Abstract
Ethinyl oestradiol (EO) is the most commonly used as a component of oral contraceptive and hormonal replacement therapy (HRT) in women. However, its excessive and prolonged use may cause cytotoxicity, including cancer of many organs. Hence, the present study was performed to produce the experimental hepatotoxicity in female albino rats. EO was administered to different groups of rats, respectively @ 250, 500 and 750 μg/kg body weight, orally, weekly for 16 and 20 weeks. One group of rats was administered with saline alone to serve as control. The rats were sacrificed after their respective experimental periods, and the livers were collected and preserved in 10% buffered formalin. Later on, the histopathological study of liver tissues was done. On the 17th week, the hepatic tissues showed severe congestion, focal areas of hemorrhage, extreme vacuolation of cytoplasm, distended sinusoids with dilated central veins. Degeneration and necrosis of hepatocytes as evidenced by increased cytoplasmic granularity, and dissolution of nuclear materials were seen. On the 21st weeks, these changes were extremely severe and quite conspicuous. Distinct fibrosis was also noticed. EO caused hepatotoxicity, the extent and severity of which were dose and time dependent, indicating that this drug at higher dose after prolonged duration (500 or 750 μg/kg, orally, weekly for 20 weeks) may cause the standard experimental hepatotoxicity in rats.
Keywords: Ethinyl estradiol, female albino rats, hepatotoxicity, histopathological study
INTRODUCTION
Estrogens are the most commonly prescribed drugs by far the two major uses are as a component of oral contraceptives (OCs) and hormonal replacement therapy (HRT) in women. OCs used to control the birth, have influenced the lives of untold millions of women since they may cause cancer in humans as well as animals. Estrogen is responsible for many illnesses in women as well as men, and its long-term use may cause deleterious effects on liver as well.[1–5] The natural estrogens, viz., estrone and estradiol are recognized carcinogens in rodents and humans. Estradiol increased the incidence of tumors of pituitary gland, mammary gland, uterus, cervix, vagina, testes, lymphoid system, or bone in various strains of rats and mice.[6] Hence, the USA Government's National Toxicology Program added estrogen to the list of known human carcinogens. It is disconcerting to think that a natural hormone (estrogen) circulating in significant amounts through the bodies of about half of the world's population (women) is a carcinogen, but it is now official.[2,4,7]
Ethinyl estradiol (EO, a semisynthetic 17 β-oestradiol) is the highly potent estrogen. The median lethal dose (LD50) of EO has been determined to be more than 1000 μg/kg body weight, orally in female albino rats.[4] EO caused the cytotoxicity, leading to cancer in the uterus and ovary of female albino rat.[2–4] Further, EO @ 500 μg/kg, orally, weekly for 8 and 12 weeks has been reported to cause liver damage in female albino rat.[8,9]
In view of the above facts, the present study was performed to produce standard hepatotoxicity by EO (estrogen) in female rats at a definite dose and period. This study has great importance as no other literature could be available on the EO induced experimental hepatotoxicity and probably no such research works have been conducted in India.
MATERIALS AND METHODS
Forty-two healthy inbred female albino rats (110--160 g) were equally divided into seven groups, each had six rats. They were kept in polypropylene cages under standard conditions with 10 h day light and 14 h darkness in the animal house of Govt. NSCB Medical College, Jabalpur. The rats were fed on standard pellet diet and drinking water ad libitum. The experimental designs and protocols for study received the approval of Institutional Animal Ethics Committee in accordance with the guidelines provided by the CPCSEA.
