Abstract
Malignant pleural effusion (MPE) usually presents in the disseminated and advanced stage of malignancy. Dyspnea is the debilitating symptom which needs palliation in these patients. Various modalities are available in the management of MPE. Careful consideration of the patient's expected survival and quality of life is needed when deciding the optimum treatment modality in such patients. In this article, different modalities of the palliative management of MPE are discussed with an attempt to derive a treatment algorithm for the management of MPE.
Keywords: Malignant pleural effusion, Pleurodesant, Pleurodesis, Talc, Tube thoracostomy
INTRODUCTION
Advanced malignancies are frequently complicated by malignant pleural effusions (MPEs). They present either synchronously or as recurrence after the completion of treatment of the primary malignancy. The pathogenesis of MPE is by hematogenous or lymphatic implantation of tumor cells or by direct extension of tumor cells from adjacent organs such as lung, breast, chest wall, or pleura.
EPIDEMIOLOGY
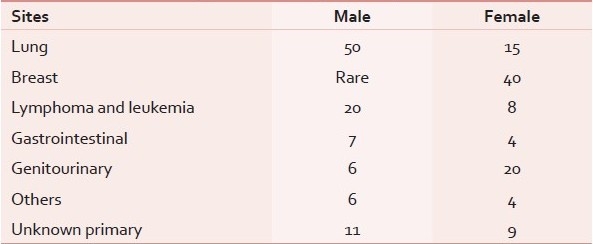
Neoplasms of lung, breast, ovary, and lymphomas constitute more than 75% of cases of MPE.[1–4] Metastatic adenocarcinoma is the most common cause.[5] In male patients, lung cancer is the most common cause and in females, breast cancer is the most common cause [Table 1].[5,6] The presence of MPE implies disseminated or advanced disease and a reduced survival. The median survival following a diagnosis of malignant pleural effusion depends on the organ of origin of primary tumor, histological type and stage, and usually ranges from 3 to 12 months. Lung cancer has the shortest, ovarian cancer has the longest, and cancer of unknown primary has an intermediate survival.[7–10]
Table 1.
Etiology of malignant pleural effusion
PRESENTATION
The main symptom of MPE is chronic shortness of breath, although cough and pain can also be debilitating. All these greatly diminish the quality of the final phase of life for patients living with cancer. The management of dyspnea is the main complain which needs palliation in MPE. Treatment goals for these patients should focus on the relief or elimination of dyspnea, restoration of normal activity and function, minimization or elimination of hospitalization, and efficient use of medical care resources.[11–13]
LABORATORY DIAGNOSIS
Chest radiographs confirm the size and location of the pleural collection. Thoracentesis is usually diagnostic and also therapeutic. Exudative and hemorrhagic collections should be considered metastatic until proved otherwise. Symptomatic relief is frequently attained by removing a large amount of fluid. Cytologically malignant cells are detected in approximately 50% of proven MPEs.[14–16] A CT scan may give information about loculations of MPE, the primary disease process, and anatomy of other organs in the thorax.
MANAGEMENT
Apart from the management of the primary disease process with chemotherapy, surgery, or radiation, the main concern of MPE is palliation of dyspnea. In this article, the issues concerning the palliation of MPE will be discussed in detail.
The palliation of dyspnea in MPE is managed by the removal of fluid from the pleural space by a least invasive procedure with minimal morbidity within the limited survival period of these patients. The methods of removing fluid from the pleural space can be simple single time aspiration, tube thoracostomy, or video-assisted thoracoscopic surgery (VATS). Tube thoracostomy and VATS can be followed by pleurodesis. The aggressiveness with which treatment is done depends mainly on two factors, i.e., the extent of symptoms caused by MPE and the performance status of the patient.
