Abstract
Organophosphorus (OP), the commonest agent for poisoning in India due to its easy availability, acts by inhibiting acetylcholinesterase at muscarinic and nicotinic receptors. Erythrocyte cholinesterase (EchE) and plasma cholinesterase (PchE) are reduced in OP poisoning, but their estimation is costly and not regularly performed. There are emerging options for new cheaper biochemical markers in relation to OP poisoning. Serum level of creatine phosphokinase (CPK) is often found to be elevated in OP poisoning. This study was conducted to see if CPK may be used as an alternative of cholinesterase levels in blood to assess the severity of OP poisoning. This was a prospective and observational study. Sixty-three patients of OP poisoning without any prior treatment, presenting within 6 hours, were selected and their clinical severity was categorized according to Peradeniya organophosphorus poisoning (POP) scale. Level of serum CPK, blood EchE and pH were measured following admission, and total dose of atropine (mg) until the final clinical outcome (complete recovery or death) was calculated. Student's t-test and Pearson's correlation coefficient was used for the assessment of statistical significance. According to POP scale, clinical severity was mild (score 0–3) in 17 (27%), moderate (score 4–7) in 32 (50.8%) and severe (score 8–11) in 14 (22.2%) patients. Serum CPK, EchE level, blood pH and total atropine dose strongly correlated with clinical severity. Our study recommends serum CPK as an alternative marker.
Keywords: Acetylcholinesterase level, creatine phosphokinase, organophosphorus poisoning, Peradeniya organophosphorus poisoning scale
INTRODUCTION
Organophosphorus (OP) compounds are arguably one of the most common causes of insecticide poisoning worldwide. In 2007, The American Association of Poison Control Centers received 96,307 calls (3.4% of all human exposures) related to pesticide exposures, many of which involved OP agents and 80 uses of Pralidoxime (2-PAM).[1] However, poison center recorded exposures to OPs from 1995 to 2004 have declined because of the United States Environmental Protection Agency phasing out commonly used household and agricultural OP agents.[2] In countries such as India and Nicaragua, OPs are easily available and cheap, hence a source of both intentional and unintentional poisonings. OP poisoning causes what is known as the “suicide impulse” which leads to high level of suicides in some sectors of the agricultural industry. The incidence of international OP-related human exposures appears to be underestimated.[3] According to the World Health Organization (WHO), 1 million serious unintentional poisonings occur every year and an additional 2 million people are hospitalized for suicide attempts with pesticides.[4] According to an Indian study, the incidence of OP poisoning was around 1.26 lakhs during the period of 12 months in 2007, as reported by Ravi et al.[5] The commonly encountered OP compounds comprise insecticides (including malathion, parathion, diazinon, fenthion, dichlorvos, chlorpyrifos, ethion), nerve gases (including soman, sarin, tabun, VX), ophthalmic agents (echothiophate, isoflurophate), antihelmintics (such as trichlorfon), herbicides [including tribufos (DEF), merphos which are tricresyl phosphate containing industrial chemicals]. Massive OP intoxication from suicidal and accidental events, such as the Jamaican ginger palsy incident in 1930, led to the discovery of the mechanism of action of OPs. They act by inhibiting the acetylcholinesterase enzyme (AchE) at muscarinic and nicotinic receptors, producing an array of symptoms like miosis, bradycardia, increased gastrointestinal motility, emesis, sweating, tachypnea, salivation, lacrimation, altered sensorium, fasciculation, bronchospasm, blurred vision, photophobia, urination and defecation. Complications include acidosis, respiratory paralysis, acute renal failure, seizures, arrythmias, aspiration, coma and even death. The causes of death in OP poisoning may be either one or a combination of the above. Early diagnosis is a key to cure. A delay of initiation of treatments limits not only outcome, but also the opportunity to use 2-PAM (cholinesterase re-activator) which prevents “aging” of the enzyme. Till now, investigations comprised serum erythrocyte cholinesterase (EchE) and plasma cholinesterase (PchE) estimation, the levels of which are reduced in OP poisoning. But estimation of their levels is costly and not regularly performed in most laboratories of our country. Besides, the kinetic study of inhibition of human AchEs by Demeton-S-methyl has shown that cholinesterase-based titration methods are not suitable for the estimation of OPs.[6] There are emerging options for new cheaper and/or easily quantifiable biochemical markers in relation to OP poisoning like creatine phosphokinase (CPK), lactate dehydrogenase (LDH), serum immunoglobulins (IgG, IgA), circulating complements (C3, C4), etc.[7] But immunoglobulin assays, apart from being costly and difficult to perform in most laboratories, are often unreliable. Several animal model studies on rat liver and fresh-water snails indicate the association between OP poisoning and CPK levels.[8] In a study, it was proposed that serum level of CPK is often found to be elevated in OP poisoning and may be used as a biomarker.[7]
With this background in mind, we undertook a study to assess the role of CPK as an alternative prognostic marker and to establish a correlation between CPK levels and the severity of OP poisoning.
