Abstract
Arsenic is an environmental pollutant and its contamination in the drinking water is considered as a serious worldwide environmental health threat. The chronic arsenic exposure is a cause of immense health distress as it accounts for the increased risk of various disorders such as cardiovascular abnormalities, diabetes mellitus, neurotoxicity, and nephrotoxicity. In addition, the exposure to arsenic has been suggested to affect the liver function and to induce hepatotoxicity. Moreover, few studies demonstrated the induction of carcinogenicity especially cancer of the skin, bladder, and lungs after the chronic exposure to arsenic. The present review addresses diverse mechanisms involved in the pathogenesis of arsenic-induced toxicity and end-organ damage.
Keywords: Arsenic, carcinogenicity, cardiovascular dysfunction, diabetes, hepatotoxicity, nephrotoxicity, neurotoxicity
INTRODUCTION
Arsenic is a naturally occurring element that ubiquitously exists in both organic and inorganic form in the environment. Arsenic contamination is an issue of concern worldwide and it is a considerable risk factor in various countries including Bangladesh, Taiwan, India, Mexico, China, Chile, Argentina, and USA. Human exposure to arsenic is either through oral route involving contaminated food and water or through inhalation that majorly covers exposure to agricultural pesticides and mining activities. According to World Health Organization (WHO) fact sheet in 1999, arsenic contamination is considered as major public health issue requiring correction measures on emergency basis.[1] The WHO guidelines describe safety limit of arsenic at 10 μg/l and a maximum permissible limit of 50 μg/l of drinking water.[2] Over 200 million people are at risk worldwide, out of which more than half are residing in Bengal Delta Plain including West Bengal and Bangladesh.[3] The arsenic content in this area has been found to be 800 μg/l of drinking water.[4] The chronic poisoning caused by high levels of arsenic in well waters has led to public health emergency in Bangladesh.[5,6] In Taiwan, chronic arsenic exposure through well waters has led to peripheral vascular disease called as black foot disease.[7] Arsenic exists in the environment as pentavalent (As5+, arsenate) and trivalent (As3+, arsenite) forms, and arsenite has been considered to be more toxic when compared with arsenate.[8] On absorption, arsenic is stored in liver, kidney, heart, and lungs. The lower amount of arsenic is observed in muscles and neuronal tissues.[9] The accumulation of arsenic in these tissues is associated with many disorders including cancer, diabetes, hepatotoxicity, neurotoxicity, and cardiac dysfunction. Arsenic metabolism is important for its toxicity and it exerts its toxicity by inhibiting around 200 enzymes involved in cellular energy pathways and DNA synthesis and repair, etc.[10] It is metabolized by reduction and methylation reactions, catalyzed by glutathione-S-transferase omega-1 (GSTO1) and arsenic (III) methyltransferase (AS3MT) involving methylation of arsenic via one-carbon metabolism by S-adenosyl methionine (SAM) as methyl donor and requiring reduced glutathione (GSH) as electron donor in reductase reaction. GSTO1 reduces methylarsonate [MA(V)] and arsenate [As(V)] to methylarsonite [MA(III)] and arsenite [As(III)], respectively, and these toxic trivalent arsenicals formed during reduction are detoxified by AS3MT to methylarsonate [MA(V)] and dimethylarsinate [DMA(V)], which are less toxic pentavalent arsenicals.[11] Acute arsenic poisoning is associated with nausea, vomiting, abdominal pain, and severe diarrhea.[10] Chronic ingestion of arsenite through contaminated water results in accumulation of arsenite and MA(III) in vital organs and tissues leading to the incidence of atherosclerosis, hypertension, ischemic heart diseases, diabetes, hepatotoxicity, nephrotoxicity, and cancer of the skin, bladder, and lungs.[12–17] The present review critically discussed the mechanisms involved in the pathogenesis of arsenic-induced toxicity and end organ damage.