Thoracentesis
Thoracentesis, or simply called aspiration of pleural fluid, is mainly used for diagnostic purposes in patients with asymptomatic MPE. Aspiration in symptomatic MPE may provide temporary relief of dyspnea, but usually failed by reaccumulation of fluid in 98–100% of patients. Repeated aspiration in these patients can be complicated with the development of iatrogenic pneumothorax, pleural fluid loculation, or contamination with subsequent empyema.[17–19] Repeated thoracenteses, therefore, should be reserved for patients who (1) have slow reaccumulation of pleural effusions after each thoracentesis, (2) have cancers that commonly respond to therapy with resolution of the associated effusions, (3) appear unlikely to survive beyond 1–3 months, and (4) cannot tolerate other more interventional procedures to control pleural fluid, such as pleurodesis.[18,19]
Tube thoracostomy
Tube thoracostomy involves making a hole in the intercostal space into the pleural cavity (blindly or with image guidance) under aseptic precautions with placement of a secured tube for continuous drainage into a water-sealed container. Tube thoracostomy can be preceded by VATS with operative visualization of the pleural cavity and pleural biopsy for diagnostic purposes. But, VATS can only be performed in those patients who can tolerate anesthesia and single lung ventilation.[20] Either large-bore (28–36 F) or small-bore (7–16 F) chest tube can be used for reliable drainage and both have equivalent results. Thoracostomy tube should not be kept for a prolonged period for fear of infection, empyema, pneumothorax, etc. The recurrence of MPE is seen in around 80% of patients within 30 days after the removal of the tube.[17] So, thoracostomy may be sufficiently therapeutic for patients with a short expected survival of 1–3 months.[18] For patients with greater survival, an alternative procedure has to be adopted to prevent reaccumulation.
A thoracostomy tube is usually inserted at the bedside under aseptic precautions in the 5th intercostal space anterior to the mid-axillary line in the triangle of safety. Loculated MPE cannot be drained by the insertion of a single chest tube. Multiple chest tubes may be needed, but this needs to be carefully decided.
Pleurodesis
Once the thoracostomy tube in the pleural space drains 150 ml per day and the lung is fully expanded which is confirmed on chest roentgenogram, the next aim is to prevent reaccumulation by another procedure.[21] Pleurodesis is the process by which the pleural space is obliterated by inflammation induced through chemical or mechanical means, to achieve definitive and long-standing pleural apposition with fibrosis. Most physicians consider an expected survival beyond 2–3 months necessary to justify the risks, discomforts, and cost of pleurodesis. So, all patients with symptomatic MPE who are with a life expectancy of 2–3 months or more should be evaluated for pleurodesis. Those patients with a very limited life expectancy can be treated only with tube thoracostomy or thoracentesis.[22]
Pleurodesis should be done in patients who are supposed to respond to the procedure. Pleurodesis usually remains unsuccessful for patients with a trapped lung, which cannot fully inflate due to scarring, adhesions, obstructive atelectasis from an endobronchial tumor, multiple pleural loculations, or extensive intrapleural tumor masses. Of the patients who are evaluated for pleurodesis, nearly 30% are unsuitable candidates for the procedure because of trapped lungs.[23]
Agents for pleurodesis
Chemical pleurodesis is the most commonly used method of pleurodesis. Many agents have been described for chemical pleurodesis including bleomycin, tetracycline/doxycycline, Corynebacterium parvum extract, silver nitrate, iodopovidone, quinacrine, interferons, interleukin-2, and several chemotherapeutics [Table 2].[24] All these pleurodesants are usually administered through a chest tube, after the drainage of the MPE with the drain amount less than 150 ml per 24 h. Bleomycin is the most widely administered antineoplastic agent with a success rate of 60–80%. It is associated with chest pain, fever, and nausea and is generally well tolerated, although it is costly. Tetracycline and doxycycline are also commonly used for pleurodesis and have similar clinical success rates, although they are associated with intense pleuritic pain.[25,26] There are also reports of acute renal failure, anaphylaxis, and acute respiratory distress syndrome (ARDS) after tetracycline pleurodesis.[27,28] Reports of serious adverse effects after intrapleural doxycycline administration are absent from the literature. Alternative methods of pleurodesis should always be considered if sensitivity is suspected.
Table 2.