MATERIALS AND METHODS
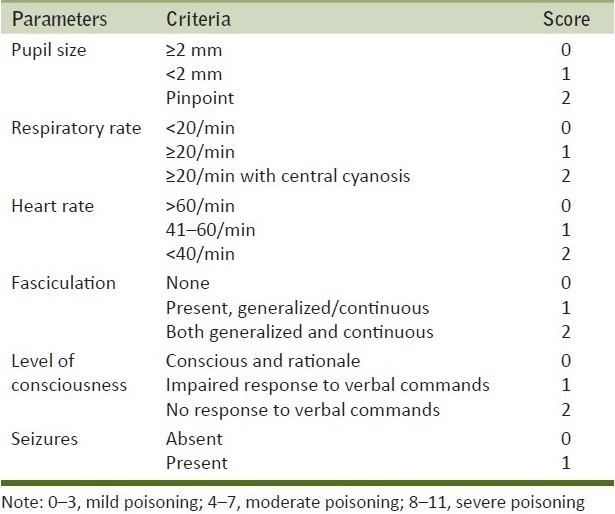
Sixty-three patients admitted in the Department of Medicine, Calcutta Medical College Hospital, within 6 hours of exposure to the poison without receiving any prior treatment were included in the study. It was a prospective and observational study conducted from 1st January 2010 to 30th June 2010. Confirmation of OP poisoning was done by seeing the packet/container. Clinical severity was categorized according to Peradeniya organophosphorus poisoning (POP) scale as shown in Table 1.[9] Patients who had history of any kind of illnesses like myopathy, chronic renal disease, epilepsy, known psychiatric illness, receiving intramuscular (I/M) injections, myocardial infarction, myocarditis, cardiopulmonary resuscitation, trauma, malignancy, autoimmune diseases, overwhelming sepsis, or on medications like statins, fibrates, aspirin, anticoagulants, frusemide, and dexamethasone were excluded from the study. They were included only if their caregivers gave informed consent. Sample was collected aseptically by a single prick after initial resuscitation, from a peripheral vein without tying any tourniquet. The levels of serum CPK, EchE, and pH were measured following admission. During the course of treatment, I/M injections were avoided. They were treated with 2-PAM (adult dose is 1-2 g intravenously followed by 0.5 g/hour infusion) and initial dose of injection atropine 2 mg followed by bolus every 5-10 min or as infusion until the signs of “atropinization” occurred, i.e., heart rate>80/min and dilatation of initially constricted pupil. The total dose of atropine (mg) until the final clinical outcome (complete recovery or death) was calculated for each patient. Just before discharging the patients from our institution or at the end of one completed week, the levels of serum CPK was re-evaluated and the responses were tabulated. RBC cholinesterase activity (normal range=10,000–14,000 IU/L) was measured by the procedure of Voss and Sachsse.[10] CPK was measured by spectrophotometric methods.[11] The normal value of serum CPK is taken to be 51–294 IU/L for males and 39–238 IU/L for females.[12] Post-mortem muscle biopsy was conducted in some patients with severe poisoning to document myonecrosis.
Table 1.
The Peradeniya organophosphorus poisoning scale
GraphPad QuickCalcs software (Graphpad Software Inc., La Jolla, CA, USA) and MS Excel were used for statistical analysis. For the description of data, mean values, percentages and standard deviations were used. Student's t-test and Pearson's Correlation coefficient were used for the assessment of statistical significance. The study got clearance from the Institutional Ethical Committee.
RESULTS
In all, 63 patients were enrolled in the study. Out of them, 42 were men and 21 were women (male:female=2:1). They were aged from 16 to 44 years (mean age=25.5 years) [shown in detail in Figure 1]. Fifty-one (80.95%) came from a rural background, with a predominantly lower and lower middle socioeconomic status according to the Modified Kuppuswamy Scale.[13] They were mostly farmers by occupation; some others were students, housewives, laborers or porters. Their level of education varied from illiterates to graduates. The commonest OP compound found to be abused among our study group was chlorpyrifos in 24 (38.1%) patients, which is an agricultural pesticide.
Figure 1.
