Abstract
An ameliorating effect of Ocimum sanctum on the toxic effect of meloxicam, a new non-steroidal anti-inflammatory drug was studied by evaluating haemato-biochemical parameters, oxidative stress, gross and histopathological changes in various organs of Wistar rats. A total of thirty-six male rats were divided in six experimental groups each comprising of six rats and numbered from G1 to G6. Meloxicam toxicity was induced by oral feeding of meloxicam at 1.2 mg/kg and 2.4 mg/kg body weight in G2 and G3 respectively for 28 days. Group G4 and G5 were fed with 1.2-mg/kg body weight and 2.4-mg/kg body weight of meloxicam along with 200 mg/kg body weight of aqueous extract of Ocimum sanctum. Group G1 serve as control while group G6 was kept as treatment control and fed only aqueous extract of Ocimum sanctum at 200 mg/kg body weight. Clinical finding showed mild diarrhea from 23rd day onwards in-group treated with 2.4-mg/kg body of meloxicam. Significant reduction of hemoglobin and packed cell volume (PCV) was observed in both the group treated with 1.2 mg/kg and 2.4-mg/kg body wt. of meloxicam. Ocimum sanctum could restore the hemoglobin and PCV value in-group treated with meloxicam at low dose level. Serum alkaline phosphatase, serum glutamic pyruvic transaminase, Serum glutamic oxaloacetic transaminase and total bilirubin were found elevated in meloxicam treated groups and indicated hepatotoxic activity of meloxicam. Ocimum sanctum could reduce hepatotoxic activity of meloxicam in group G4 receiving meloxicam at lower dose rate along with Ocimum sanctum failed to regulate creatinine level in meloxicam treated groups. In meloxicam toxicity elevated Lipid peroxidation values was noticed in liver and kidneys, while superoxide dismutase and glutathione did not revealed any change. Stomach and intestine revealed hemorrhagic gastroenteritis and ulcers. Perivascular necrosis with infiltration with inflammatory cells was evident in liver. Interstitial nephritis, myocardial necrosis and spongiform encephalopathy were important lesions. The Ocimum sanctum could only counteract the toxic effect of meloxicam in liver and gastrointestinal tract.
Keywords: Meloxicam toxicity, Ocimum sanctum, oxidative stress, Wistar rats
INTRODUCTION
Meloxicam, a new non-steroidal anti-inflammatory drug (NSAID) is widely used in humans, cattle, buffalo, goats and dogs. Meloxicam is a COX-2 (cyclo-oxygenase) inhibitor at its lowest therapeutic dose and is an anti-inflammatory by inhibiting prostanoid synthesis in inflammatory cells[1]. Meloxicam inhibits COX-2 about 12 times more selectively than COX-1.[2] Meloxicam is reported to be safer as it produce significantly lower incidence of gastrointestinal adverse effects as compared to diclofenac sodium.[3] Most NSAIDs are absorbed well from gastric and intestinal mucosa. They are bound to plasma albumin and distributed all over the body evenly. Most NSAIDs are metabolized in the liver by oxidation and converted to inactive metabolites, which are then typically excreted in the urine while some drugs are partially excreted in bile. Meloxicam, as NSAID, either alone or with antimicrobial drugs, is indicated for use in ruminants for the treatment of pneumonia, pleuritis, laminitis, myositis, sprain, mastitis, prolapse of uterus, premature labour etc. The principle side effect of meloxicam is gastrointestinal irritation (vomiting, diarrhea and ulceration). Rare but important side effects include liver and kidney toxicity[4].
The plant Ocimum sanctum belongs to family “Labitae” and is a well-known pot herb regarded as sacred by Hindus. Ocimum sanctum (Tulsi) is a medicinal plant commonly grown in India. Different parts of this plant have been reported to exhibit several medicinal properties.[5] Ocimum sanctum has been reported to possess antihepatotoxicity property,[6] and two triterpenes from the leaves have been shown to possess hepatoprotective effect against carbon tetrachloride induced damage in rats.[7] The present study is formulated to study sub acute toxicity of meloxicam in Wistar rats. An attempt is also made to evaluate protective property of Ocimum sanctum during meloxicam toxicity.
MATERIALS AND METHODS
Chemicals
Meloxicam pure base powder was obtained from M/s. Cadila Pharmaceutical Ltd., Dholka and Sodium Carboxy Methyl Cellulose pure base powder was obtained from M/s Fischer scientific, Mumbai. All the biochemical kits and chemicals used in the study were of analytical grade.
Herbal medicine
Aqueous extract of dried leaves of Ocimum sanctum was prepared as follows and was used as herbal medicine. The leaves of Ocimum sanctum were collected from neighborhood and identified based on morphological characters. The leaves were dried in shadow at room temperature and powered. The powder was stored in glass bottle in cool and dry place away from direct sunlight and used for preparation of aqueous extract. The aqueous extract was prepared by the method described earlier.[8] The extractability percentage was 6.25 and extract was dark brown in color. Extract was finally stored in desiccators in a cool and dry place.