Agents of pleurodesis

Although talc is the most effective pleurodesant, it is not without complications with rare reports indicating that the incidence of ARDS can be as high as 1–9% due to intense pleuritis.[29] There are studies from across the world showing conflicting results of the deposition of talc in the alveoli of the lung with subsequent ARDS.[30–32] The literature also advocates the fact that the complication may be related to large doses of talc[33,34] although this hypothesis is questioned.[20,35,36] Some authors have the opinion that the different geographic variations in complications of talc pleurodesis are due to the size of the particles of the talc used in different studies.[37–39]
A prospective, randomized trial[40] concluded that bleomycin is as effective as talc, but the agent is extremely costly. One report of ARDS and subsequent death after bleomycin pleurodesis has been described.[41] A recent meta-analysis comparing talc, bleomycin, tetracycline/doxycycline, C. parvum extract, and mitozatrone did not find one agent that was significantly better than the others, although the trend was toward better success with talc.[42] Shaw et al.[43] in a Cochrane meta-analysis found that talc was associated with a significantly better chance of success than any other agent. The rate of complications was similar regardless of the pleurodesant. Fever and chest pain were the most common adverse events. These two meta-analyses point that talc is the most effective pleurodesant.[42,43]
Procedure of pleurdesis
Talc is used either in the form of poudrage or slurry. Poudrage is defined as the surgical application of a slightly irritating powder, such as an aerosolized drug applied during a thoracoscopic procedure (i.e., talc poudrage), to the opposing pleural surfaces in order to secure adhesion. Slurry is defined as a watery mixture of insoluble matters in a liquid (usually talc in 5% dextrose with a local anesthetic) and is introduced through a chest tube.[44] Most studies report an overall effectiveness of 60–90% at 30 days for treating dyspnea and MPE.[20,45] Different studies show that the effectiveness of talc poudrage is more than 90%.[20]
To accurately compare the effectiveness of talc poudrage versus talc slurry, there have been three randomized prospective trials, all of which have shown a similar efficacy.[44,46,47] The largest trial, which included 482 patients, found that for all patients, the rate of recurrence was similar after 30 days for either group, but the success rate of poudrage was significantly higher than talc slurry in the subset of patients with lung or breast cancer. However, there was a slightly higher mortality and rate of respiratory complications in the poudrage group.[46]
Conventional pleurodesis
In the traditional approach to chest-catheter pleurodesis, a short-term catheter is inserted intrapleurally, either blindly or by image guidance for the drainage of the pleural fluid and instillation of a sclerosing agent. The tube is removed when minimal fluid remains to be drained. Many studies showed that small-bore (9–14 F) catheters are as effective as conventional large-bore (20–32 F) chest tubes.[48–54] Most experts recommend that the sclerosant be instilled only when catheter drainage has decreased to less than 150 ml/day.[8] When talc is used as a pleurodesant, it is used as a slurry. Six gram of talc is instilled, as a slurry, in a 200-ml saline solution, to which 20 ml of 7.5% ropivacaine is added to decrease associated pain. The tube is clamped for 8 h, and the patient turned in different positions. After 8 h, the drain is connected to slow suction of 20 cm of H2O for 24 h.[44] At the end of the procedure, the patient can be discharged from the hospital. The tube is left in place, until less than 100 ml[44] to 150 ml[22] of fluid is drained in 24 h. Chest roentgenograms are obtained 1 month after the procedure and then monthly for 3 months. Further follow-up is needed as per the type of tumor and the onset of respiratory symptoms.
Video-assisted thoracoscopic surgery pleurodesis
Talc poudrage is performed by videothoracoscopy, under general anesthesia and selective one-lung ventilation. Any residual fluid is aspirated, loculations are divided when present, pleural biopsies are taken if necessary, and lung reexpansion is confirmed. Using a disposable gas-propelled atomizer, 6 g of talc is insufflated and uniformly distributed onto the pleural surface. A chest drain is inserted and positioned toward the apex and a small-bore catheter (10 F), with a three-way stopcock is placed in the posterior costovertebral gutter. The apex chest tube is removed when the chest is expanded and the output drops below 150 ml. The patient is discharged with the small-bore catheter in place for outpatient management. Chest X-rays are obtained at 1 and 2 weeks. If pleurodesis is achieved, the pleural catheter is removed, usually 2 weeks after the procedure.[44]
A recent prospective randomized study was done to compare the efficacy, safety, and outcome of thoracoscopic talc poudrage (TTP) versus povidone iodine pleurodesis (PIP) through a thoracostomy tube as palliative treatment of MPE due to metastatic breast cancer.[55] A total of 42 patients were prospectively enrolled (22 received talc poudrage and 20 patients received pleurodesis by instilling povidone iodine through a thoracostomy tube, as a bedside procedure). There were no in-hospital deaths. TTP was associated with severe pleuritic chest pain (18% vs. 0%, P = 0.2), fever (18% vs. 5%), and longer hospital stay (P = 0.009). Both groups achieved good symptom control. The recurrence of MPE requiring intervention was not significant in the two groups (2 vs. 3 patients, P = ns). Although the study was small, it can be concluded that povidone iodine is easily available, cost effective and safe, can be given through a thoracostomy tube and can be repeated if necessary. Povidone iodine is a good alternative to TTP. A larger randomized study, inclusion of talc slurry as an arm, and inclusion of MPE arising out of other malignancies are necessary to come to a conclusion about the efficacy of povidone iodine.
