Abstract
Rotenone, a botanical insecticide is known to cause apoptosis in various cell types. Trans-resveratrol, a natural phytophenol present in red grapes and wine, is also well documented for its antioxidant, anti-inflammatory, anti-mutagenic, and anticarcinogenic activities. Therefore, the present investigations were carried out to assess the protective effect of trans-resveratrol against rotenone-induced cell death in human breast adenocarcinoma (MCF-7) cells. MCF-7 cells were exposed with various concentrations of rotenone for 24 h, and the loss in percent cell viability was evaluated by MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide] and neutral red uptake (NRU) assays. A significant decrease in percent cell viability in MCF-7 cells was observed at 50 μM and above concentrations of rotenone, as compared to untreated control. Furthermore, various concentrations (5, 10, and 25 μM) of trans-resveratrol were used to see its protective role on cell viability in rotenone-induced cell death in MCF-7 cells. Pre- or post- treatment of trans-resveratrol for 24 h was given to the cells. The data exhibited a significant dose dependent increase in the percent cell viability under pre- and post-treatment conditions. However, post-treatment of trans-resveratrol for 24 h after rotenone exposure to the cells was relatively less effective. Overall, the results suggest that trans-resveratrol significantly protects MCF-7 cells from rotenone-induced cell death. This model can be used as an effective and economical alternative to animal models for screening the antioxidant activity of a variety of natural compounds/drugs.
Keywords: Cytotoxicity, MCF--7 cells, rotenone, trans-resveratrol
INTRODUCTION
Rotenone, a specific inhibitor of mitochondrial complex-I, is used as a botanical insecticide for at least 150 years to control crop pests.[1] Being a cell respiratory enzyme inhibitor, it acts as a stomach poison in insects.[2] It is well known to inhibit biochemical process at the cellular level, and can block electron transfer from complex to ubiquinone, resulting in a blockage of the oxidative phosphorylation, and an increase of the reactive oxygen species (ROS).[3,4] A number of studies have evaluated the effects of rotenone both in vitro[5] and in vivo.[3] Studies have shown that rotenone is capable to induce apoptosis in various cells derived from human B-cell lymphomas,[6] promyelocytic leukemias,[7] and neuroblastomas.[8] Recently, reports have also shown that rotenone induce oxidative stress mediated cytotoxicity in PC12 cells,[9] and in MCF-7 cells.[4]
Trans-resveratrol (trans-3,4’,5-tryhydroxystilbene), a natural phytophenol is reportedly present in high concentrations in red grapes and wine. Pharmacological activities of trans-resveratrol is well known through its antioxidant,[10,11] anti-inflammatory,[12] antimutagenic,[13] and anticarcinogenic[14] activities. Protective potential of trans-resveratrol has also been demonstrated by several investigators in different cell lines and primary cell cultures[15–18] including PC12 cells.[19] Moreover, the neuroprotective potential of trans-resveratrol has been extensively studied in both in vitro and in vivo models.[19–21] However, the protective potential of trans-resveratrol against rotenone-induced cytotoxicity in MCF-7 cells, a human breast cell line has not been reported. The MCF-7 cells have been chosen because this cell line has been extensively used for the toxicological and/or pharmacological studies.[22–24] Thus, the present studies were carried out to determine the antioxidant potential of trans-resveratrol against rotenone-induced cytotoxicity in MCF-7 cells.
MATERIALS AND METHODS
Cell culture
MCF-7 cells, were cultured in minimum essential medium (MEM), supplemented with 10% fetal bovine serum (FBS), 0.2% sodium bicarbonate and antibiotic-antimycotic solution (100×; 1 ml/100 ml of medium, HiMedia, India) following the method described by Musarrat et al.[25] Culture were maintained at 37°C in 5% CO2---95% atmosphere under high humid conditions. Medium was changed twice weekly and the culture was split at a ratio of 1:5 once a week. Prior to experiment, each batches of cells was assessed for cell viability by trypan blue dye exclusion test,[26] and batches showing more than 95% cell viability were used in the study. Cells of passage number between 8 and 10 were used in the study.
