding on immune response to BCG vaccination. Lancet. 1989;1:295–7 [DOI] [PubMed] [Google Scholar]
- 314.Pabst HF, Spady DW, Pilarski LM, Carson MM, Beeler JA, Krezolek MP. Differential modulation of the immune response by breast- or formula-feeding of infants. Acta Paediatr. 1997;86:1291–7 [DOI] [PubMed] [Google Scholar]
- 315.Turck D. Later effects of breastfeeding practice: the evidence. Nestle Nutr Workshop Ser Pediatr Program. 2007;60:31–9, discussion 9–42 [DOI] [PubMed] [Google Scholar]
- 316.Kull I, Wickman M, Lilja G, Nordvall SL, Pershagen G. Breast feeding and allergic diseases in infants-a prospective birth cohort study. Arch Dis Child. 2002;87:478–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Oddy WH, Holt PG, Sly PD, Read AW, Landau LI, Stanley FJ, Kendall GE, Burton PR. Association between breast feeding and asthma in 6 year old children: findings of a prospective birth cohort study. BMJ. 1999;319:815–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Saarinen UM, Kajosaari M. Breastfeeding as prophylaxis against atopic disease: prospective follow-up study until 17 years old. Lancet. 1995;346:1065–9 [DOI] [PubMed] [Google Scholar]
- 319.Young KA, Parrish LA, Zerbe GO, Rewers M, Deane KD, Michael Holers V, Norris JM. Perinatal and early childhood risk factors associated with rheumatoid factor positivity in a healthy paediatric population. Ann Rheum Dis. 2007;66:179–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Jacobsson LT, Jacobsson ME, Askling J, Knowler WC. Perinatal characteristics and risk of rheumatoid arthritis. BMJ. 2003;326:1068–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Brugman S, Visser JT, Hillebrands JL, Bos NA, Rozing J. Prolonged exclusive breastfeeding reduces autoimmune diabetes incidence and increases regulatory T-cell frequency in bio-breeding diabetes-prone rats. Diabetes Metab Res Rev. 2009;25:380–7 [DOI] [PubMed] [Google Scholar]
- 322.Mayer EJ, Hamman RF, Gay EC, Lezotte DC, Savitz DA, Klingensmith GJ. Reduced risk of IDDM among breast-fed children. The Colorado IDDM Registry. Diabetes. 1988;37:1625–32 [DOI] [PubMed] [Google Scholar]
- 323.Barclay AR, Russell RK, Wilson ML, Gilmour WH, Satsangi J, Wilson DC. Systematic review: the role of breastfeeding in the development of pediatric inflammatory bowel disease. J Pediatr. 2009;155:421–6 [DOI] [PubMed] [Google Scholar]
- 324.Koletzko S, Sherman P, Corey M, Griffiths A, Smith C. Role of infant feeding practices in development of Crohn's disease in childhood. BMJ. 1989;298:1617–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Davis MK, Savitz DA, Graubard BI. Infant feeding and childhood cancer. Lancet. 1988;2:365–8 [DOI] [PubMed] [Google Scholar]
- 326.Simard JF, Costenbader KH, Hernan MA, Liang MH, Mittleman MA, Karlson EW. Early life factors and adult-onset rheumatoid arthritis. J Rheumatol. 2010;37:32–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Hide DW. The clinical expression of allergy in breast-fed infants. Adv Exp Med Biol. 1991;310:475–80 [DOI] [PubMed] [Google Scholar]
- 328.Juvonen P, Mansson M, Andersson C, Jakobsson I. Allergy development and macromolecular absorption in infants with different feeding regimens during the first three days of life. A three-year prospective follow-up. Acta Paediatr. 1996;85:1047–52 [DOI] [PubMed] [Google Scholar]
- 329.Mallet E, Henocq A. Long-term prevention of allergic diseases by using protein hydrolysate formula in at-risk infants. J Pediatr. 1992;121:S95–100 [DOI] [PubMed] [Google Scholar]