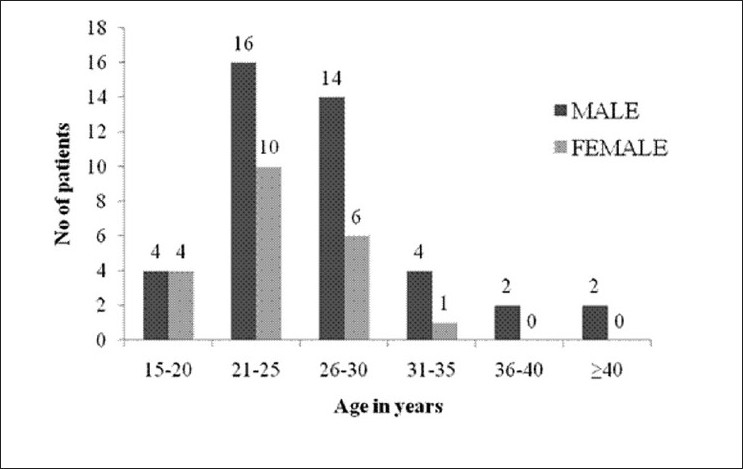
Bar diagram showing age and sex distribution of the study population (n=63)
Maximum patients presented with vomiting [55 (87.3%)], followed by miosis [51 (80.95%)], bradycardia [44 (69.84%)], salivation [44 (69.84%)], sweating [34 (53.97%)], altered sensorium [33 (52.38%)], tachypnea [23 (36.5%)], fasciculation [16 (25.39%)], etc. [Figure 2]. Two patients presented with tachycardia (not shown in diagram).
Figure 2.
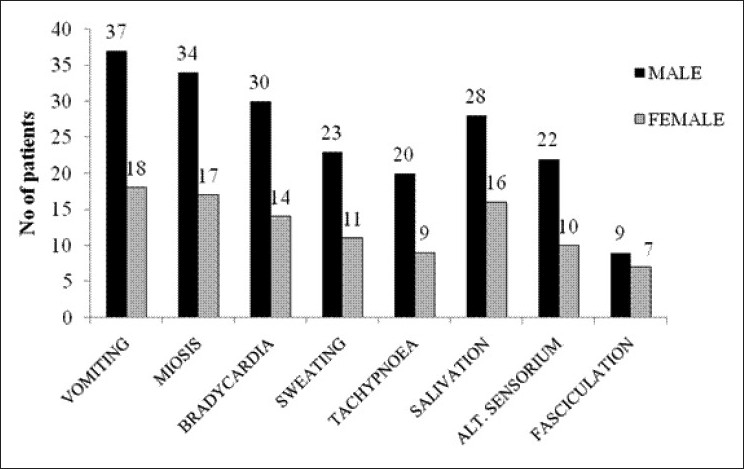
Bar diagram showing frequency of initial symptoms of the study population (n=63)
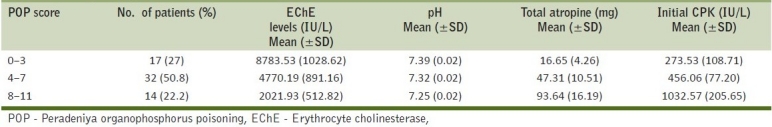
According to POP score, 17 out of 63 patients (27%), 32 out of 63 (50.8%) and 14 out of 63 (22.2%) were considered as mild, moderate and severe cases of poisoning, respectively [Figure 3]. Their EChE levels (in IU/L), pH values, total dose of atropine (in mg) and CPK levels (in IU/L) were also calculated on admission [details shown in Table 2]. We observed that as the POP score increases, the serum CPK value also increases and there is concomitant fall in arterial pH and increase in the total atropine requirement.
Figure 3.
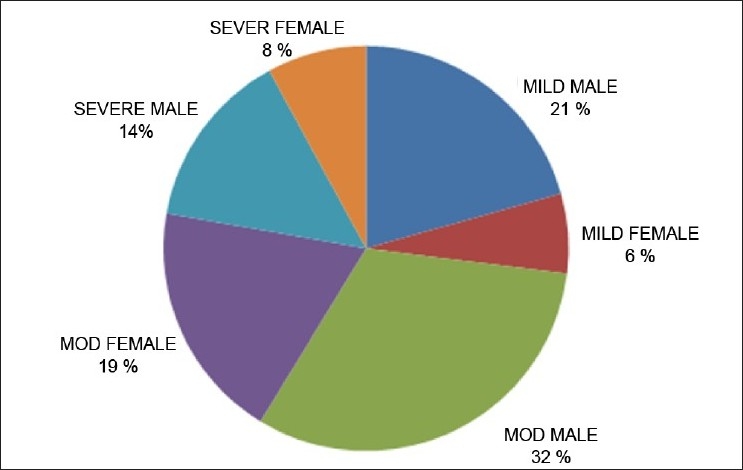
Pie diagram showing the categorization of patients according to POP score (Note: POP score 0–3, mild poisoning; 4–7, moderate poisoning; 8–11, severe poisoning)
Table 2.