Experimental design
To identify the cytotoxic doses, the MCF-7 cells were exposed to various concentrations of rotenone (1--1000 μM) for 24 h. Under identical setup, biologically safe doses of trans-resveratrol were also detected in MCF-7 cells. Further, the experiments were conducted using rotenone (100 μM) and trans-resveratrol at 5, 10, and 25 μM for 24 h each. Responsiveness of MCF-7 cells to trans-resveratrol was determined by performing the following two treatment schedules; (a) cells treated with trans-resveratrol for 24 h prior to rotenone insult (pre-treatment group); (b) cells treated with trans-resveratrol for 24 h after 24 h of rotenone exposure (post-treatment group). Influence of trans-resveratrol was estimated by comparing the values of treatment groups with the non-treated cells exposed to rotenone alone.
Mitochondrial activity by MTT assay
MTT assay was performed following the protocols of Siddiqui et al.[27] In brief, cells (1 × 104) were seeded in 96-well culture plates and allowed to adhere properly for 24 h at 37 °C in a CO2 incubator (5% CO2---95% atmosphere at high humidity). After respective exposures, MTT (5 mg/ml of stock in PBS) was added (10 μl/well containing 100 μl of cell suspension) 4 h prior to the completion of exposure period. After the incubation period, the reaction mixture was carefully aspirated and 200 μl of dimethyl sulfoxide was added to each well with gentle mixing to ensure complete homogeneity. Subsequently, after 10 min, the color was read at 550 nm, using a multiwell microplate reader (Zynth 200, Germany).
Neutral red uptake assay
Neutral red uptake (NRU) assay was performed following the protocols of Siddiqui et al.[27] In brief, cells (1 × 104) were seeded in 96-well culture plates and allowed to adhere properly for 24 h at 37 °C in a CO2 incubator (5% CO2---95% atmosphere at high humidity). On the completion of respective incubation periods, the test solution was aspirated and cells were washed with PBS twice. Cells were then incubated for 3 h in medium supplemented with neutral red (50 μg/ml). Then medium was washed off rapidly with a solution containing 0.5% formaldehyde and 1% calcium chloride. The cells were then subjected to further incubation further for 20 min at 37 °C, in a mixture of acetic acid (1%) and ethanol (50%), to extract the dye. The plates were then read at 540 nm using multiplate reader (Zynth 200, Germany).
Statistical analysis
Results are expressed as the mean ± standard error of three independent experiments with six replicates each. Statistical analysis was performed using one-way analysis of variance (ANOVA) and Dunnett post-hoc test to compare the inter- and intra-group findings. The values depicting P < 0.05 were considered as statistically significant.
RESULTS AND DISCUSSION
The results of cytotoxicity assessment of rotenone are presented in Figures 1 and 2. Statistically significant decrease in percent cell viability (% control) was initiated following 24 h exposure of rotenone at 50 μM concentration. A concentration-dependent decrease in cell viability was observed at 50 μM and higher concentrations, whereas, 25 μM and lower concentrations did not exhibit any adverse effect in rotenone exposed MCF-7 cells [Figure 1]. Similar pattern of concentration dependent effects was also observed with NRU assay [Figure 2]. Under identical experimental setup, none of the doses (5, 10, and 25 μM) of trans-resveratrol was found to be cytotoxic in 24 h exposed MCF-7 cells by MTT [Figure 3] and NRU [Figure 4] assays.
Figure 1.