- 330.Sasai K, Furukawa S, Kaneko K, Yabuta K, Baba M. Fecal IgE levels in infants at 1 month of age as indicator of atopic disease. Allergy. 1994;49:791–4 [DOI] [PubMed] [Google Scholar]
- 331.Savilahti E, Tuomikoski-Jaakkola P, Jarvenpaa AL, Virtanen M. Early feeding of preterm infants and allergic symptoms during childhood. Acta Paediatr. 1993;82:340–4 [DOI] [PubMed] [Google Scholar]
- 332.Selcuk ZT, Caglar T, Enunlu T, Topal T. The prevalence of allergic diseases in primary school children in Edirne, Turkey. Clin Exp Allergy. 1997;27:262–9 [DOI] [PubMed] [Google Scholar]
- 333.Strachan DP, Anderson HR, Johnston ID. Breastfeeding as prophylaxis against atopic disease. Lancet. 1995;346:1714. [DOI] [PubMed] [Google Scholar]
- 334.Tariq SM, Stevens M, Matthews S, Ridout S, Twiselton R, Hide DW. Cohort study of peanut and tree nut sensitisation by age of 4 years. BMJ. 1996;313:514–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Calder PC, Krauss-Etschmann S, de Jong EC, Dupont C, Frick JS, Frokiaer H, Heinrich J, Garn H, Koletzko S, Lack G, et al. Early nutrition and immunity: progress and perspectives. Br J Nutr. 2006;96:774–90 [PubMed] [Google Scholar]
- 336.Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, Collet JP, Vanilovich I, Mezen I, Ducruet T, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285:413–20 [DOI] [PubMed] [Google Scholar]
- 337.Hopkins RG, Failla ML. Chronic intake of a marginally low copper diet impairs in vitro activities of lymphocytes and neutrophils from male rats despite minimal impact on conventional indicators of copper status. J Nutr. 1995;125:2658–68 [DOI] [PubMed] [Google Scholar]
- 338.Keen CL, Lonnerdal B, Golub MS, Uriu-Hare JY, Olin KL, Hendrickx AG, Gershwin ME. Influence of marginal maternal zinc deficiency on pregnancy outcome and infant zinc status in rhesus monkeys. Pediatr Res. 1989;26:470–7 [DOI] [PubMed] [Google Scholar]
- 339.Kochanowski BA, Sherman AR. Cellular growth in iron-deficient rats: effect of pre- and postweaning iron repletion. J Nutr. 1985;115:279–87 [DOI] [PubMed] [Google Scholar]
- 340.Prentice AM. The thymus: a barometer of malnutrition. Br J Nutr. 1999;81:345–7 [PubMed] [Google Scholar]
- 341.Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. Br J Nutr. 2007;98 Suppl 1:S11–6 [DOI] [PubMed] [Google Scholar]
- 342.Chandra RK. Protein-energy malnutrition and immunological responses. J Nutr. 1992;122:597–600 [DOI] [PubMed] [Google Scholar]
- 343.Lyra JS, Madi K, Maeda CT, Savino W. Thymic extracellular matrix in human malnutrition. J Pathol. 1993;171:231–6 [DOI] [PubMed] [Google Scholar]
- 344.Parent G, Chevalier P, Zalles L, Sevilla R, Bustos M, Dhenin JM, Jambon B. In vitro lymphocyte-differentiating effects of thymulin (Zn-FTS) on lymphocyte subpopulations of severely malnourished children. Am J Clin Nutr. 1994;60:274–8 [DOI] [PubMed] [Google Scholar]
- 345.Kuvibidila S, Dardenne M, Savino W, Lepault F. Influence of iron-deficiency anemia on selected thymus functions in mice: thymulin biological activity, T-cell subsets, and thymocyte proliferation. Am J Clin Nutr. 1990;51:228–32 [DOI] [PubMed] [Google Scholar]
- 346.Dhur A, Galan P, Christides JP, Polier de Courcy G, Preziosi P, Hercberg S. Effect of folic acid deficiency upon lymphocyte subsets from lymphoid organs in mice. Comp Biochem Physiol A Comp Physiol. 1991;98:235–40 [DOI] [PubMed] [Google Scholar]
- 347.Malpuech-Brugere C, Nowacki W, Gueux E, Kuryszko J, Rock E, Rayssiguier Y, Mazur A. Accelerated thymus involution in magnesium-deficient rats is related to enhanced apoptosis and sensitivity to oxidative stress. Br J Nutr. 1999;81:405–11 [PubMed] [Google Scholar]