Survival after pleurodesis
The most difficult question to answer is the improvement of survival with pleurodesis. As such, pleurodesis is purely a palliative procedure and it only decreases the mortality out of respiratory compromise and thus improving quality of life and survival. Multiple clinical factors have been used to estimate survival after pleurodesis, including the organ of origin of malignancy, cell type (adenocarcinoma, squamous, small cell, etc.), stage of the tumor, characteristics of the pleural fluid, and performance level. Unfortunately, in spite of careful selection, 32% of patients do not survive 30 days after pleurodesis.[19,56–59] The American Thoracic Society/European Respiratory Society guideline for the management of MPE recommends that pleurodesis should be limited to patients with pleural fluid pH values greater than 7.30, because of the direct correlation between low pH and poor short-term survival.[19,60–63] Among the criteria now in common use, performance status is the most important for estimating postpleurodesis survival.[64]
SURGERY FOR MALIGNANT PLEURAL EFFUSION
Thoracostomy with pleurectomy and decortication are effective means of pleurodesis. However, these operations have a mortality of 10% in excess and a high morbidity, especially prolonged air leaks.[65,66] It is only in some selected patients, with a high functional reserve and a trapped lung, that the operation may provide a benefit.
SUMMARY
MPEs present usually in a disseminated and advanced stage of malignancy. Different methods exist for the palliation of dyspnea in these patients. Careful consideration of the patient's expected survival and quality of life is needed when deciding between simple thoracentesis, thoracostomy, or pleurodesis, owing to their limited survival and varying performance status [Figure 1]:
Figure 1.

Flow chart in the management of malignant pleural effusion
Thoracentesis – repeated aspiration may be the only method in patients with a poor performance status with a very limited life span.
Thoracostomy – survival of at least 1 month is required to justify the risks and morbidity of tube thoracostomy. Small-bore tube thoracostomy has similar efficacy to large-bore tube thoracostomy.
Pleurodesis – for patients with expected survival of more than 2–3 months, pleurodesis should be considered. Talc is the most effective, cheap, and easily available pleurodesant. For patients who can tolerate general anesthesia and single lung ventilation, a VATS procedure helps in diagnosis and simultaneously draining the effusion, freeing the lung from adhesions if necessary and simultaneously performing talc pleurodesis. For patients unable to tolerate an operation but with an expandable lung, bedside tube thoracostomy to drain the effusion till the drain output is less than 100 ml followed by talc slurry pleurodesis has a similar efficacy to VATS talc poudrage.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Henschke CI, Yankelevitz DF, Davis SD. Pleural diseases: Multimodality imaging and clinical management. Curr Probl Diagn Radiol. 1991;20:155–81. doi: 10.1016/0363-0188(91)90020-3. [DOI] [PubMed] [Google Scholar]
- 2.Storey DD, Dines DE, Coles DT. Pleural effusion: A diagnostic dilemma. JAMA. 1976;236:2183–6. [PubMed] [Google Scholar]
- 3.Martinez-Moragon E, Aparicio J, Sanchis J, Menendez R, Cruz Rogado M, Sanchis F. Malignant pleural effusion: Prognostic factors for survival and response to chemical pleurodesis in a series of 120 cases. Respiration. 1998;65:108–13. doi: 10.1159/000029240. [DOI] [PubMed] [Google Scholar]