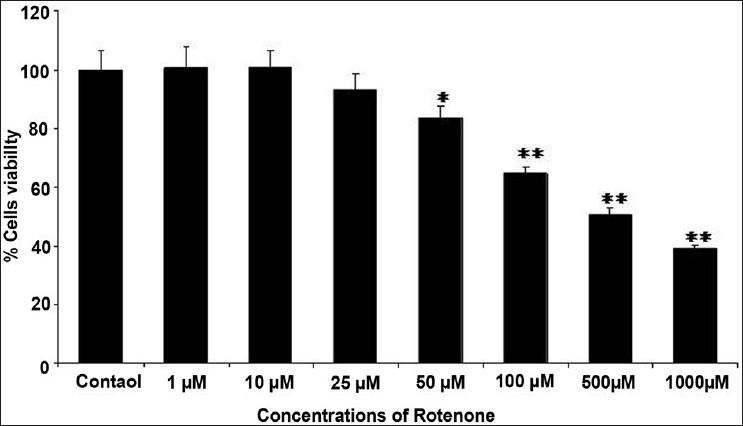
Cytotoxicity assessment by MTT assay in MCF-7 cells following the exposure of various concentrations (1--1000 μM) of rotenone for 24 h. Values are mean ± SE of three independent experiments. *P < 0.05, **P < 0.01 vs control.
Figure 2.
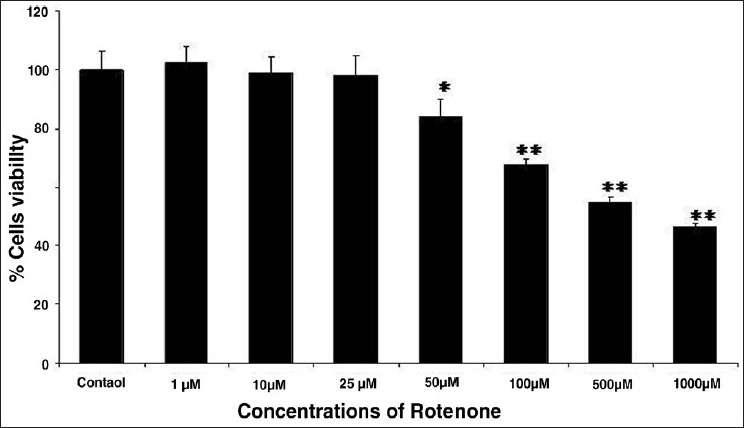
Cytotoxicity assessment by NRU assay in MCF-7 cells following the exposure of various concentrations (1--1000 μM) of rotenone for 24 h. Values are mean ± SE of three independent experiments. *P < 0.05, **P < 0.01 vs control.
Figure 3.
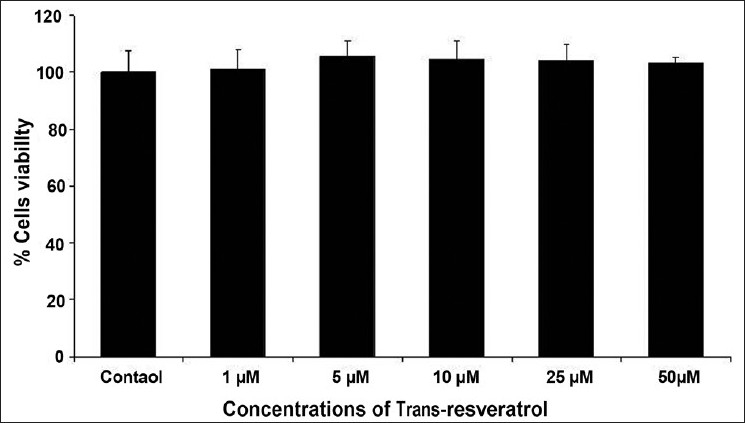
Cytotoxicity assessment by MTT assay in MCF-7 cells following the exposure of various concentrations (1--50 μM) of trans-resveratrol for 24 h. Values are mean ± SE of three independent experiments.
Figure 4.