Experimental animals
Thirty-six male Wistar rats were divided into six groups of 6 animals each. All the animals were maintained in polypropylene cages, (47 × 34 × 18 cm). The maximum number of animals in each cage was six. Dried rice husk was used as a bedding material. Bedding material was changed every alternate day. All the animals were kept under standard managemental conditions as per the norms of Committee for the purpose of control and supervision on experiments on animals (CPCSEA), and were maintained under a controlled environment with temperature at 23 ± 2°C, relative humidity at 55 ± 5%, and a 12-hours/12 hours light/dark cycle throughout the experiment. The animals were fed on a standard pellet diet. The pellet diet consisted of 22.02% crude protein, 4.25% crude oil, 3.02% crude fiber, 7.5% ash and 1.38% sand silica. They were given ad-lib feed and wholesome drinking water throughout the experiment. The necessary Institute Animal Ethical committee approval was obtained.
Design of experiment
The rats were acclimatized for 15 days to the environment, before the start of the experiment. Each group of rat was given separate identification mark by using picric acid. A total of thirty-six male rats, were divided in to six groups (G1, G2, G3, G4, G5 and G6) with six rats in each group. Group ‘G1’ served as control and was treated with vehicle, 0.5 per cent sodium carboxy methyl cellulose at 1 ml. Group ‘G2’ and ‘G3’, which received meloxicam at 1.2 mg/kg body weight. and 2.4 mg /kg body weight, respectively. Group ‘G4’ and G5 received meloxicam at 1.2 mg/kg body weight and 2.4 mg /kg body weight, respectively along with 200 mg/kg of aqueous extract of Ocimum sanctum and Group G6 was treated with 200 mg of aqueous extract of Ocimum sanctum per oral. Vehicle used for diluting meloxicam to obtain the desired concentration was 0.5 per cent Sodium Carboxy Methyl Cellulose in distilled water.
Collection of material for hematological and biochemical parameters
The rats from all experimental groups were sacrificed on 28th day of experiment. Prior to sacrifice the rats were fasted for 12 hours. Blood was collected on the day of sacrifice (28th day) from retro orbital plexus with the help of capillary tube in two vials, one coated with Ethylenediaminetetraacetic acid (EDTA) as an anticoagulant and in second vial without anticoagulant, later used for serum separation. Haematology parameters like Hemoglobin (Hb), PCV, Total Erythrocyte Count (TEC), Total Leukocyte Count (TLC), Differential Leukocyte Count (DLC) and haematocrit values were studied by standard routine procedures.[9] Blood samples collected in vials without anticoagulant and were kept undisturbed. The serum was separated and stored at –20°C for subsequent analysis. SGOT, SGPT, ALP, Glucose, Total Protein, Albumin, Globulin, Creatinine, Cholesterol and Triglyceride estimations were carried out by analytical kits supplied by RFCL, Haridwar, Uttarakhand, India on clinical chemistry semi auto analyzer.
Animals were sacrificed by cervical dislocation at the end of experiment. A detailed post mortem examination was carried out. The representative tissues - liver, kidney, heart lung, spleen, brain and spinal cord were fixed in 10% buffered formalin immediately on removal. They were subjected for histopathological examination following standard procedure.[10] Tissues such as liver and kidney were collected in ice cold containers for studying oxidative stress and lipid peroxidation parameters viz. LPO,[11] Reduced glutathione (GSH)[12] and SOD.[13]
Statistical analysis
The data generated was analyzed with one way analysis of variance (ANOVA).[14] The level of significance was set at P < 0.05.
RESULTS AND DISCUSSION
Clinical observations
The rats from group G2 receiving 1.2 mg/kg of meloxicam did not exhibit any clinical symptoms up to the end of experiment, while the rats receiving 2.4 mg/kg body weight of meloxicam showed mild diarrhea on 23rd day of experiment which continued till the end of experiment. The group G4 and G5 receiving 1.2 mg/kg and 2.4-mg/kg body weight of meloxicam respectively, along with aqueous extract of Ocimum sanctum did not show any clinical symptom. Group G6 also remained normal throughout the period of experiment. The gastrointestinal disorder due to subacute meloxicam toxicity has been reported earlier,[15] which could be the outcome of gastroenteritis as observed in present study.