Peradeniya organophosphorus poisoning scores, mean serum erythrocyte cholinesterase levels (IU/L), pH, total dose of atropine (in mg) and serum CPK levels (IU/L) of the patients on admission (n=63)
We found high degree of correlation between initial CPK value and POP score, serum EChE levels, arterial pH values and total dose of atropine (in mg) [details shown in Table 3 and Figures 4–7]. The correlation was found to be statistically significant (P < 0.001) in each case.
Table 3.
Correlation between the initial creatine phosphokinase value (in IU/L) and POP score, EChE (in IU/L), pH and total dose of atropine (in mg)

Figure 4.
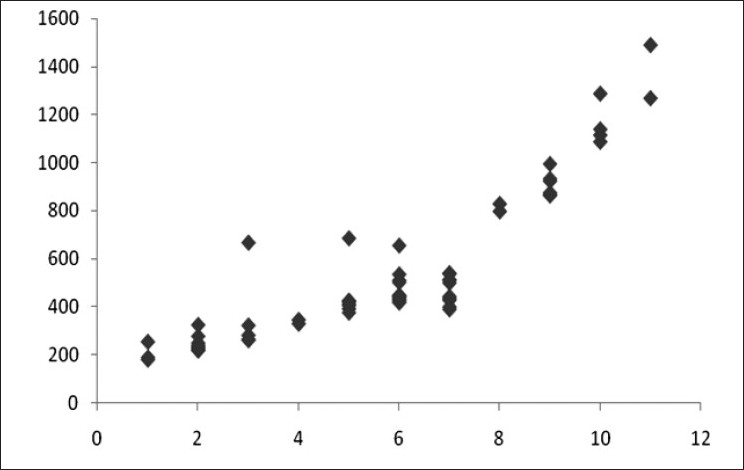
Scatter diagram showing correlation of CPK (in IU/L) with POP score (r=0.874)
Figure 7.
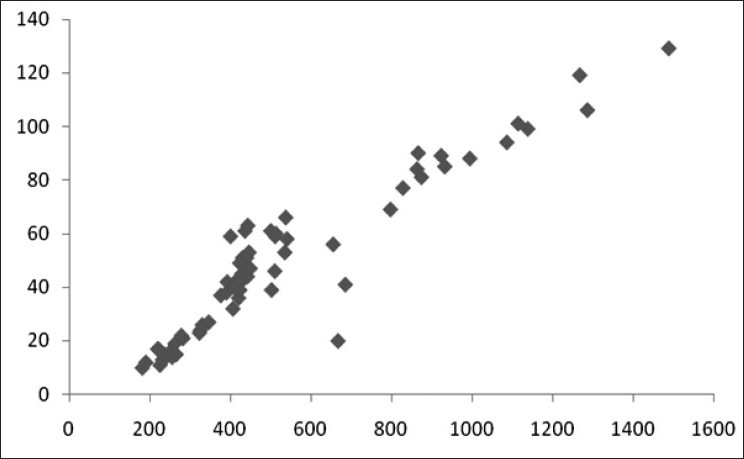
Scatter diagram showing correlation of CPK (in IU/L) and total dose of atropine (in mg) (r=0.936)
Figure 5.
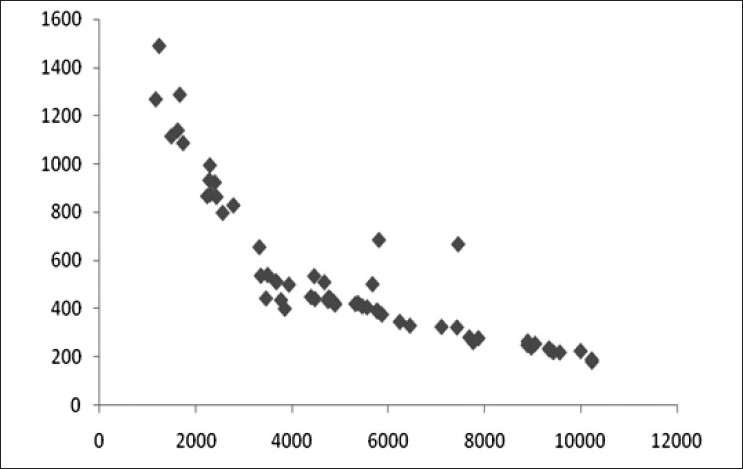
Scatter diagram showing correlation of CPK (in IU/L) and EChE (in IU/L) (r=–0.832)
Figure 6.