ARSENIC-INDUCED CARDIOVASCULAR DYSFUNCTION
Long-term exposure to inorganic arsenic may cause various cardiovascular disorders such as atherosclerosis, hypertension, ischemic heart diseases, and ventricular arrhythmias.[12–14,18] Arsenite stimulates nicotinamide adenine dinucleotide phosphate (NADPH) oxidase present in the plasma membrane of vascular endothelial cells and vascular smooth muscle cells (VSMC) to increase the generation of reactive oxygen species (ROS) such as superoxides and hydrogen peroxide.[19,20] ROS generated during arsenite exposure couples with nitric oxide (NO) to form peroxynitrite, a strong oxidant implicated in the upregulation of inflammatory mediator such as cyclooxygenase-2.[21] ROS generated during arsenite exposure increases the expression of atherosclerosis related genes such as heme oxygenase-1 (HO-1), monocyte chemo-attractant protein (MCP-1), and interleukin-6 (IL-6) and thus its exposure promotes the attachment, penetration, and migration of monocytes in VSMC.[22] Arsenic alters focal adhesion proteins in VSMCs leading to their proliferation and migration.[23] Further, arsenic increases the synthesis of inflammatory mediators such as leukotriene E4 (LTE4) and prostacyclin, tumor necrosis factor-alpha and nuclear factor kappa B in vascular endothelial cells to induce the pathogenic process of atherosclerosis.[24,25] Moreover, arsenic causes neurogenic inflammation of the blood vessel by increasing the release of substance P and endothelial neurokinin-1.[26] Furthermore, arsenic activates protein kinase C alpha, which causes phosphorylation of beta-catenin and thus reverses the association between vascular endothelial cadherin and beta-catenin, along with the formation of actin stress fibers resulting in increased intercellular gap formation and permeability of the endothelium.[27] Arsenite has been reported to decrease the activity of endothelial nitric oxide synthase (eNOS) and Akt/protein kinase B, which subsequently decreases the bioavailability of NO that may lead to vascular endothelial dysfunction and associated cardiovascular complications.[28,29] Arsenite mediates vasoconstriction of the blood vessels by phosphorylating myosin light chain kinase (MLCK) and increases calcium sensitization leading to hypertension.[30] Chronic exposure to arsenic induces oxidative stress and alters the release of vasoactive mediators in blood vessel leading to elevation of blood pressure.[31] Arsenic trioxide develops ventricular arrhythmia by inducing prolonged Q-T interval and action potential duration.[18,32] Taken together, it may be suggested that arsenic induces cardiovascular dysfunction by inducing high oxidative stress, reducing the activation of eNOS and enhancing the phosphorylation of MLCK, which may be targeted for preventing arsenic exposure-associated cardiovascular complications [Figure 1]. Recently, a couple of studies from our laboratory demonstrated that treatment with either bis-(maltolato) oxovanadium, an inhibitor of protein tyrosine phosphatase, or rosiglitazone, an agonist of peroxisome proliferator activated receptor-gamma (PPAR-γ) markedly ameliorated sodium arsenite-induced vascular endothelial dysfunction in rats by enhancing the integrity of vascular endothelium, improving endothelium-dependent relaxation and reducing oxidative stress.[33,34]
Figure 1.