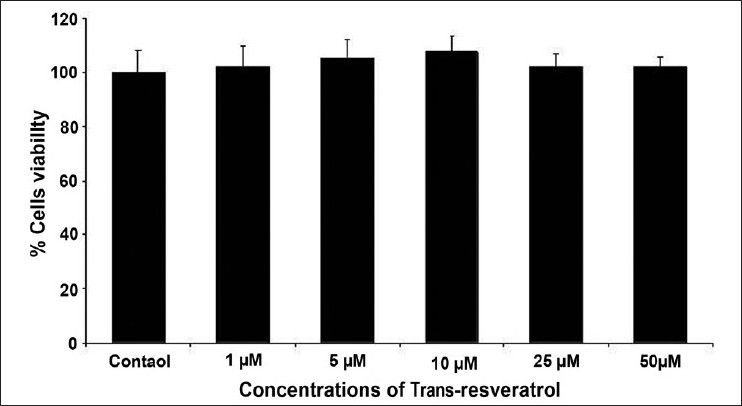
Cytotoxicity assessment by NRU assay in MCF-7 cells following the exposure of various concentrations (1--50 μM) of trans-resveratrol for 24 h. Values are mean ± SE of three independent experiments.
Result highlights of protective potential of trans-resveratrol against toxic insult of rotenone (100 μM) in MCF-7 cells are depicted in Figures 5 and 6. Statistically significant (6%, 20%, and 28% of rotenone control) increase in percent cell viability was recorded following 5, 10, and 25 μM concentrations of trans-resveratrol, respectively, in pre-treatment schedule for 24 h in MCF-7 cells. In post-treatment group, a similar trend of trans-resveratrol mediated protection was observed. However, the magnitude of protection was comparatively lower at all the doses used, i.e., 7% and 10% of rotenone control at 10 and 25 μM trans-resveratrol respectively, whereas, trans-resveratrol at 5 μM could not protect MCF-7 cells against rotenone-induced cell death. Although, the trans-resveratrol mediated increase in percent cell viability was statistically significant (P < 0.05 and P < 0.01), when compared with rotenone (100 μM) exposed group, but the recovery response of pre-treatment of trans-resveratrol even at highest dose, i.e., 25 μM was significantly (P < 0.01) lower than unexposed control cells [Figure 5]. In case of NRU assay, the loss of percent cell viability was significantly increased by 10% and 12% at 10 μM and 25 μM of trans-resveratrol, respectively, compared with the cells exposed to 100 μM concentration of rotenone. The trend of recovery at all the concentrations of trans-resveratrol was almost similar as in case of MTT assay. However, trans-resveratrol treated MCF-7 cells after rotenone exposure could not get significantly increase, except 25 μM (P < 0.05) of trans-resveratrol when compared with rotenone (100 μM) exposed cells [Figure 6].
Figure 5.
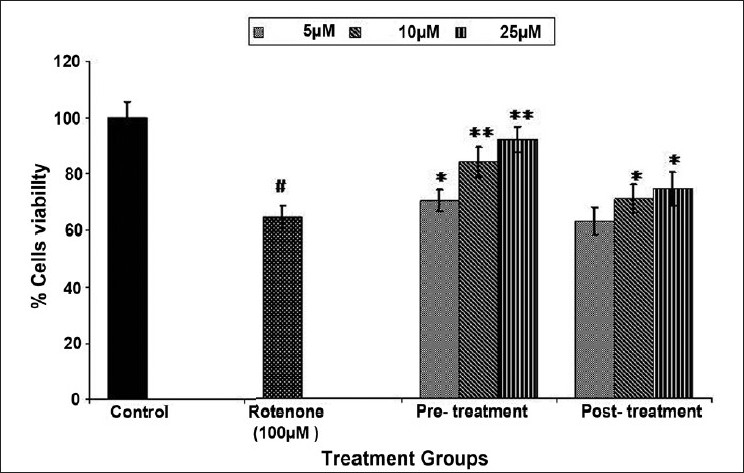
Protective potential of trans-resveratrol in MCF-7 cells exposed to 100 μM of rotenone for 24 h by MTT assay. Values are mean ± SE of three independent experiments. #P < 0.01 vs control and *P < 0.05, **P < 0.01 vs rotenone exposure.