The required amount of EO as Lynoral tablets (each tablet containing 0.05 mg of EO only) was purchased, and its suspension was prepared in distilled water mixed with a pinch of Gum acacia powder. EO was administered @ 250, 500, and 750 μg/kg, orally, weekly for 16 weeks to the rats of groups 2, 3, and 4, respectively; while the same doses of EO were administered for 20 weeks to the rats of groups 5, 6, and 7, respectively. However, the rats of group 1 were administered with normal saline (also containing a pinch of Gum acacia powder) to serve as control. After end of the experiments, the rats were sacrificed by cervical decapitation (euthanized scientifically) on the 1st week (Group 1), 17th week (Groups 2--4) and 21st week (Groups 5--7), and the liver of each rat was collected and preserved in 10% buffered formalin. Later on, the hepatic tissues were processed and stained with hemotoxylin and eosin (H and E) stain as per the method described by Culling.[10] Then the uniform and optimum damage as standard hepatotoxicity in the hepatic tissues was observed, microscopically.
RESULTS AND DISCUSSION
On the 17th week, the hepatic tissues of group 2 (EO @ 250 μg/kg, orally, weekly for 16 weeks) showed severely congested blood vessels. The sinusoids were seen as distended and central veins were dilated. Degeneration and necrosis of hepatocytes as evidenced by increased cytoplasmic granularity was noticed. In group 3 (EO @ 500 μg/kg, orally, weekly for 16 weeks), besides the above histopathological changes, the hepatic tissues revealed vascular hyperemia and necrotic foci in the hepatocytes with dissolution of nuclear material and extreme vacuolation of cytoplasm [Figure 1]. In group 4 (EO @ 750 μg/kg, orally, weekly for 16 weeks), the histopathological changes in liver tissues were more marked. On the 21st week, the above changes were extremely severe and quite conspicuous in the hepatic tissues of rats of groups 5, 6, and 7 administered with EO @ 250, 500, and 750 μg/kg, orally, weekly for 20 weeks, respectively. In groups 6 [Figure 2] and 7 [Figure 3], the blood vessels were highly congested and hepatocytes revealed degeneration and necrosis in larger areas. Distinct fibrosis was also noticed.
Figure 1.
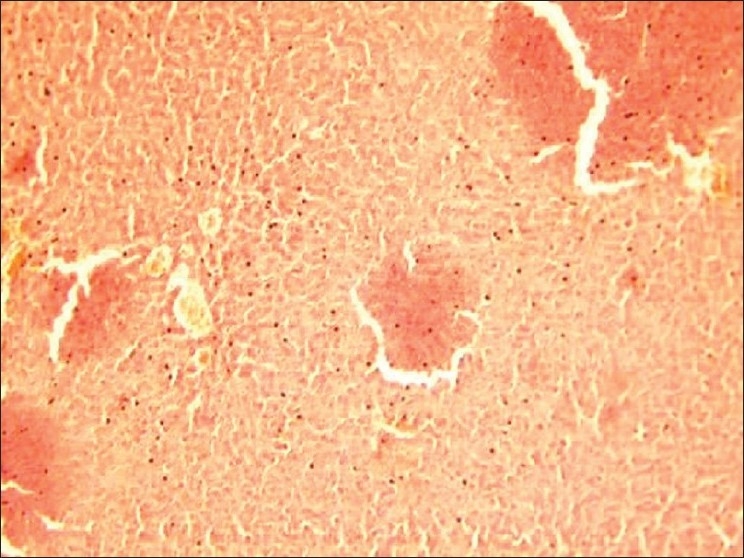
(Group 3---EO @ 500 μg/kg, orally, weekly for 16 weeks): Liver of rat on 17th week is showing marked congested blood vessels, distended sinusoids and dilated central veins; degeneration and necrosis of hepatocytes as evidenced by increased cytoplasmic granularity, extreme vacuolation of cytoplasm and dissolution of nuclear material are also seen (H and E, ×100).
Figure 2.