Pathological mechanisms involved in arsenic-induced cardiovascular dysfunction
ARSENIC-INDUCED DIABETES MELLITUS
The prolonged exposure to arsenic causes decreased expression of PPAR-γ, which may reduce the sensitivity of insulin that is responsible for the induction of type II diabetes by arsenic.[35] Arsenite replaces a phosphate group from adenosine triphosphate (ATP) forming ADP-arsenate, which slows down the metabolism of glucose, interrupts the production of energy, and interferes with the ATP-dependent insulin secretion.[36] Moreover, arsenite has high affinity for sulfhydryl groups and hence form covalent bonds with the disulfide group of insulin, insulin receptors, glucose transporter, and enzymes involved in glucose metabolism.[36] An interesting biphasic response in glucose metabolism due to arsenic exposure was found through glucocorticoid receptor-mediated transcription with hyperglycemic effect at low concentration (<120 ppb) and hypoglycemia at high concentration (>120 ppb).[37] In addition, the chronic exposure to arsenic may cause hypoglycemia due to significant decrease in glucose-6-phosphatase activity in both liver and kidney.[38] Sodium arsenite has been suggested to downregulate the expression of insulin mRNA.[39] The long-term exposure to inorganic arsenic increases oxidative stress leading to overexpression of various stress mediators such as NF-κB, c-Jun-N-terminal kinase/stress-activated protein kinase (JNK/SAPK) and hexosamine that cause insulin resistance and dysfunction of beta cells of islets of Langerhans.[40] The trivalent arsenicals such as inorganic arsenic [iAs (III)], dimethylarsinous acid [DMA (III)], and monomethylarsonous acid [MMA (III)] suppress the phosphorylation of Akt/protein kinase B by inhibiting the activity of 3-phosphoinositide-dependent kinase-I (PDK-1) that causes significant inhibition of insulin-dependent glucose uptake and hence results in hyperglycemia.[40,41] Together, these studies suggest that decreased expression of PPAR-γ, interference in ATP-dependent insulin secretion, altered glucocorticoid receptor mediated transcription, and inhibition of PDK-1 are involved in the induction of arsenic-associated diabetes, which can serve as potential targets to modulate arsenic-induced diabetes [Figure 2].
Figure 2.
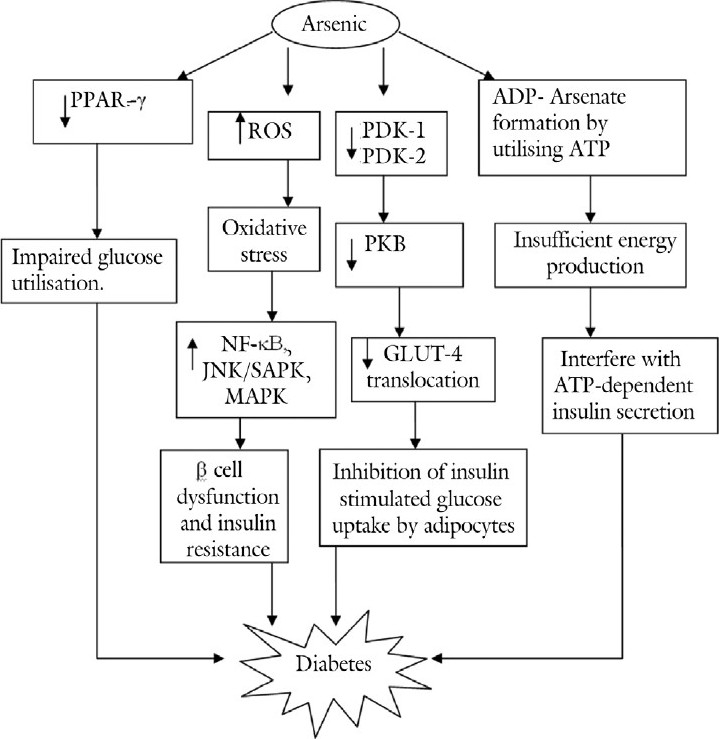
Pathological mechanisms involved in arsenic-induced diabetes
ARSENITE-INDUCED NEUROTOXICITY
Brain is a soft target for arsenic toxicity as it freely crosses blood-brain barrier.[42,43] Arsenic exposure is associated with wide range of neurological complications in humans such as impaired memory, poor concentration, Parkinson's disease, Guillain-Barre like neuropathy, verbal comprehension, encephalopathy, and peripheral neuropathy.[44–49] The mechanism postulated for arsenic-induced neurotoxicity majorly involve oxidative stress with increased reactive oxygen species, lipid peroxides along with decrease in superoxide dismutase, and reduced glutathione levels.[50] Arsenic exposure has been reported to alter metabolism of various neurotransmitters such as monoamines, acetylcholine, gamma amino butyric acid, and glutamate.[51] In a recent study, a significant reduction in monoamines such as adrenaline, nor-adrenaline, dopamine, and serotonin has been observed in corpus striatum, frontal cortex, and hippocampus areas of brain on chronic arsenic exposure.[42] Arsenite-mediated neurotoxicity involves induction of apoptosis in the cerebral neurons by activating p38 mitogen-activated protein kinase (p38MAPK) and JNK3 pathways.[52] Moreover, arsenic exposure induces neurotoxicity by causing destabilization and disruption of cytoskeletal framework, eventually leading to axonal degeneration.