Figure 6.
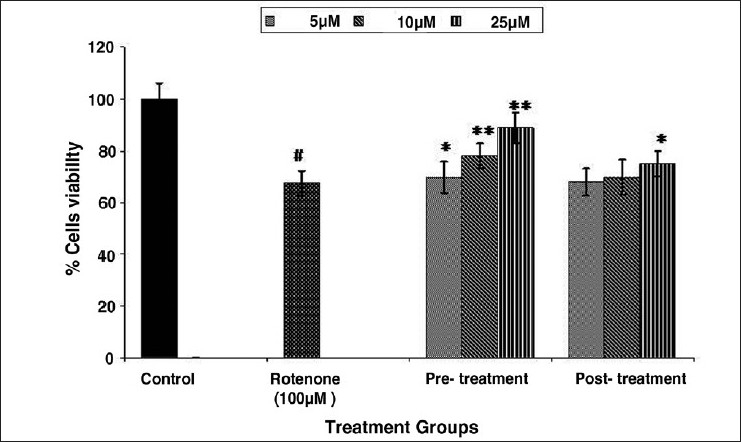
Protective potential of trans-resveratrol in MCF-7 cells exposed to 100 μM of rotenone for 24 h by NRU assay. Values are mean ± SE of three independent experiments. #P < 0.01 vs control and *P < 0.05, **P < 0.01 vs rotenone exposure.
An essential task in pharmacology is the search and development of new drugs for the prevention and treatment of diseases.[28,29] Natural compounds could be the suitable candidates in this respect.[30,31] Moreover, in recent years it has been shown that trans-resveratrol offers protection against different toxins.[32,33]
The present study shows that rotenone induce significant decrease in cell viability in a concentration dependent manner in MCF-7 cells following 24 h exposure based on MTT and NRU assays, as the endpoints for cytotoxicity assessment. Similar trend in the decrease in cell viability was obtained by MTT and NRU assays. A possible explanation for this is that the cytotoxicity of rotenone is mediated through the both mitochondrial and lysosomal damage. Both the parameters have been used as direct measurements of cell viability by different investigators.[9,34]
Our results are in accordance with the previous findings that the rotenone-induce cytoxicity in various types of cells, including the MCF-7 cells.[4,9,35] Rotenone-induced oxidative stress and death of dopaminergic neurons in chronic midbrain slice model is documented.[36] Evidences suggest that complex-I may be a source of ROS generation, since, there is a site of electron leakage in complex-I upstream of the rotenone-binding site, which partially inhibits the complex I following rotenone exposure.[9,37]
The protective potential of trans-resveratrol studied with pre- and post-treatment schedules ascertained the therapeutic applicability of trans-resveratrol in MCF-7 cells, under experimental exposure of rotenone. A dose dependent synergistic protective potential could be observed under all treatment groups i.e. pre, and post. In general, pre-treatment group has shown higher restoration than post-treatment group by MTT and NRU assays. This might be due to pre-treatment of trans-resveratrol induced increase in the level of antioxidant and anti-inflammatory enzymes in MCF-7 cells. These kinds of pre-treatment of trans-resveratrol have already been studied by different investigators showing better protection than post-treatment.[19,27] The pre-treatment of many other antioxidants and anti-inflammatory drugs have also been demonstrated showing better protection than therapeutics.[27,38]
Increase in cell viability was concentration dependent but the recovery response of trans-resveratrol at higher concentrations, i.e., 10 and 25 μM was more protective that 5 μM of trans-resveratrol. Trans-resveratrol is well known for its anti-inflammatory and antioxidant activities, therefore, our data on the protective effects of trans-resveratrol are well corelated with previous in vitro studies demonstrating the antioxidative effects of this drug on toxicant-induced apoptosis in PC12 cells,[39,40] β-amyloid neurotoxicity,[41] and in oxygen-glucose deprivation.[42–44] Studies performed in rodents also suggested that this drug has a good protective role against toxicants.[45,46]
It is concluded that the study has significance in understanding the cytotoxicity of rotenone and also the protection against the cytotoxic activity by trans-resveratrol at cellular level, using MCF-7 cells as a model for simple, easy and inexpensive screening the antioxidant potential of a variety of natural compounds/pharmaceuticals.