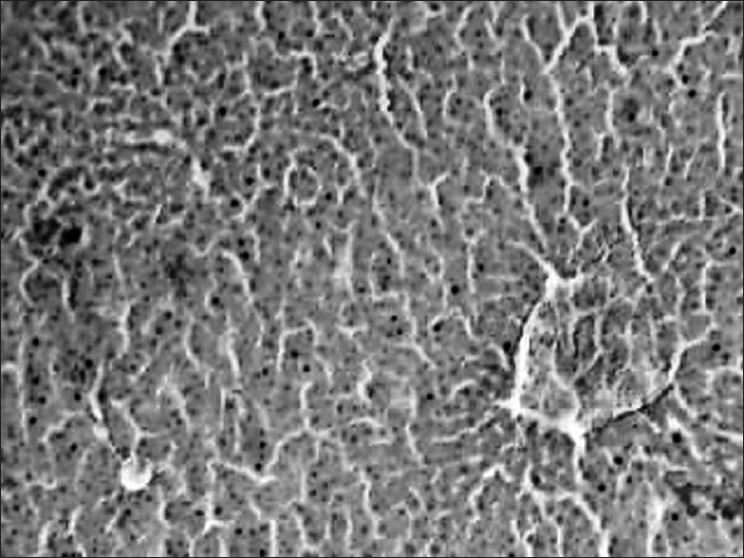
Liver of rat on 21st week is showing extremely severe and quite conspicuous changes as compared to group 3 on 17th week; highly congested blood vessels, and degenerated and necrotic hepatocytes in larger areas with distinct fibrosis are also noticed (H & E, ×100).
Figure 3.
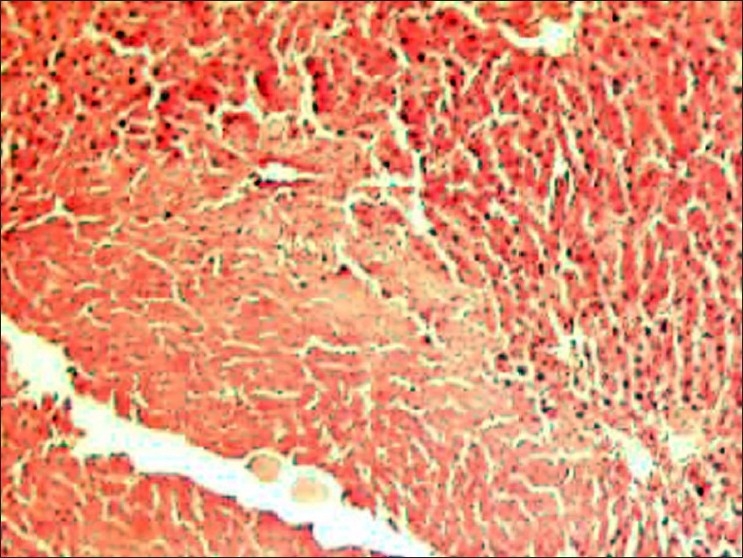
(Group 7---EO @ 750 μg/kg, orally, weekly for 20 weeks): Liver of rat on 21st week is showing extremely severe and quite conspicuous changes as compared to group 3 on 17th week; highly congested blood vessels, and degenerated and necrotic hepatocytes in larger areas with distinct fibrosis are also noticed (H and E, ×100).
The corroborating findings observed in the present study have been reported in female rat liver after administration of EO (500 μg/kg, orally, weekly for 8 and 12 weeks).[8,9] Similarly, EO (750 μg/kg, orally, weekly for 8--24 weeks) has been found to cause uterine and ovarian cytotoxicity, leading to cancer in albino rat.[2–4] Excessive and long-term use of hormonal contraceptives (e.g., estrogen) has already been reported to cause liver damage.[1,11] Furthermore, estrogen has been found to play a critical role in female hepatocarcinogenesis.[12] It is, however, stated that probably no research work on experimental hepatotoxicity induced by EO (estrogen) has been done in India since no literature could be available in this regard.