- 4.Hausheer FH, Yarbro JW. Diagnosis and treatment of malignant pleural effusion. Cancer Metastasis Rev. 1987;6:23–40. doi: 10.1007/BF00047607. [DOI] [PubMed] [Google Scholar]
- 5.Awasthi A, Gupta N, Srinivasan R, Nijhawan R, Rajwanshi A. Cytopathological spectrum of unusual malignant pleural effusions at a tertiary care centre in north India. Cytopathology. 2007;18:28–32. doi: 10.1111/j.1365-2303.2007.00382.x. [DOI] [PubMed] [Google Scholar]
- 6.Johnston WW. The malignant pleural effusion: A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56:905–9. doi: 10.1002/1097-0142(19850815)56:4<905::aid-cncr2820560435>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Sears D, Hajdu SI. The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol. 1987;31:85–97. [PubMed] [Google Scholar]
- 8.Molengraft van de FJJM, Vooijs GP. Survival of patients with malignancy-associated effusions. Acta Cytol. 1989;33:911–6. [PubMed] [Google Scholar]
- 9.Bonnefoi H, Smith IE. How should cancer presenting as a malignant effusion be managed? Br J Cancer. 1996;74:832–5. doi: 10.1038/bjc.1996.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbruzzese JL, Abbruzzese MC, Hess KR, Raber MN, Lenzi R, Frost P, et al. Unknown primary carcinoma: Natural history and prognostic factors in 657 consecutive patients. J Clin Oncol. 1994;12:1272–80. doi: 10.1200/JCO.1994.12.6.1272. [DOI] [PubMed] [Google Scholar]
- 11.Assi Z, Caruso JL, Herndon J, Patz EF., Jr Cytologically proved malignant pleural effusions: distribution of transudates and exudates. Chest. 1998;113:1302–4. doi: 10.1378/chest.113.5.1302. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med. 2000;162:1987–2001. doi: 10.1164/ajrccm.162.5.ats8-00. [DOI] [PubMed] [Google Scholar]
- 13.Antunes G, Neville E. Management of malignant pleural effusions. Thorax. 2000;55:981–3. doi: 10.1136/thorax.55.12.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irani DR, Underwood RD, Johnson EH, Greenberg SD. Malignant pleural effusions: A clinical cytopathologic study. Arch Intern Med. 1987;147:1133–6. [PubMed] [Google Scholar]
- 15.Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: Analysis of 414 cases. Mayo Clin Proc. 1985;60:158–64. doi: 10.1016/s0025-6196(12)60212-2. [DOI] [PubMed] [Google Scholar]
- 16.Salyer WR, Eggleston JC, Erozan YS. Efficacy of pleural needle biopsy and pleural fluid cytopathology in the diagnosis of malignant neoplasm involving the pleura. Chest. 1975;67:536–9. doi: 10.1378/chest.67.5.536. [DOI] [PubMed] [Google Scholar]
- 17.Tassi GF, Cardillo G, Marchetti GP, Carleo F, Martelli M. Diagnostic and therapeutical management of malignant pleural effusion. Ann Oncol. 2006;17(Suppl 2):ii11–2. doi: 10.1093/annonc/mdj911. [DOI] [PubMed] [Google Scholar]
- 18.Maskell NA, Butland RJ. BTS guidelines for the investigation of a unilateral pleural effusion in adults. Thorax. 2003;58(Suppl 2):ii8–17. doi: 10.1136/thorax.58.suppl_2.ii8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antony VB, Loddenkemper R, Astoul P, Boutin C, Goldstraw P, Hott J, et al. Management of malignant pleural effusions. Eur Respir J. 2001;18:402–19. doi: 10.1183/09031936.01.00225601. [DOI] [PubMed] [Google Scholar]
- 20.De Campos JR, Vargas FS, DeCampos Werebe E, Cardoso P, Teixeira LR, Jatene FB, et al. Thoracoscopy talc poudrage: A 15-year experience. Chest. 2001;119:801–6. doi: 10.1378/chest.119.3.801. [DOI] [PubMed] [Google Scholar]