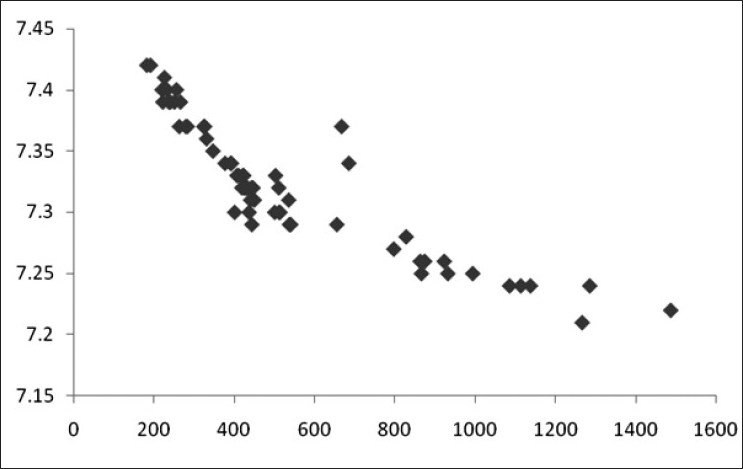
Scatter diagram showing correlation of CPK (in IU/L) and pH (r=–0.888)
One individual with mild poisoning (POP score=2) had a disproportionately high CPK level of 685 IU/L which can be explained as due to consumption of alcohol along with the poison. Another individual with moderate exposure (POP=4) was found to have an initial CPK level of 667 IU/L, which was due to vigorous muscle exercise prior to exposure.
During our study, we encountered various complications like metabolic acidosis of varying degree [44 (69.84%)], respiratory paralysis [10 (15.87%)], coma [7 (11.11%)], seizures [5 (7.94%)], intermediate syndrome [5 (7.94%)], acute renal failure (ARF) [4 (6.35%)], and arrhythmias [2 (3.17%)] [vide Figure 8].
Figure 8.
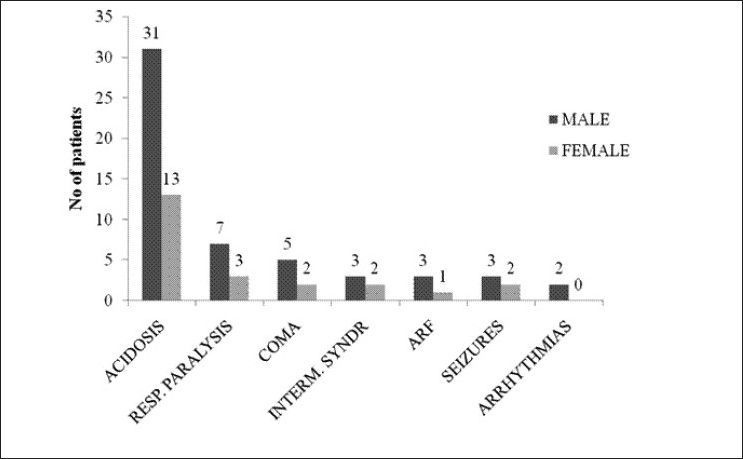
Bar diagram showing complications developing during the course of the study in the study population (n=63)
Ten out of 63 patients (19.4%) expired in our study [Figure 9]. The cause of death included respiratory paralysis, metabolic, acidosis, acute respiratory distress syndrome (ARDS), ARF, CNS depression, or a combination of these. Two patients with intermediate syndrome expired.
Figure 9.
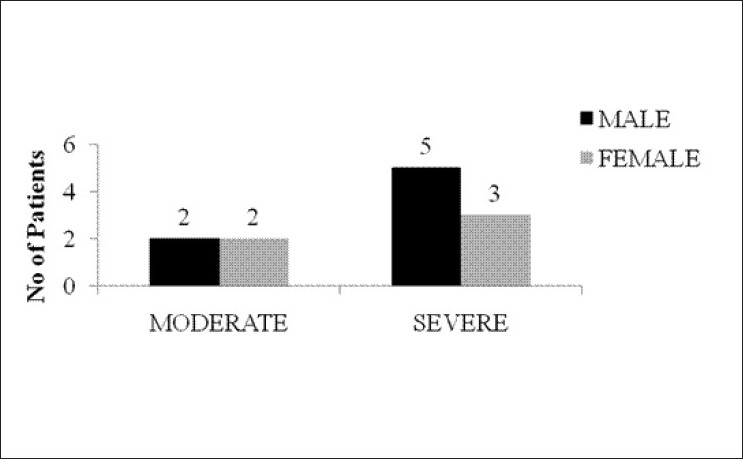
Bar diagram showing mortality of patients during the study (n=63)
The final CPK level was estimated for 61 patients before discharge or after completion of 1 week, whichever was earlier. Two patients (initial CPK 1138 IU/L and 1086 IU/L) died out of complications during the course of therapy on day 3 and day 5, respectively. It was found that mortality was more in patients with high initial CPK values.