Conclusively, estrogen @ 250, 500, and 750 μg/kg body weight, orally, weekly for 16 and 20 weeks caused hepatotoxicity in female rats. The extent and severity of hepatic damage were found to be dose and time dependent, indicating that at higher dose for prolonged period (500 or 750 μg/kg, orally, weekly for 20 weeks), EO successively increases the intensity of damage and may cause the standard experimental hepatotoxicity in rats.
Excessive estrogen is trapped in different specific target organs (such as uterus, ovary, liver, etc.) due to stagnation, which over-stimulates the cell division, leading to abnormal growth or tissue damage. After the hormone binds to its receptors in a cell, it turns on hormone-responsive genes that promote DNA synthesis and cell proliferation. If a cell happens to have cancer-causing mutations, those cells will also proliferate and develop into tumors. In metabolizing carcinogenic estrogen, the reactions produce intermediates capable of producing oxygen radicals that can damage the cell's fats, proteins, and DNA. Unrepaired DNA damage can turn into a mutation, leading to cancer.[2,4,5,7,9]
ACKNOWLEDGMENTS
The authors are thankful to the Dean, Govt. NSCB Medical College, Jabalpur for providing laboratory facilities. The first author as a D.Sc. research scholar also acknowledges gratefully to the RDVV, Jabalpur, MP.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
Part of D.Sc. thesis of the first author; paper was presented in 39th Annual Conference of ‘Indian Pharmacology Society’ held on 21st-- 23rd December, 2006 at Jaipur, Rajasthan, India.
REFERENCES
- 1.Loose DS, Stancel GM. Estrogens and progestins. In: Brunton LL, editor. Goodman and Gilman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill Co; 2006. pp. 1541–71. [Google Scholar]
- 2.Sharma M. Studies on estrogen induced uterine and ovarian carcinogenesis and effect of roImmu in rat [PhD thesis] Jabalpur, India: RDVV; 2008. [Google Scholar]
- 3.Sharma M, Pandey G. Effect of ProImmu, a herbal drug on estrogen caused uterine and ovarian cytotoxicity. Biomed. 2010;5:57–62. [Google Scholar]
- 4.Sharma M, Pandey G, Khanna A. estrogen induced uterine damage in rats. Toxicol Int. 2009;161:5–7. [Google Scholar]
- 5.Pandey G, Sharma M. Median lethal dose, acute and chronic toxicities of ethinyl oestradiol estrogen. Natl J Life Sci. 2008;52:291–4. [Google Scholar]
- 6.Liehr JG. Genotoxicity of the steroidal estrogens oestrone and oestradiol: possible mechanism of uterine and mammary cancer development. Hum Reprod Update. 2001;7:273–81. doi: 10.1093/humupd/7.3.273. [DOI] [PubMed] [Google Scholar]
- 7.Estrogen and cancer. [Last cited on 2006]. Available from: http://www.womenshealth.com .
- 8.Pandey G, Sharma M. Efficacy of OptiLiv, a herbal formulation against estrogen induced liver damage in female rats. Int J Green Pharm. 2007;1:39–41. [Google Scholar]
- 9.Pandey G, Sharma M, Pandey SP, Shrivastav AB. Hepatic tissue regeneration by OptiLiv in estrogen induced hepatotoxicity. Ind Res Comm. 2008;2:47–52. [Google Scholar]
- 10.Culling CF. Hand book of histological techniques. 2nd ed. London: Butterworth and Co Ltd; 1963. pp. 25–172. [Google Scholar]
- 11.Sheth AR, Adarkar MA, Rao SS, Virkar KD, Kora S. Liver function tests in women using oral contraceptives. Indian J Med Res. 1967;55:1205–12. [PubMed] [Google Scholar]
- 12.Gayathri R, Priya DK, Gunassekaran GR, Sakthisekaran D. Ursolic acid attenuates oxidative stress-mediated hepatocellular carcinoma by diethylnitrosamine in male Wistar rats. Asian Pac J Cancer Prev. 2009;10:933–8. [PubMed] [Google Scholar]