- 21.Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer. 2006;54:1–9. doi: 10.1016/j.lungcan.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Heffner JE, Klein JS. Recent advances in the diagnosis and management of malignant pleural effusions. Mayo Clin Proc. 2008;83:235–50. doi: 10.4065/83.2.235. [DOI] [PubMed] [Google Scholar]
- 23.Steger V, Mika U, Toomes H, Walker T, Engel C, Kyriss T, et al. Who gains most? A 10-year experience with 611 thoracoscopic talc pleurodeses. Ann Thorac Surg. 2007;83:1940–5. doi: 10.1016/j.athoracsur.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 24.Dikensoy O, Light RW. Alternative widely available, inexpensive agents for pleurodesis. Curr Opin Pulm Med. 2005;11:340–4. doi: 10.1097/01.mcp.0000166587.24127.91. [DOI] [PubMed] [Google Scholar]
- 25.Emad A, Rezaian GR. Treatment of malignant pleural effusions with a combination of bleomycin and tetracycline. A comparison of bleomycin or tetracycline alone versus a combination of bleomycin and tetracycline. Cancer. 1996;78:2498–501. doi: 10.1002/(sici)1097-0142(19961215)78:12<2498::aid-cncr8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Moragón E, Aparicio J, Rogado MC, Sanchis J, Sanchis F, Gil-Suay V. Pleurodesis in malignant pleural effusions: A randomized study of tetracycline versus bleomycin. Eur Respir J. 1997;10:2380–3. doi: 10.1183/09031936.97.10102383. [DOI] [PubMed] [Google Scholar]
- 27.Smythe WR, Bavaria JE. Tetracycline pleurodesis-associated acute renal failure. Chest. 1993;104:1274–6. doi: 10.1378/chest.104.4.1274. [DOI] [PubMed] [Google Scholar]
- 28.DiBardino DJ, Vanatta JM, Fagan SP, Awad SS. Acute respiratory failure after pleurodesis with doxycycline. Ann Thorac Surg. 2002;74:257–8. doi: 10.1016/s0003-4975(01)03518-4. [DOI] [PubMed] [Google Scholar]
- 29.Janssen JP, Collier G, Astoul P, Tassi GF, Noppen M, Rodriguez-Panadero F, et al. Safety of pleurodesis with talc poudrage in malignant pleural effusion: A prospective cohort study. Lancet. 2007;369:1535–9. doi: 10.1016/S0140-6736(07)60708-9. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy L, Harley RA, Sahn SA, Strange C. Talc slurry pleurodesis. Pleural fluid and histologic analysis. Chest. 1995;107:1707–12. doi: 10.1378/chest.107.6.1707. [DOI] [PubMed] [Google Scholar]
- 31.Werebe EC, Pazetti R, Milanez de Campos JR, Fernandez PP, Capelozzi VL, Jatene FB, et al. Systemic distribution of talc after intrapleural administration in rats. Chest. 1999;115:190–3. doi: 10.1378/chest.115.1.190. [DOI] [PubMed] [Google Scholar]
- 32.Mathlouthi A, Chabchoub A, Labbene N, Amara A, Ghorbel A, Kacem S, et al. Etude anatomopathologique du talcage pleural. Rev Mal Resp. 1992;9:617–21. [PubMed] [Google Scholar]
- 33.Rinaldo JE, Owens JR, Rogers MR. Adult respiratory distress syndrome after intrapleural instillation of talc. J Thorac Cardiovasc Surg. 1983;85:523–6. [PubMed] [Google Scholar]
- 34.Kennedy L, Sahn SA. Talc pleurodesis for the treatment of pneumothorax and pleural effusion. Chest. 1994;106:1215–22. doi: 10.1378/chest.106.4.1215. [DOI] [PubMed] [Google Scholar]
- 35.Bouchama A, Chastre JC, Gaudichet A, Soler P, Gibert C. Acute pneumonitis with bilateral pleural effusion after talc pleurodesis. Chest. 1984;86:795–7. doi: 10.1378/chest.86.5.795. [DOI] [PubMed] [Google Scholar]
- 36.Rehse DH, Aye RW, Florence MG. Respiratory failure following talc pleurodesis. Am J Surg. 1999;177:437–40. doi: 10.1016/s0002-9610(99)00075-6. [DOI] [PubMed] [Google Scholar]
- 37.Ferrer J, Villarino MA, Tura JM, Traveria A, Light RW. Talc preparations used for pleurodesis vary markedly from one preparation to another. Chest. 2001;119:1901–5. doi: 10.1378/chest.119.6.1901. [DOI] [PubMed] [Google Scholar]