Hematological parameters
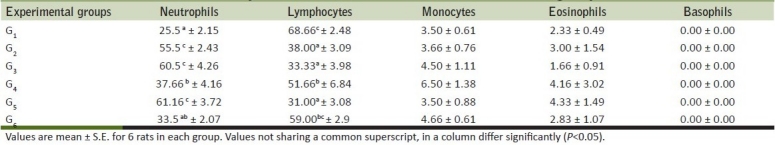
Hematological parameters like Hb concentration, TLC, PCV and TEC were estimated in control and treatment groups and are presented in Tables 1 and 2. The significant reduction in hemoglobin values of rats receiving of meloxicam indicated deleterious effect of meloxicam on blood cells. PCV values showed significant decrease in G2 and G3 group as compared to control. There was marked elevation in neutrophils count of rats from G2, G3 and G5 groups. Lymphocytes count showed relative decrease in-group G2, G3and G5 as a result of neutrophilia in these groups. The monocyte and eosinophils fluctuated within the normal range. Further, it was observed that the Ocimum sanctum could counteract untoward effect of meloxicam up to 1.2 mg/kg body weight. When meloxicam was fed orally at 2 mg/kg for 16 days, Alencar, et al.[16] reported anemia in meloxicam intoxicated dogs and substantiated the findings of present experiment. The increase in leucocyte count recorded in the present study could be attributed to ulcerogenic effect of meloxicam as observed in present study and as reported earlier.[16] Ocimum sanctum supplementation indicated protective role of Ocimum sanctum at low dose toxicity of meloxicam in the rats.
Table 1.
Mean hemoglobin, total erythrocyte count, packed cell volume and total leucocyte count of rats from various treatment groups
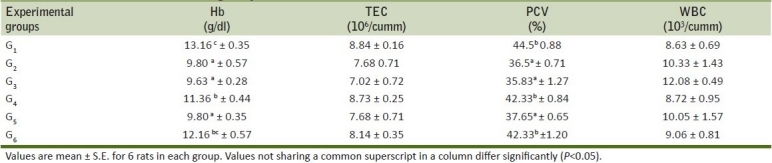
Table 2.
Mean differential leucocyte count of rats from various treatment groups
Serum biochemical parameters
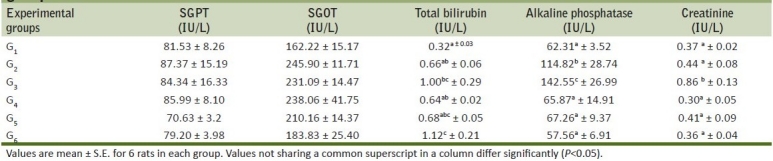
The serum biochemical parameter studied during the present experiment is presented in Table 3. The observations revealed that the rats treated with 1.2 mg (G2) and 2.4 mg (G3) of meloxicam resulted in two-fold elevation of alkaline phosphatase value, significantly increased serum bilirubin, serum creatinine when compared with control group. The group G4 and G5 receiving meloxicam and Ocimum sanctum showed relatively reduced values as compared to group treated with meloxicam alone. The elevated levels suggests hepatotoxic and nephrotoxic effect of meloxicam. Similar serum biochemical changes in case of a female patient with rheumatoid arthritis who developed acute cytolytic hepatitis due to meloxicam was reported earlier.[17] Dogs treated with meloxicam at 1 mg and 2-mg/kg body weight had elevation of serum creatinine[18] and is in agreement with the present findings. Serum biochemical parameters of Ocimum sanctum treated rats were not elevated, in turn indicating its protective action.
Table 3.
Mean SGPT, SGOT, total bilirubin and alkaline phosphatase of rats from various treatment groups
The values of parameters like LPO, GSH and antioxidant enzymes like SOD, activities of experimental rats were shown in Tables 4 and 5. The values of LPO in liver and kidney in G2 and G3 groups were found significantly elevated as compared with control group showed significant difference. Present observation is in accordance with Villages et al.,[19] who also observed elevation in oxidative stress parameters at higher dose of meloxicam (15 mg/kg) in rats. The other parameters studied did not showed any significant difference between the groups. However, there was an increase in the glutathione content and SOD values of meloxicam toxicated rats.
Table 4.
Mean tissue lipid peroxidase on liver and kidneys of rats from various treatment groups
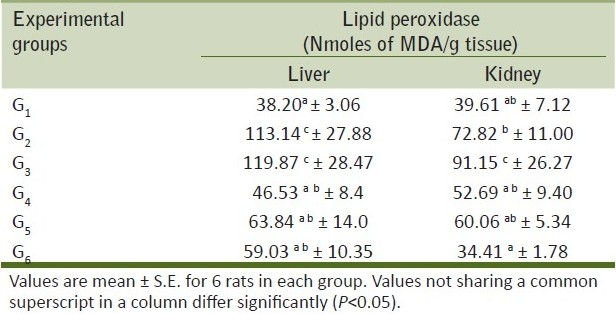
Table 5.
Mean reduced glutathione and super oxide dismutase on liver and kidneys of rats from various treatment groups
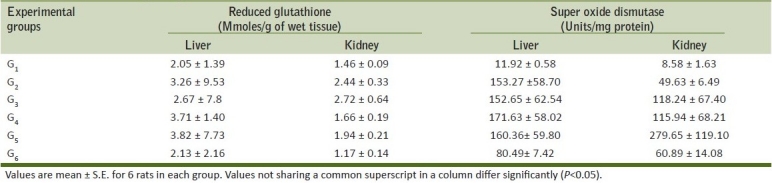
Gross findings
Grossly, rats from group G2 showed congestion of glandular stomach. The mucus membrane of intestine was thickened and become rough. Rats from group G3 showed hemorrhages in glandular stomach [Figure 1], congestion and subserosal hemorrhages in intestine [Figure 2]. Congestion and small necrotic area was seen on liver surface. Similar changes were also recorded in-group G5. Group G4 did not show any detectable macroscopic lesion. Control groups G1 and G6 also did not show any gross pathological lesions.