ACKNOWLEDGEMENT
Financial support from Abdul Rahman Al-Jeraisy Chair for DNA Research and the Global Consortium for the Science of Medicinal Plants (GCSMP), King Saud University, Riyadh, KSA is greatly acknowledged.
Footnotes
Source of Support: Abdul Rahman Al-Jeraisy Chair for DNA Research and the Global Consortium for the Science of Medicinal Plants (GCSMP), King Saud University, Riyadh, KSA
Conflict of Interest: The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Lee J, Huang MS, Yang IC, Lai TC, Wang JL, Pang VF, et al. Essential roles of caspases and their upstream regulators in rotenone-induced apoptosis. Biochem Biophys Res Commun. 2008;371:33–8. doi: 10.1016/j.bbrc.2008.03.149. [DOI] [PubMed] [Google Scholar]
- 2.Fields PG, Arnason JT, Philogene BJ, Aucoin RR, Morand P, Soucy-Breau C. Phototoxins as Insecticides and Natural Plant Defenses. Mem ent Soc Can. 1991;IS9:29–38. [Google Scholar]
- 3.Radad K, Rausch WD, Gille G. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Neurochem Int. 2006;49:379–86. [Google Scholar]
- 4.Deng YT, Huang HC, Lin JK. Rotenone induces apoptosis in MCF-7 human breast cancer cell-mediated ROS through JNK and p38 signaling. Mol Carcinog. 2010;49:141–51. doi: 10.1002/mc.20583. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Qi C, Fan GH, Zhou HY, Chen SD. PACAP protects neuronal differentiated PC12 cells against the neurotoxicity induced by a mitochondrial complex I inhibitor, rotenone. FEBS Lett. 2005;579:4005–11. doi: 10.1016/j.febslet.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Armstrong JS, Hornung B, Lecane P, Jones DP, Knox SJ. Rotenone-induced G2/M cell cycle arrest and apoptosis in a human B lymphoma cell line PW. Biochem Biophys Res Commun. 2001;289:973–8. doi: 10.1006/bbrc.2001.6054. [DOI] [PubMed] [Google Scholar]
- 7.Tada-Oikawa S, Hiraku Y, Kawanishi M, Kawanishi S. Mechanism for generation of hydrogen peroxide and change of mitochondrial membrane potential during rotenone-induced apoptosis. Life Sci. 2003;73:3277–88. doi: 10.1016/j.lfs.2003.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Chung WG, Miranda CL, Maier CS. Epigallocatechin gallate (EGCG) potentiates the cytotoxicity of rotenone in neuroblastoma SH-SY5Y cells. Brain Res. 2007;1176:133–42. doi: 10.1016/j.brainres.2007.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddiqui MA, Kashyap MP, Khanna VK, Yadav S, Al-Khedhairy AA, Musarrat J, et al. Association of dopamine DA-D2 receptor in rotenone-induced cytotoxicity in PC12 cells. Toxicol Ind Health. 2010;26:533–42. doi: 10.1177/0748233710377776. [DOI] [PubMed] [Google Scholar]
- 10.Miller NJ, Rice-Evans CA. Antioxidant activity of resveratrol in red wine. Clin Chem. 1995;41:1789. [PubMed] [Google Scholar]
- 11.Ozkan OV, Yuzbasioglu MF, Ciralik H, Kurutas EB, Yonden Z, Aydin M, et al. Resveratrol, a natural antioxidant, attenuates intestinal ischemia/reperfusion injury in rats. Tohoku J Exp Med. 2009;218:251–8. doi: 10.1620/tjem.218.251. [DOI] [PubMed] [Google Scholar]
- 12.Oh YC, Kang OH, Choi JG, Chae HS, Lee YS, Brice OO, et al. Anti-inflammatory effect of resveratrol by inhibition of IL-8 production in LPS-induced THP-1 cells. Am J Chin Med. 2009;37:1203–14. doi: 10.1142/S0192415X09007600. [DOI] [PubMed] [Google Scholar]
- 13.Subbiah U, Raghunathan M. Chemoprotective action of resveratrol and genistein from apoptosis induced in human peripheral blood lymphocytes. J Biomol Struct Dyn. 2008;25:425–34. doi: 10.1080/07391102.2008.10507191. [DOI] [PubMed] [Google Scholar]