- 38.Maskell NA, Lee YC, Gleeson FV, Hedley EL, Pengelly G, Davies RJ. Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med. 2004;170:377–82. doi: 10.1164/rccm.200311-1579OC. [DOI] [PubMed] [Google Scholar]
- 39.Sahn SA, Light RW. Pro/con editorial: Talc should/should not be used for pleurodesis. Am J Respir Crit Care Med. 2000;162:2023–6. doi: 10.1164/ajrccm.162.6.pc09-00a. [DOI] [PubMed] [Google Scholar]
- 40.Zimmer PW, Hill M, Casey K, Harvey E, Low DE. Prospective randomized trial of talc slurry vs bleomycin in pleurodesis for symptomatic malignant pleural effusions. Chest. 1997;112:430–9. doi: 10.1378/chest.112.2.430. [DOI] [PubMed] [Google Scholar]
- 41.Audu PB, Sing RF, Mette SA, Fallahnejhad M. Fatal diffuse alveolar injury following use of intrapleural bleomycin. Chest. 1993;103:1638. doi: 10.1378/chest.103.5.1638a. [DOI] [PubMed] [Google Scholar]
- 42.Tan C, Sedrakyan A, Browne J, Swift S, Treasure T. The evidence on the effectiveness of management for malignant pleural effusion: a systematic review. Eur J Cardiothorac Surg. 2006;29:829–38. doi: 10.1016/j.ejcts.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 43.Shaw P, Agarwal R. Pleurodesis for malignant pleural effusions. Cochrane Database Sys Rev. 2004;(1):CD002916. doi: 10.1002/14651858.CD002916.pub2. [DOI] [PubMed] [Google Scholar]
- 44.Stefani A, Natali P, Casali C, Morandi U. Talc poudrage versus talc slurry in the treatment of malignant pleural effusion. A prospective comparative study. Eur J Cardiothorac Surg. 2006;30:827–32. doi: 10.1016/j.ejcts.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Brega-Massone PP, Lequaglie C, Magnani B, Ferro F, Cataldo I. Chemical pleurodesis to improve patients’ quality of life in the management of malignant pleural effusions: The 15 year experience of the National Cancer Institute of Milan. Surg Laparosc Endosc Percutan Technol. 2004;14:73–9. doi: 10.1097/00129689-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Yim AP, Chan AT, Lee TW, Wan IY, Ho JK. Thoracoscopic talc insufflations versus talc slurry for symptomatic malignant pleural effusion. Ann Thorac Surg. 1996;62:1655–8. [PubMed] [Google Scholar]
- 47.Dresler CM, Olak J, Herndon JE, 2nd, Richards WG, Scalzetti E, Fleishman SB, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909–15. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patz EF., Jr Malignant pleural effusions: Recent advances and ambulatory sclerotherapy. Chest. 1998;113:74–77S. doi: 10.1378/chest.113.1_supplement.74s. [DOI] [PubMed] [Google Scholar]
- 49.Clementsen P, Evald T, Grode G, Hansen M, Krag Jacobsen G, Faurschou P. Treatment of malignant pleural effusion: Pleurodesis using a small percutaneous catheter: A prospective randomized study. Respir Med. 1998;92:593–6. doi: 10.1016/s0954-6111(98)90315-8. [DOI] [PubMed] [Google Scholar]
- 50.Hsu WH, Chiang CD, Chen CY, Kwan PC, Hsu JY. Ultrasound-guided small-bore elecath tube insertion for the rapid sclerotherapy of malignant pleural effusion. Jpn J Clin Oncol. 1998;28:187–91. doi: 10.1093/jjco/28.3.187. [DOI] [PubMed] [Google Scholar]
- 51.Patz EF, Jr, McAdams HP, Erasmus JJ, Goodman PC, Culhane DK, Gilkeson RC, et al. Sclerotherapy for malignant pleural effusions: A prospective randomized trial of bleomycin vs doxycycline with small-bore catheter drainage. Chest. 1998;113:1305–11. doi: 10.1378/chest.113.5.1305. [DOI] [PubMed] [Google Scholar]