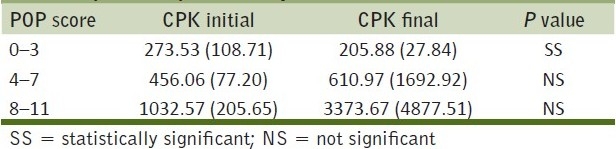
Table 4 shows the initial and final CPK levels in IU/L according to the POP scores and the P value for each group. While the reduction in CPK values with treatment in mild cases was significant, the changes in the moderate (P>0.05) and the severe (P>0.05) groups were not so. This was probably due to the complications that occurred along the course of stay in some patients in the latter groups.
Table 4.
Comparison of the initial and final CPK levels (in IU/L) of the patients
While recording the final readings, we noted some aberrations in patients who developed complications. A young female (initial CPK=513 IU/L) who had several episodes of vomiting and convulsions on the 4th day of admission showed a final CPK level of 769 IU/L, and a 35-year-old male who showed a final CPK level of 858 IU/L had suffered from one episode of convulsion along with ARF, both managed successfully. A very interesting finding was noted when three persons with severe poisoning showed final CPK values of 12,564 IU/L (initial CPK=797 IU/L), 11,005 IU/L (initial CPK=932 IU/L) and 10,583 IU/L (initial CPK=923 IU/L), respectively, and one individual with moderate poisoning had final CPK value of 9858 IU/L (initial CPK=428 IU/L) due to development of intermediate syndrome. One patient with intermediate syndrome expired on day 3 (initial CPK 1138 IU/L).
CPK values on follow-up did not correlate with POP score, serum EChE levels (in IU/L), arterial pH values, total dose of atropine (in mg) and initial CPK levels (in IU/L).
Post-mortem muscle biopsy done in two patients without intermediate syndrome showed muscle fiber necrosis.
DISCUSSION
OP insecticides are arguably one of the commonest causes of morbidity and mortality due to poisoning worldwide, especially in developing countries like India. The morbidity and mortality outcome depends on time lag between exposure and the onset of management. With increase in use of OP compounds for agricultural and industrial purposes and due to easy access and low cost, they are becoming a major source of health hazard. So, it is cardinal to recognize the entire spectrum of the symptoms. Identification, risk stratification, early diagnosis and prompt treatment of OP poisoning victims are equally vital. Symptoms are classified into muscarinic and nicotinic receptors based on the receptor involved. Muscarinic features include excessive salivation, lacrimation, urination, diarrhea, gastrointestinal cramps, emesis, blurred vision, miosis, bradycardia, and wheezing. Nicotinic features include fasciculation, paresis or paralysis, hypertension and tachycardia. Central receptor features include anxiety, confusion, seizures, psychosis and ataxia. Three types of paralysis are noticed. Type I is due to continued depolarization at neuro-muscular junction, type II due to intermediate syndrome and type III due to delayed polyneuropathy.
The presence of muscle fiber necrosis in OP poisoning has been already demonstrated in animal experimental studies by Calore et al.[14] A study conducted by Sharma et al. has shown that CPK was elevated in a fraction of their cases who had severe poisoning. It has been shown that there is rhabdomyolysis in “intermediate syndrome” and consequently there is raised CPK level.[15]
But our study showed that serum CPK level is elevated even in the absence of intermediate syndrome, provided the patient is severely poisoned, presumably due to muscle fiber necrosis, as shown by muscle biopsy in two of our patients. If there is ongoing injury to the muscle due to development of complications, the CPK level continues to be elevated. Since half-life of CPK is about 1.5 days, it normalizes within 5–6 days of a single insult to the muscle.[16]
It has been shown by Senanayeke et al. that the POP score can efficiently predict the severity, morbidity and mortality of OP poisoned patients.[9] This scale uses high respiratory rate (>20/minute ) and the presence of cyanosis. This approach is likely to cause difficulties as severe OP poisoning may cause either central respiratory depression with a reduced respiratory rate or tachypnea in the context of bronchorrhea, bronchoconstriction or respiratory muscle weakness.[17] Also, in our study, we found that sometimes POP scores can be deceptive and even a person with relatively low POP score can develop complication/s and/or expire prematurely.