- 348.Nodera M, Yanagisawa H, Wada O. Increased apoptosis in a variety of tissues of zinc-deficient rats. Life Sci. 2001;69:1639–49 [DOI] [PubMed] [Google Scholar]
- 349.Howard JK, Lord GM, Matarese G, Vendetti S, Ghatei MA, Ritter MA, Lechler RI, Bloom SR. Leptin protects mice from starvation-induced lymphoid atrophy and increases thymic cellularity in ob/ob mice. J Clin Invest. 1999;104:1051–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Mitsumori K, Takegawa K, Shimo T, Onodera H, Yasuhara K, Takahashi M. Morphometric and immunohistochemical studies on atrophic changes in lympho-hematopoietic organs of rats treated with piperonyl butoxide or subjected to dietary restriction. Arch Toxicol. 1996;70:809–14 [DOI] [PubMed] [Google Scholar]
- 351.Mittal A, Woodward B. Thymic epithelial cells of severely undernourished mice: accumulation of cholesteryl esters and absence of cytoplasmic vacuoles. Proc Soc Exp Biol Med. 1985;178:385–91 [DOI] [PubMed] [Google Scholar]
- 352.Smith IF, Latham MC, Azubuike JA, Butler WR, Phillips LS, Pond WG, Enwonwu CO. Blood plasma levels of cortisol, insulin, growth hormone and somatomedin in children with marasmus, kwashiorkor, and intermediate forms of protein-energy malnutrition. Proc Soc Exp Biol Med. 1981;167:607–11 [DOI] [PubMed] [Google Scholar]
- 353.Chevalier P, Sevilla R, Zalles L, Sejas E, Belmonte G. Effect of zinc supplementation on nutritional immune deficiency. Nutr Res. 1996;16:369–79 [Google Scholar]
- 354.Chevalier P, Sevilla R, Sejas E, Zalles L, Belmonte G, Parent G. Immune recovery of malnourished children takes longer than nutritional recovery: implications for treatment and discharge. J Trop Pediatr. 1998;44:304–7 [DOI] [PubMed] [Google Scholar]
- 355.West LJ. Defining critical windows in the development of the human immune system. Hum Exp Toxicol. 2002;21:499–505 [DOI] [PubMed] [Google Scholar]
- 356.Leonhardt M, Lesage J, Dufourny L, Dickes-Coopman A, Montel V, Dupouy JP. Perinatal maternal food restriction induces alterations in hypothalamo-pituitary-adrenal axis activity and in plasma corticosterone-binding globulin capacity of weaning rat pups. Neuroendocrinology. 2002;75:45–54 [DOI] [PubMed] [Google Scholar]
- 357.Kochanowski BA, Sherman AR. Iron status of suckling rats as influenced by maternal diet during gestation and lactation. Br J Nutr. 1983;49:51–7 [DOI] [PubMed] [Google Scholar]
- 358.Rothenbacher H, Sherman AR. Target organ pathology in iron-deficient suckling rats. J Nutr. 1980;110:1648–54 [DOI] [PubMed] [Google Scholar]
- 359.Langley-Evans SC, Phillips GJ, Jackson AA. Fetal exposure to low protein maternal diet alters the susceptibility of young adult rats to sulfur dioxide-induced lung injury. J Nutr. 1997;127:202–9 [DOI] [PubMed] [Google Scholar]
- 360.Devereux G. The increase in allergic disease: environment and susceptibility. Proceedings of a symposium held at the Royal Society of Edinburgh, 4th June 2002. Clin Exp Allergy. 2003;33:394–406 [DOI] [PubMed] [Google Scholar]
- 361.Vercelli D. Mechanisms of the hygiene hypothesis–molecular and otherwise. Curr Opin Immunol. 2006;18:733–7 [DOI] [PubMed] [Google Scholar]
- 362.Martino DJ, Prescott SL. Silent mysteries: epigenetic paradigms could hold the key to conquering the epidemic of allergy and immune disease. Allergy. 2010;65:7–15 [DOI] [PubMed] [Google Scholar]
- 363.Schaub B, Lauener R, von Mutius E. The many faces of the hygiene hypothesis. J Allergy Clin Immunol. 2006;117:969–77, quiz 78 [DOI] [PubMed] [Google Scholar]