Figure 1.
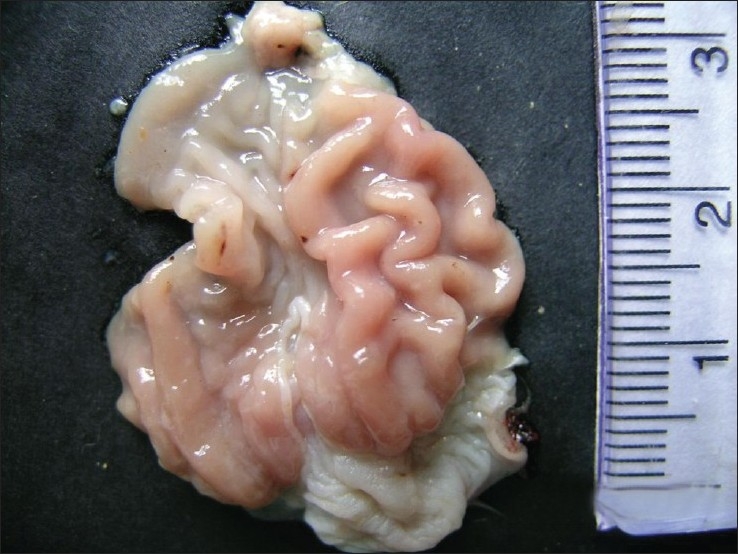
Hemorrhagic spot in the glandular stomach of meloxicam treated group G3
Figure 2.
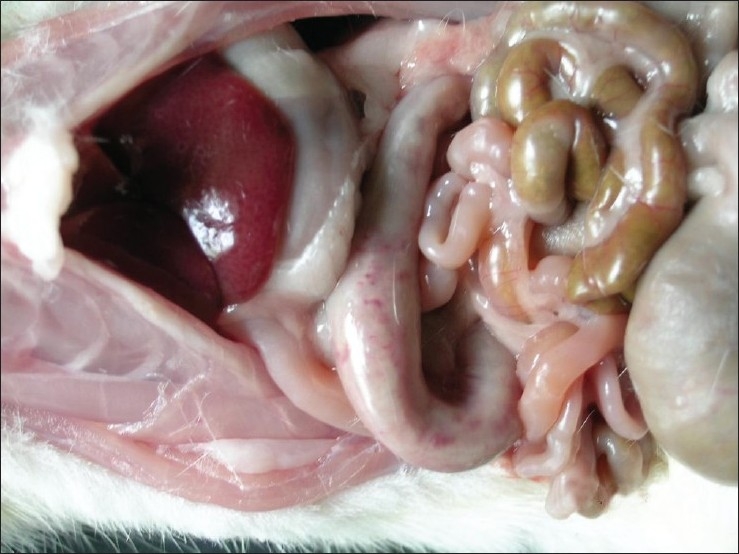
Subserosal hemorrhages in duodenum of group
Microscopic findings
Liver from group G2 revealed varied degree of changes from granular degenerative changes to necrosis of hepatocytes [Figure 3]. Infiltration of inflammatory cells and fibrous tissue in perivascular, perilobular and periportal areas of liver was seen [Figure 4]. Liver from group G3 revealed granular degenerative changes and necrosis of hepatocytes from perivascular areas. Mild congestion in liver and proliferation of Kupffer cell was pronounced in the liver from group G4 and G5.
Figure 3.
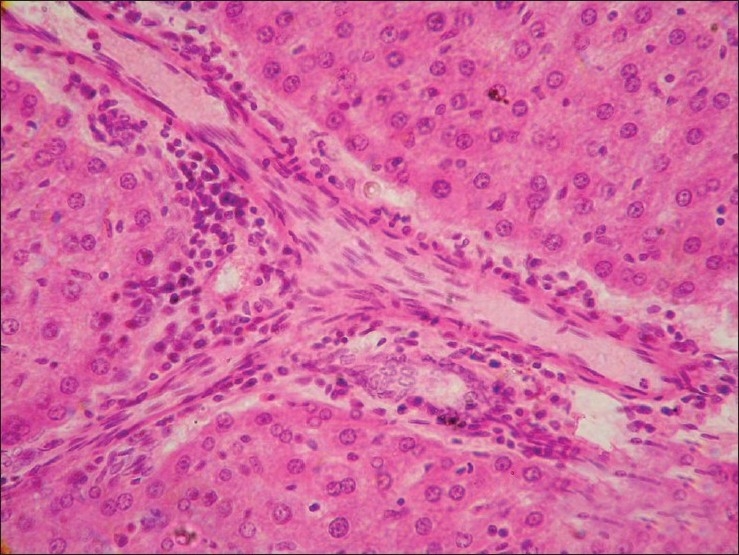
Section of liver (Group G2) showing vacuolar degenerative changes and necrosis of hepatocytes (H and E, × 200)
Figure 4.