The quest for newer biomarkers in relation to OP poisoning started quite a long time back. OP-labeled albumin in human plasma and blood β-glucuronidase have been suggested by some researchers.[18–20] Measurement of paraoxonase status, on the other hand, has been proposed as a reliable marker to assess susceptibility to OP poisoning.[21,22] Meconium and urine have also been studied for estimation of biomarkers in children susceptible to chronic OP exposure in utero.[23] Studies on these biomarkers involved small number of human subjects or experimental animals, so they might not be reliable when applied to larger populations. These markers are also costly and their estimation is difficult in developing countries.
Acidosis is a frequently encountered complication of OP poisoning, and it was previously suggested that both the degree and type of acidosis can be a predictor of outcome in OP poisoning.[24] Our study also demonstrated acidosis as a common complication. It is documented that acidosis itself can cause modest elevations in CPK levels in blood, which implies that CPK can be falsely high in case of acidosis.[25] But significant acidosis being a complication of severe poisoning, CPK values also correlated with degree of acidosis along with severity of poisoning.
Follow-up serum CPK level, measured in those individuals who were severely poisoned or those who developed complications, continued to remain elevated even after 1 week, whereas the values in recovering patients showed a tendency to decrease. The initial CPK level was also high in those who developed complications later on. So, serial measurement of serum CPK level might be helpful in predicting as well as assessing the prognosis of patients of OP poisoning.
CONCLUSION
So, to conclude, serum CPK level can be an efficient biomarker in case of acute OP poisoning not only due to its easy availability and low cost, but also because serial monitoring of its level during the entire course of therapy can predict the prognosis. However, the main disadvantage with this marker is its non-specificity. So, exclusion of other causes of raised CPK in a patient of acute OP poisoning is required. Also, further studies with greater number of patients are required to support our observation, since our study was conducted with a relatively small number of patients, and in only one center located in eastern India, which might prejudice any comment on its efficacy and reliability among other ethnic groups.
Key Messages: Serum creatine phosphokinase can be a simple, cheap and widely available biomarker for acute OP poisoning. It can be of particular importance in developing countries, where other costly biomarkers are difficult to estimate.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bronstein AC, Spyker DA, Cantilena LR, Jr, Green JL, Rumack BH, Heard SE. 2007 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 25th Annual Report. Clin Toxicol (Phila) 2008;46:927–1057. doi: 10.1080/15563650802559632. [DOI] [PubMed] [Google Scholar]
- 2.Sudakin DL, Power LE. Organophosphate exposures in the United States: a longitudinal analysis of incidents reported to poison centers. J Toxicol Environ Health A. 2007;70:141–7. doi: 10.1080/15287390600755224. [DOI] [PubMed] [Google Scholar]
- 3.Corriols M, Marín J, Berroteran J, Lozano LM, Lundberg I, Thörn A. The Nicaraguan Pesticide Poisoning Register: constant underreporting. Int J Health Serv. 2008;38:773–87. doi: 10.2190/HS.38.4.k. [DOI] [PubMed] [Google Scholar]
- 4.Jeyaratnam J. Acute pesticide poisoning: A major global health problem. World Health Stat Q. 1990;43:139–44. [PubMed] [Google Scholar]
- 5.Ravi G, Rajendiran C, Thirumalaikolundusubramanian P, Babu N. Presented at 6th Annual congress of Asia Pacific Association of Medical Toxicology. Bangkok, Thailand: 2007. Poison control, training and research center, Institute of Internal Medicine, Government General Hospital, Madras Medical College, Chennai, India. [Google Scholar]
- 6.Bazire A, Gillon E, Lockridge O, Vallet V, Nachon F. The kinetic study of the inhibition of human cholinesterases by demeton-S-methyl shows that cholinesterase-based titration methods are not suitable for this organophosphate. Toxicol In Vitro. 2011;25:754–9. doi: 10.1016/j.tiv.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal SB, Bhatnagar VK, Agarwal A, Agarwal U, Venkaiah K, Nigam SK, et al. Impairment in clinical indices in acute organophosphate insecticide poisoning patients in India. Internet J Toxicol. 2007;4:1. [Google Scholar]