- 52.Sahin U, Unlu M, Akkaya A, Ornek Z. The value of small-bore catheter thoracostomy in the treatment of malignant pleural effusions. Respiration. 2001;68:501–5. doi: 10.1159/000050558. [DOI] [PubMed] [Google Scholar]
- 53.Parulekar W, Di Primio G, Matzinger F, Dennie C, Bociek G. Use of small-bore vs large-bore chest tubes for treatment of malignant pleural effusions. Chest. 2001;120:19–25. doi: 10.1378/chest.120.1.19. [DOI] [PubMed] [Google Scholar]
- 54.Sartori S, Tombesi P, Tassinari D, Ceccotti P, Nielsen I, Trevisani L, et al. Sonographically guided smallbore chest tubes and sonographic monitoring for rapid sclerotherapy of recurrent malignant pleural effusions. J Ultrasound Med. 2004;23:1171–6. doi: 10.7863/jum.2004.23.9.1171. [DOI] [PubMed] [Google Scholar]
- 55.Mohsen TA, Zeid AA, Meshref M, Tawfeek N, Redmond K, Ananiadou OG, et al. Local iodine pleurodesis versus thoracoscopic talc insufflation in recurrent malignant pleural effusion: A prospective randomized control trial. Eur J Cardiothorac Surg. 2011;40:282–6. doi: 10.1016/j.ejcts.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Jones GR. Treatment of recurrent malignant pleural effusion by iodized talc pleurodesis. Thorax. 1969;24:69–73. doi: 10.1136/thx.24.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marom EM, Patz EF, Jr, Erasmus JJ, McAdams HP, Goodman PC, Herndon JE. Malignant pleural effusions: Treatment with small-bore-catheter thoracostomy and talc pleurodesis. Radiology. 1999;210:277–81. doi: 10.1148/radiology.210.1.r99dc04277. [DOI] [PubMed] [Google Scholar]
- 58.Schulze M, Boehle AS, Kurdow R, Dohrmann P, Henne-Bruns D. Effective treatment of malignant pleural effusion by minimal invasive thoracic surgery: Thoracoscopic talc pleurodesis and pleuroperitoneal shunts in 101 patients. Ann Thorac Surg. 2001;71:1809–12. doi: 10.1016/s0003-4975(01)02586-3. [DOI] [PubMed] [Google Scholar]
- 59.Bernard A, de Dompsure RB, Hagry O, Favre JP. Early and late mortality after pleurodesis for malignant pleural effusion. Ann Thorac Surg. 2002;74:213–7. doi: 10.1016/s0003-4975(02)03599-3. [DOI] [PubMed] [Google Scholar]
- 60.Good JT, Jr, Taryle DA, Maulitz RM, Kaplan RL, Sahn SA. The diagnostic value of pleural fluid pH. Chest. 1980;78:55–9. doi: 10.1378/chest.78.1.55. [DOI] [PubMed] [Google Scholar]
- 61.Gottehrer A, Taryle DA, Reed CE, Sahn SA. Pleural fluid analysis in malignant mesothelioma: Prognostic implications. Chest. 1991;100:1003–6. doi: 10.1378/chest.100.4.1003. [DOI] [PubMed] [Google Scholar]
- 62.Heffner JE, Heffner JN, Brown LK. Multilevel and continuous pleural fluid pH likelihood ratios for evaluating malignant pleural effusions. Chest. 2003;123:1887–94. doi: 10.1378/chest.123.6.1887. [DOI] [PubMed] [Google Scholar]
- 63.Heffner JE, Nietert PJ, Barbieri C. Pleural fluid pH as a predictor of survival for patients with malignant pleural effusions. Chest. 2000;117:79–86. doi: 10.1378/chest.117.1.79. [DOI] [PubMed] [Google Scholar]
- 64.Burrows CM, Mathews WC, Colt HG. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: An assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest. 2000;117:73–8. doi: 10.1378/chest.117.1.73. [DOI] [PubMed] [Google Scholar]
- 65.Fry WA, Khandekar JD. Parietal pleurectomy for malignant pleural effusion. Ann Surg Oncol. 1995;2:160–4. doi: 10.1007/BF02303632. [DOI] [PubMed] [Google Scholar]
- 66.Soysal O, Karaoğlanoğlu N, Demiracan S, Topçu S, Taştepe I, Kaya S, et al. Pleurectomy/ decortication for palliation in malignant pleural mesothelioma: Results of surgery. Eur J Cardiothorac Surg. 1997;11:210–3. doi: 10.1016/s1010-7940(96)01008-1. [DOI] [PubMed] [Google Scholar]