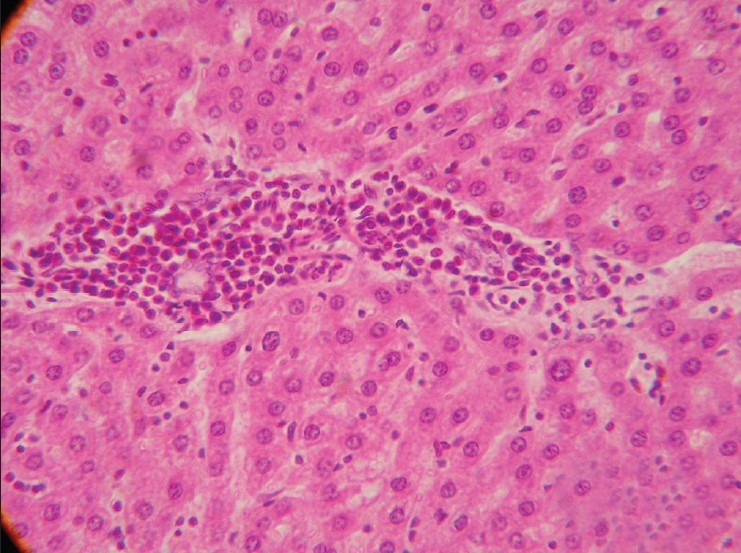
Section of liver (Group G2) showing infiltration of inflammatory cells and fibrous tissue in perivascular, perilobular and periportal areas of liver. (H and E, × 200)
Stomach from Group G2 revealed intact mucosal epithelium with mild infiltration of round cells in between the glands. Stomach from group G3 showed ulceration, hemorrhages and desquamation of mucosal epithelium at many places [Figure 5]. Stomach from group G4 and G5 revealed similar changes as that of group G2. Intact intestinal mucosa with mild submucosal infiltration was seen in rats from group G2 [Figure 6], while intestine group G3 showed hydropic degenerative changes in intestinal epithelium, hemorrhages and desquamation of the epithelial mucosa [Figure 7]. The group G4 and G5 did not show any detectable abnormality in intestine. Kidney from group G2 showed granular degenerative changes in the tubular epithelium, while kidneys from group G3 revealed extensive degenerative changes of tubular epithelium and interstitial nephritis [Figure 8], Hemorrhages were the prominent findings. The kidneys from G4 rats did not show any changes other than mild degenerative changes of tubular epithelium. In-group G5, severe granular degenerative changes were observed in tubular epithelium leading occlusion of tubular lumen. Glomerular tuft of kidney from group G5 showed hemorrhages. The section of brain from group G3 showed perivascualr edema, gliosis [Figure 9], and necrosis of neurons. Section of brain from other groups did not reveal any histological abnormalities. Focal myocardial necrosis and infiltration of inflammatory cells in between the cardiac muscles was evident in hear of rats from group G3 [Figure 10]. Section of heart from other groups did not reveal any histological abnormalities.
Figure 5.
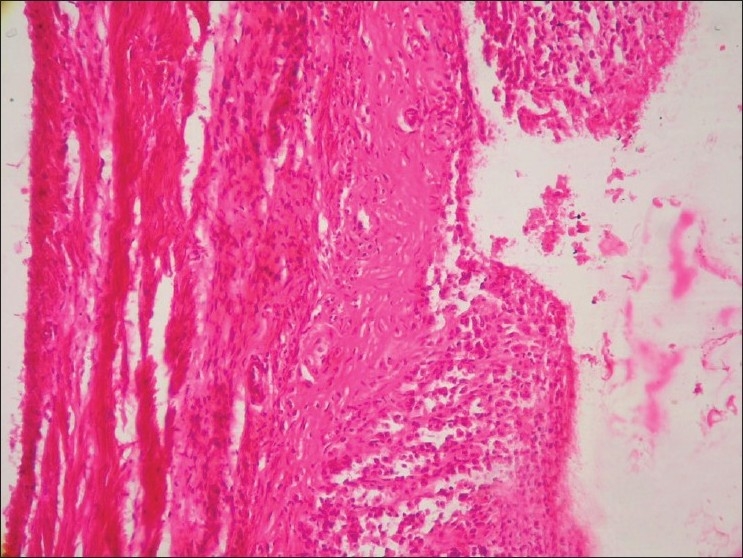
Section of glandular stomach (Group G3) showing ulcer (H and E, ×100)
Figure 6.