- 14.Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao ZB, Hu GY. Trans-resveratrol, a red wine ingredient, inhibits voltage-activated potassium currents in rat hippocampal neurons. Brain Res. 2005;1056:68–75. doi: 10.1016/j.brainres.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Kim HI, Kim TH, Song JH. Resveratrol inhibits Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2005;1045:134–41. doi: 10.1016/j.brainres.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006;1111:41–7. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- 18.Zamin LL, Dillenburg-Pilla P, Argenta-Comiran R, Horn AP, Simao F, Nassif M, et al. Protective effect of resveratrol against oxygen-glucose deprivation in organotypic hippocampal slice cultures: Involvement of PI3-K pathway. Neurobiol Dis. 2006;24:170–82. doi: 10.1016/j.nbd.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Siddiqui MA, Kashyap MP, Kumar V, Al-Khedhairy AA, Musarrat J, Pant AB. Protective potential of trans-resveratrol against 4-hydroxynonenal induced damage in PC12 cells. Toxicol In Vitro. 2010;24:1592–8. doi: 10.1016/j.tiv.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 20.Zaidi SF, Ahmed K, Yamamoto T, Kondo T, Usmanghani K, Kadowaki M, et al. Effect of resveratrol on Helicobacter pylori-induced interleukin-8 secretion, reactive oxygen species generation and morphological changes in human gastric epithelial cells. Biol Pharm Bull. 2009;32:1931–5. doi: 10.1248/bpb.32.1931. [DOI] [PubMed] [Google Scholar]
- 21.Robb EL, Stuart JA. Trans-Resveratrol as a neuroprotectant. Molecules. 2010;15:1196–212. doi: 10.3390/molecules15031196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Zhang J, Su X, Mason RP. In vitro and in vivo assessment of CdTe and CdHgTe toxicity and clearance. J Biomed Nanotechnol. 2008;4:524–8. doi: 10.1166/jbn.2008.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar RA, Papaïconomou N, Lee JM, Salminen J, Clark DS, Prausnitz JM. In vitro cytotoxicities of ionic liquids: effect of cation rings, functional groups, and anions. Environ Toxicol. 2009;24:388–95. doi: 10.1002/tox.20443. [DOI] [PubMed] [Google Scholar]
- 24.Wei RG, Zhao YX, Liu PY, Qin ZF, Yan SS, Li Y, et al. Determination of environmentally relevant exposure concentrations of polybrominated diphenyl ethers for in vitro toxicological studies. Toxicol In Vitro. 2010;24:1078–85. doi: 10.1016/j.tiv.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Musarrat J, Wilson JA, Abou-Issa H, Wani AA. O(6)-alkylguanine DNA alkyltransferase activity levels in normal, benign and malignant human female breast. Biochem Biophys Res Commun. 1995;208:688–96. doi: 10.1006/bbrc.1995.1393. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui MA, Singh G, Kashyap MP, Khanna VK, Yadav S, Chandra D, et al. Influence of cytotoxic doses of 4-hydroxynonenal on selected neurotransmitter receptors in PC-12 cells. Toxicol In Vitro. 2008;22:1681–8. doi: 10.1016/j.tiv.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui MA, Kashyap MP, Al-Khedhairy AA, Musarrat J, Khanna VK, Yadav S, et al. Protective potential of 17{beta}-estradiol against co-exposure of 4-hydroxynonenal and 6-hydroxydopamine in PC12 cells. Hum Exp Toxicol. 2011;30:860–9. doi: 10.1177/0960327110382130. [DOI] [PubMed] [Google Scholar]