[53] The deficiency of thiamine (vitamin B1) is well known to induce neuronal complications. It is worthwhile to note that arsenic causes thiamine deficiency and inhibits pyruvate decarboxylase, which elevates blood pyruvate and hence causes encephalopathy.[47] Arsenic-induced oxidative stress in the brain causes oxidative DNA damage and subsequent brain cell death and induces the degeneration of dopaminergic neurons resulting in Parkinson like symptoms.[45,46] Acute arsenic toxicity decreases acetyl cholinesterase activity and hence causes cholinergic crisis like situation with altered mental status and weakness, which can be associated with peripheral neuropathy, neuropsychiatric abnormalities, and extrapyramidal disorders.[54] Moreover, arsenic affects the peripheral nervous system by disrupting the neuroskeletal integrity and thus markedly diminishes the nerve conduction velocity in the peripheral nerves to cause peripheral neuropathy.[49] The exposure to arsenic and its metabolites monomethylarsonic acid and monomethylarsonous acid suppresses the NMDA receptors in hippocampus, which play a pivotal role in synaptic plasticity, learning, and memory, leading to neurobehavioral disorders and cognitive dysfunction.[55,56] The chronic arsenic exposure is associated with morphological changes in axons and nerve fibers of the striatum which disturbs central structural organization.[57] Hence, oxidative stress, induction of thiamine deficiency, and inhibitions of pyruvate decarboxylase, acetyl cholinesterase, reduction in biogenic monoamines seem to play a pivotal role in arsenic-induced neurotoxicity [Figure 3]. The animal models of arsenic toxicity are associated with inconsistent neurotoxicity because of varying doses, duration, and different salts of arsenic used in various studies. However, these have been able to provide deep insight into pathophysiological mechanisms involved in arsenic induced neurotoxicity.
Figure 3.

Pathological mechanisms involved in arsenic-induced neurotoxicity
ARSENIC-INDUCED NEPHROTOXICITY AND HEPATOTOXICITY
Arsenic concentrates in the kidney during its urinary elimination that affects the function of proximal convoluted tubules.[58,59] Arsenic-induced oxidative stress increases the expression of HO-1 and MAPK, which by regulating various transcription factors such as activator protein-1 (AP-1), activating transcription factor-2 (ATF-2), and Elk-1 lead to renal toxicity.[59,60] Acute renal dysfunction due to arsenic exposure is characterized by acute tubular necrosis and cast formation with increase in blood urea nitrogen and creatinine levels.[61] This arsenic-induced renal toxicity can be attenuated by Curcuma aromatica and Corchorus olitorius.[62,63] The kidney and liver are the primary targets for arsenic-induced toxicity, where the highest level of arsenic is detected in the liver than kidney.[64] Arsenite increases the generation of ROS, which enhances lipid peroxidation and cellular damage in both hepatic and renal tissue.[65] Chronic arsenic-mediated oxidative stress activates JNK and p38 MAPK and induces apoptosis in the hepatocytes.[66–68] Further, arsenic-induced oxidative stress induces hepatic apoptosis by upregulation of pro-apoptotic proteins.[69] A recent study has well documented that arsenite-induced apoptotic progression is aggravated by folate deficiency.[70] Arsenic exposure leads to the incidence of hepatotoxicity as manifested by increase in the levels of total bilirubin, alanine aminotransferase, aspartate aminotransferase, and malionaldehyde.[71] Hence, oxidative stress, apoptosis, and upregulation of transcription factors such as AP-1, ATF-2, and Elk-1 are the prospective target sites for arsenite-induced nephrotoxicity and hepatotoxicity [Figure 4].