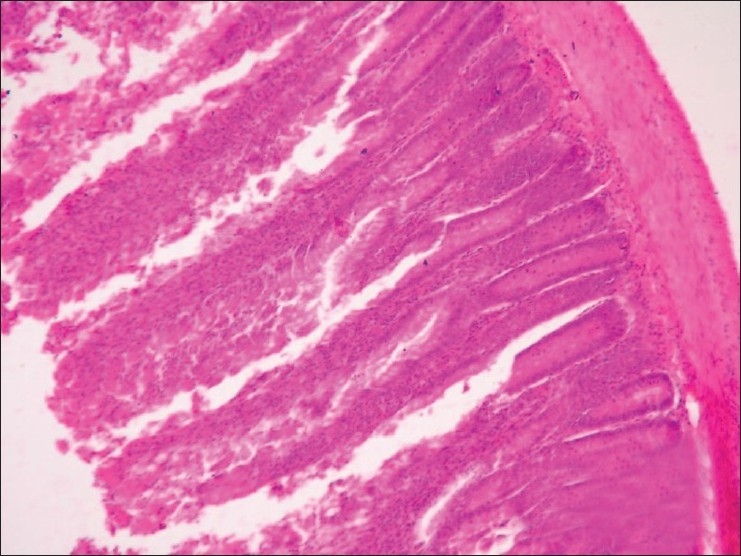
Section of intestine (Group G2) showing infiltration of inflammatory cells and hydropic degeneration in mucosal epithelium (H and E, ×100)
Figure 7.
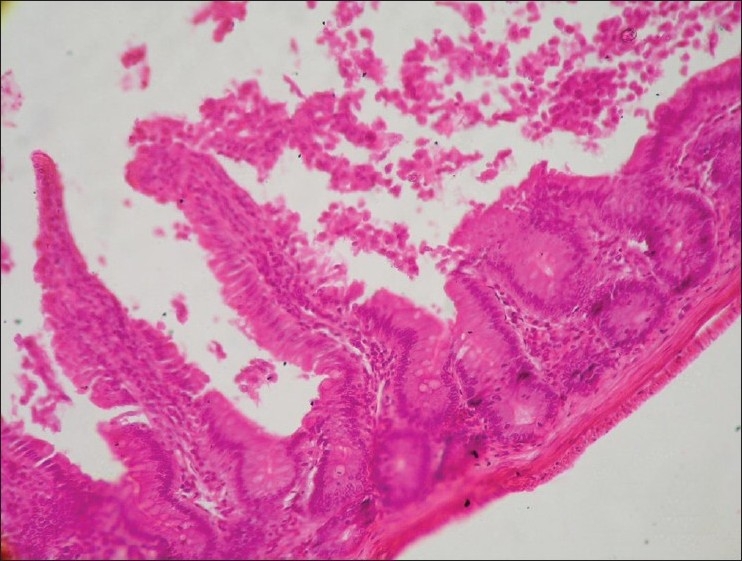
Section of intestine (Group G3) showing hydropic degeneration in mucosal epithelium of intestine and sloughing (H and E, ×100)
Figure 8.
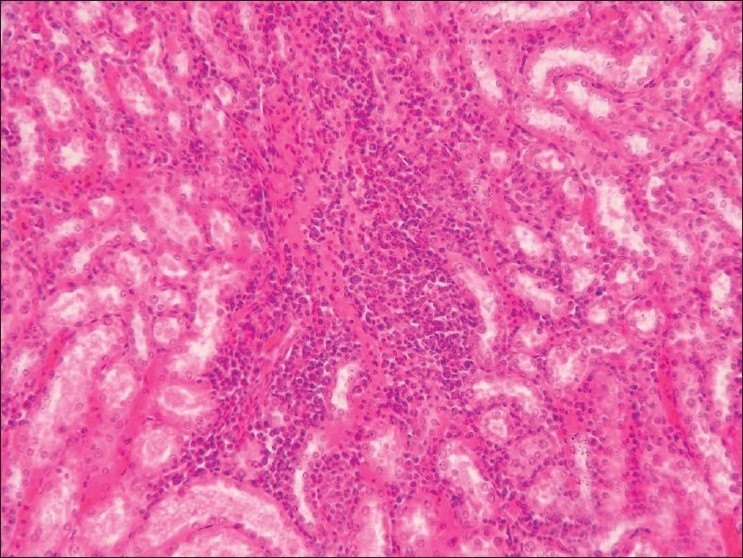
Section of kidney (Group G3) showing interstitial nephritis and hemorrhages (H and E, ×100)
Figure 9.
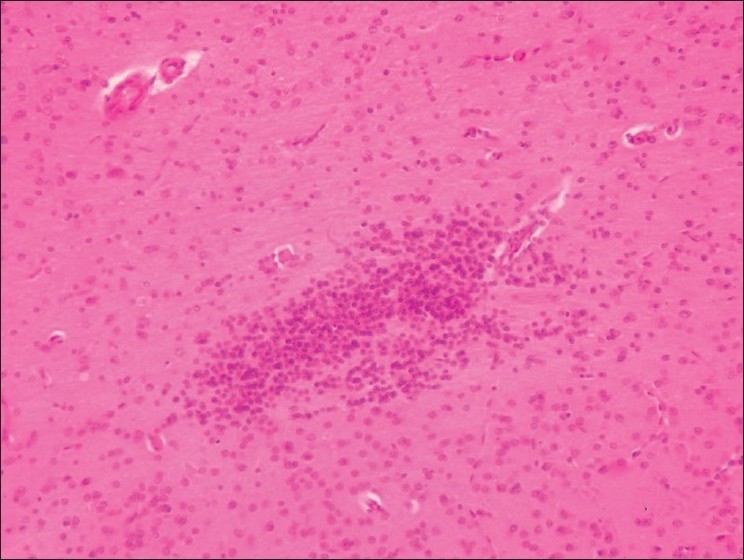
Section of brain (Group G3) showing gliosis and perivascualr edema (H and E, ×100)
Figure 10.