- 28.Krantic S, Mechawar N, Reix S, Quirion R. Molecular basis of programmed cell death involved in neurodegeneration. Trends Neurosci. 2005;28:670–6. doi: 10.1016/j.tins.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Anekonda TS, Reddy PH. Neuronal protection by sirtuins in Alzheimer's disease. J Neurochem. 2006;96:305–13. doi: 10.1111/j.1471-4159.2005.03492.x. [DOI] [PubMed] [Google Scholar]
- 30.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 31.Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29:632–9. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–3. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- 33.Anekonda TS. Resveratrol-a boon for treating Alzheimer's disease? Brain Res Rev. 2006;52:316–26. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez L, Mitjans M, Infante MR, Vinardell MP. Assessment of the potential skin irritation of lysine-derivative anionic surfactants using mouse fibroblasts and human keratinocytes as an alternative to animal testing. Pharma Res. 2004;21:1637–41. doi: 10.1023/b:pham.0000041459.63362.6f. [DOI] [PubMed] [Google Scholar]
- 35.Yoon IS, Au Q, Barber JR, Ng SC, Zhang B. Development of a high-throughput screening assay for cytoprotective agents in rotenone-induced cell death. Anal Biochem. 2010;407:205–10. doi: 10.1016/j.ab.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res Mol Brain Res. 2005;134:109–18. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Fato R, Bergamini C, Bortolus M, Maniero AL, Leoni S, Ohnishi T, et al. Differential effects of mitochondrial Complex I inhibitors on production of reactive oxygen species. Biochim Biophys Acta. 2009;1787:384–92. doi: 10.1016/j.bbabio.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddiqui MA, Kashyap MP, Kumar V, Tripathi VK, Khanna VK, Yadav S, et al. Differential protection of pre-, co- and post-treatment of curcumin against hydrogen peroxide in PC12 cells. Hum Exp Toxicol. 2011;30:192–8. doi: 10.1177/0960327110371696. [DOI] [PubMed] [Google Scholar]
- 39.Gelinas S, Martinoli MG. Neuroprotective effect of estradiol and phytoestrogens on MPP+-induced cytotoxicity in neuronal PC12 cells. J Neurosci Res. 2002;70:90–6. doi: 10.1002/jnr.10315. [DOI] [PubMed] [Google Scholar]
- 40.Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic Biol Med. 2003;34:1100–10. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 41.Savaskan E, Olivieri G, Meier F, Seifritz E, Wirz-Justice A, Muller-Spahn F. Red wine ingredient resveratrol protects from beta-amyloid neurotoxicity. Gerontology. 2003;49:380–3. doi: 10.1159/000073766. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, et al. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–74. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- 43.Raval AP, Dave KR, Perez-Pinzón MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–7. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- 44.Lu KT, Chiou RY, Chen LG, Chen MH, Tseng WT, Hsieh HT, et al. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J Agric Food Chem. 2006;54:3126–31. doi: 10.1021/jf053011q. [DOI] [PubMed] [Google Scholar]
- 45.Wang Q, Yu S, Simonyi A, Rottinghaus G, Sun GY, Sun AY. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res. 2004;29:2105–12. doi: 10.1007/s11064-004-6883-z. [DOI] [PubMed] [Google Scholar]
- 46.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, Kocak A, Yologlu S. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem. 2007;294:137–44. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]