- 8.Vanneste Y, Lison D. Biochemical changes associated with muscle fibre necrosis after experimental organophosphate poisoning. Hum Exp Toxicol. 1993;12:365–70. doi: 10.1177/096032719301200504. [DOI] [PubMed] [Google Scholar]
- 9.Senanayake N, de Silva HJ, Karalliedde L. A scale to assess severity in organophosphorus intoxication: POP scale. Hum Exp Toxicol. 1993;12:297–9. doi: 10.1177/096032719301200407. [DOI] [PubMed] [Google Scholar]
- 10.Voss G, Sachsse R. Red cell and plasma cholinesterase activities in microsamples of human and animal blood determined simultaneously by a modified acetylcholine/DTNB procedure. Toxicol Appl Pharmacol. 1970;16:764–72. doi: 10.1016/0041-008x(70)90082-7. [DOI] [PubMed] [Google Scholar]
- 11.Rosalki SB. An improved procedure for serum creatine phosphokinase determination. J Lab Clin Med. 1967;69:696–05. [PubMed] [Google Scholar]
- 12.Kratz A, Sluss PM, Januzzi JL, Lewandrowski KB. Appendices: laboratory values of clinical importance. In: Kasper DL, Fauci AS, Longo DL, Braunwald E, Hauser SL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 16th ed. Vol. 2. New York: McGraw-Hill; 2005. pp. A1–9. [Google Scholar]
- 13.Mishra D, Singh HP. Kuppuswamy's socioeconomic status scale — A revision. Indian J Pediatr. 2003;70:273–4. doi: 10.1007/BF02725598. [DOI] [PubMed] [Google Scholar]
- 14.Calore EE, Sesso A, Puga FR, Cavaliere MJ, Calore NM, Weg R. Sarcoplasmic lipase and non-specific esterase inhibition in myofibers of rats intoxicated with the organophosphate isofenphos. Exp Toxicol Pathol. 1999;51:27–33. doi: 10.1016/S0940-2993(99)80054-2. [DOI] [PubMed] [Google Scholar]
- 15.De Wilde V, Vogelaers D, Colardyn F, Vanderstraeten G, Van den Neucker K, De Bleecker J, et al. Postsynaptic neuromuscular dysfunction in organophosphate induced intermediate syndrome. Klin Wochenschr. 1991;69:177–83. doi: 10.1007/BF01665865. [DOI] [PubMed] [Google Scholar]
- 16.Sahjian M, Frakes M. Crush Injuries: Pathophysiology and Current Treatment. Nurse Pract. 2007;32:13–8. doi: 10.1097/01.NPR.0000287464.81259.8b. [DOI] [PubMed] [Google Scholar]
- 17.Eddleston M, Mohamed F, Davies JO, Eyer P, Worek F, Sheriff MH, et al. Respiratory failure in acute organophosphorus pesticide self-poisoning. QJM. 2006;99:513–22. doi: 10.1093/qjmed/hcl065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peeples ES, Schopfer LM, Duysen EG, Spaulding R, Voelker T, Thompson CM, et al. Albumin, a new biomarker of organophosphorus toxicant exposure, identified by mass spectrometry. Toxicol Sci. 2005;83:303–12. doi: 10.1093/toxsci/kfi023. [DOI] [PubMed] [Google Scholar]
- 19.Li B, Ricordel I, Schopfer LM, Baud F, Mégarbane B, Nachon F, et al. Detection of adduct on tyrosine 411 of albumin in humans poisoned by dichlorvos. Toxicol Sci. 2010;116:23–31. doi: 10.1093/toxsci/kfq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soltaninejad K, Shadnia S, Afkhami-Taghipour M, Saljooghi R, Mohammadirad A, Abdollahi M. Blood β-glucuronidase as a suitable biomarker at acute exposure of severe organophosphorus poisoning in human. Hum Exp Toxicol. 2007;26:963–6. doi: 10.1177/0960327107085349. [DOI] [PubMed] [Google Scholar]
- 21.Costa LG, Cole TB, Vitalone A, Furlong CE. Measurement of paraoxonase (PON1) status as a potential biomarker of susceptibility to organophosphate toxicity. Clin Chim Act. 2005;352:37–47. doi: 10.1016/j.cccn.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Sirivarasai J, Kaojarern S, Yoovathaworn K, Sura T. Paraoxonase (PON1) polymorphism and activity as the determinants of sensitivity to organophosphates in human subjects. Chem Biol Interact. 2007;168:184–92. doi: 10.1016/j.cbi.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 23.Wessels D, Barr DB, Mendola P. Use of Biomarkers to Indicate Exposure of children to organophosphate pesticides: Implications for a longitudinal study of children's environmental health. Environ Health Perspect. 2003;111:1939–46. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu JH, Chou CY, Liu YL, Liao PY, Lin PW, Lin HH, et al. Acid-base interpretation can be the predictor of outcome among patients with acute organophosphate poisoning before hospitalization. Am J Emerg Med. 2008;26:24–30. doi: 10.1016/j.ajem.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Warburton D, Singer DB, Oh W. Effects of acidosis on the activity of creatine phosphokinase and its isoenzymes in the serum of newborn infants. Pediatrics. 1981;68:195–7. [PubMed] [Google Scholar]