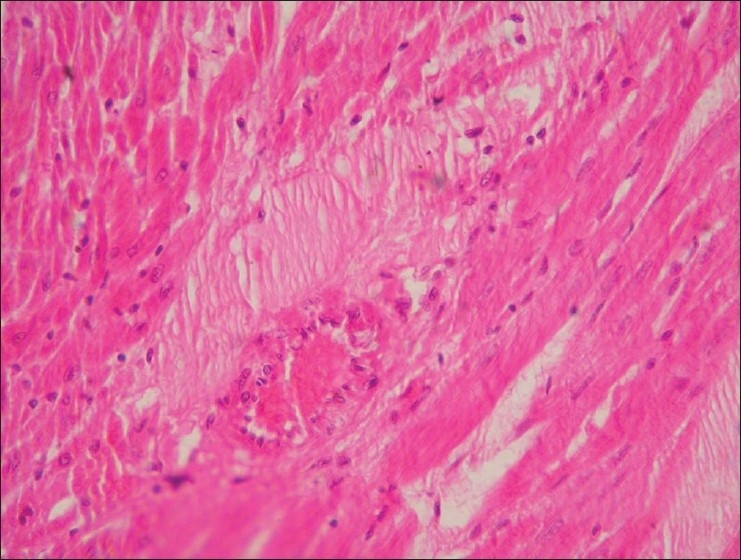
Section of heart (Group G3) showing myocardial infarct (H and E, ×100)
Histomorphological observations from group G4 indicated hepatoprotective role of Ocimum sanctum against meloxicam toxicity at low dose level and confirms the findings of hepatoprotective action of Ocimum sanctum as reported earlier.[20] The findings of gastric hemorrhages and intestinal ulceration due to meloxicam toxicity is reported earlier[16] and confirmed the findings of present study. Castro, et al.[21] also reported mononuclear infiltration in stomach of meloxicam treated dogs. The stomach and intestinal sections from groups receiving meloxicam and extract of Ocimum sanctum did not show any microscopic lesions in the stomach and intestine. This points out towards a protective action of Ocimum sanctum, which confirms the earlier findings of Mandal et al.[22] who reported anitulcerogenic properties of Ocimum sanctum in rats induced by aspirin. Inhibition of gastric secretion and ulceration in albino rats by Ocimum sanctum was reported earlier,[23] and could be the possible mechanism of protection by Ocimum sanctum. Khan et al.[24] reported interstitial nephritis and nephritic syndrome as side effect of NSAIDs due to transient interference in electrolytic homeostasis. The Ocimum sanctum could not counteract nephrotoxic effect of meloxicam in present experiment. Sections of heart from group G3 revealed focal myocardial necrosis and infiltration of inflammatory cells, which could be the outcome of sub acute meloxicam toxicity. Group G5 revealed myocardial infarcts and focal necrotic area in the section of heart which might be due to cumulative toxic action of meloxicam on cardiac muscle.
From the present study we could conclude that Meloxicam at both dose levels causes anemia, hepatotoxicty, nephrotoxicity and produces lesion in GI tract. The lesions can also be noticed in the heart and brain. Supplementation with Ocimum sanctum could counteract the toxic effect only at lower dose level. Even though many previous studies observed the toxicity of meloxicam, the basic mechanism of the toxicity is not understood thoroughly. A further detailed study at molecular level is needed to know the exact mechanism of meloxicam toxicity, and the protective mechanism of Ocimum sanctum could be explored.
ACKNOWLEDGMENT
Research presented in this manuscript was supported by grant from Department of Veterinary Pathology, Nagpur Veterinary College, Nagpur, India.
Footnotes
Source of Support: Grant from Department of Veterinary Pathology, Nagpur Veterinary College, Nagpur, India.
Conflict of Interest: None declared.
REFERENCES
- 1.Fleischmann R, Iqbal I, Slobodin G. Meloxicam. Expert Opin Pharmacother. 2002;3:1501–12. doi: 10.1517/14656566.3.10.1501. [DOI] [PubMed] [Google Scholar]
- 2.Ogino K, Harada Y, Hatanaka K. The 69th Annu. Meet Nagasaki, Japan: 1996. Inhibitory effect of Meloxicam on COX-2. Jap Pharmacol Soc. [Google Scholar]
- 3.Hawkey C, Kahan A, Steinbrück K, Alegre C, Baumelou E, Bégaud B, et al. Gastrointestinal tolerability of meloxicam compared to diclofenac in osteoarthritis patients.International MELISSA Study Group. Meloxicam Large-scale International Study Safety Assessment. Br J Rheumatol. 1998;37:937–45. doi: 10.1093/rheumatology/37.9.937. [DOI] [PubMed] [Google Scholar]
- 4.Vane JR, Botting RM. Mechanism of action of aspirin-like drugs. Semin Arthritis Rheum. 1997;26(6 Suppl 1):2–10. doi: 10.1016/s0049-0172(97)80046-7. [DOI] [PubMed] [Google Scholar]
- 5.Kirtikar KR, Basu BD. Published by LB Basu. Allahabad: 1965. In :Ocimum sanctum in Indian Medicinal Plants. [Google Scholar]
- 6.Bhargava KP, Singh N. Anti-stress activity of Ocimum sanctum Linn. Ind J Med Res. 1981;73:443–51. [PubMed] [Google Scholar]
- 7.Rajasekaran M. Ph.D. Thesis. India: University of Madras; 1988. Antifertility and some pharmacological studies on Triterpines. [Google Scholar]
- 8.Rosenthaler . The Chemical investigation of plants. 1st ed. London: Bell and sons; 1930. p. 36. [Google Scholar]
- 9.Benjamin MM. Outline of Veterinary Clinical Pathology. Kalyani Pubkishers New Delhi. 1985 [Google Scholar]
- 10.Luna AG. Manaul of histological staining methods of the Armed Force Institute of Pathology. 3rd ed. London: Mc Graw Hill Book Co; 1968. pp. 24–5. [Google Scholar]
- 11.Shafiq-Ur-Rehman Lead induced regional lipid peroxidation in brain. Toxico Lett. 1984;21:333–7. doi: 10.1016/0378-4274(84)90093-6. [DOI] [PubMed] [Google Scholar]
- 12.Sedlak J, Lindsay RH. Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellmans reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 13.Marklund S, Marklund G. Involvement of the super oxide anion radical in the autooxidation of pyrogallol and a convenient assay for super oxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 14.Snedecor GW, Cochran WG. Statistical Method. 8th ed. New Delhi: Oxford and IBH Publishing Company; 1995. [Google Scholar]
- 15.MacDonald TM, Morant SV, Goldstein JL, Burke TA, Pettitt D. Channeling bias and the incidence of gastrointestinal hemorrhage in users of meloxicam, coxibs, and older, non-specific non-steroidal anti-inflammatory drugs. Gut. 2003;52:1265–70. doi: 10.1136/gut.52.9.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alencar MM, Pinto MT, Oliveira DM, Pessoa AW, De P, Candido IA, et al. Margin of safety of Meloxicam in dogs: deleterious effects on blood cells and gastrointestinal tract. Cienc Rural. 2003;33:525–32. [Google Scholar]
- 17.Staerkel P, Horsmans Y. Meloxicam-induced liver toxicity. Acta Gastroenterol Belg. 1999;62:255–6. [PubMed] [Google Scholar]
- 18.Alencar MM, Pinto MT, Oliveira DM, Pessoa AW, De P, Candido IA, et al. Evaluation of the pharmacological activity of meloxicam on renal function in dogs. Cienc Animal. 2002;12:25–33. [Google Scholar]
- 19.Villegas I, Alarcón de la Lastra C, La Casa C, Motilva V, Martín MJ. Effect of food intake and oxidative stress on intestinal lesions caused by meloxicam and piroxicam in rats. Eur J Pharmacol. 2001;414:79–86. doi: 10.1016/s0014-2999(00)00883-9. [DOI] [PubMed] [Google Scholar]
- 20.Sankaran JR. An all India multicentric clinical survey on a herbal clue Tefroli for Hepatitis. J National Integ Med Assoc. 1984;26:255–61. [Google Scholar]
- 21.Castro MB, Develey FF, Azarias RE, Mourao GB, Marcos HR, Barbosa AM, et al. Clinical, gastroscopical and histopathological findings in dogs treated with meloxiam. Ars Veterinaria. 1999;15:154–9. [Google Scholar]
- 22.Mandal S, Das DN, De K, Ray K, Roy G, Chaudhuri SB, et al. Ocimum sanctum Linn-a study on gastric ulceration and gastric secretion in rats. Indian J Physiol Pharmacol. 1993;37:91–2. [PubMed] [Google Scholar]
- 23.Sen P. Therapeutic potentials of Tulsi: from experience to facts. Drugs News & Views. 1993;1:15–21. [Google Scholar]
- 24.Khan KN, Venturini CM, Bunch RT, Brassard JA, Koki AT, Morris DL, et al. Interspecies differences in renal localization of cyclooxygenase isoforms: Implications in nonsteroidal anti-inflammatory drug-related nephrotoxicity. Toxicol Pathol. 1998;26:612–20. doi: 10.1177/019262339802600504. [DOI] [PubMed] [Google Scholar]