Figure 4.

Pathological mechanisms involved in arsenic-induced hepatotoxicity and nephrotoxicity
Arsenic-induced carcinogenicity
The trivalent form of arsenic exhibits greater genotoxic effects than the pentavalent counterparts as it could be easily taken up by the cells.[72] Although the exact molecular mechanism of arsenic carcinogenicity is not well understood, arsenic has been shown to possess tumor-promoting properties by inducing intracellular signal transduction, activating transcription factors, and changing the expression of genes that are involved in cell growth, proliferation, and malignant transformation. Further, it is has been postulated that arsenic induces MAPK signal transduction, which activates transcription factors such as AP-1 and NF-kB to alter various gene expression profile that may account for the induction of arsenic-associated carcinogenicity.[73] Arsenic causes focal adhesion kinase activation, which mediates several downstream signaling pathways such as integrin, Src, Rho, Grb2, EGFR, ERK, and cadherins. These pathways are involved in cell adhesion, cell migration, cell survival, cell cycle control, carcinogenesis, and tumor cell necrosis.[74] DMA(V) and TMAO(V) generate oxidative stress and cause an elevation of 8-hydroxydeoxyguanosine, a marker of oxidative DNA damage, which stimulates cell proliferation and induces carcinogenicity.[75,76] Arsenic provokes proliferation of bladder epithelial cells and upregulates proto-onco-gene expression such as c-fos, c-jun, and EGR-1, which may collectively contribute to bladder cancer.[77] Smoking has been shown to potentiate the effect of arsenic on the risk of bladder and lung cancer because both can act synergistically to cause DNA damage.[78,79] Arsenic induces skin cancer by acting synergistically with sunlight and blocking DNA repair, stimulating angiogenesis, altering DNA methylation patterns, dysregulating cell cycle control, and blocking physiological apoptosis.[80] Oxidative stress seems to be the main culprit for arsenic-induced carcinogenicity, which can be prevented by antioxidants such as vitamin E, melatonin, and curcumin.[81,82] Taken together, various possible modes of carcinogenic action of arsenic proposed till date are increased oxidative stress, direct genotoxic effects, altered expression of growth factors, and altered DNA repairing mechanisms [Figure 5].
Figure 5.

Pathological mechanisms involved in arsenic-induced carcinogenicity
CONCLUSION
The chronic exposure to arsenic through contaminated water may account for various health intimidations. Arsenic increases oxidative stress, upregulates proinflammatory cytokines and inflammatory mediators, inactivates eNOS, and causes phosphorylation of MLCK to induce cardiovascular abnormalities. The decreased expression of PPAR-γ, interference in ATP-dependent insulin secretion, altered glucocorticoid receptor-mediated transcription, and inhibition of PDK-1 are the pathological events associated with arsenic-induced diabetes. Further, oxidative stress, inhibition of pyruvate decarboxylase, and acetyl cholinesterase seem to play a pivotal role in arsenic-induced neurotoxicity. Moreover, arsenic induces nephrotoxicity and hepatotoxicity by increasing oxidative stress and apoptosis. Furthermore, arsenic exposure may cause carcinogenicity as it increases oxidative DNA damage and chromosomal aberration and interferes with cellular signaling pathways. Targeting and modulating the aforementioned key pathological signaling mechanisms may provide novel pharmacological interventions to halt arsenic exposure-associated disorders.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Fact sheet no. 210. Geneva: WHO; 1999. WHO (World Health Organization) [Google Scholar]
- 2.Steinmaus C, Carrigan K, Kalman D, Atallah R, Yuan Y, Smith AH. Dietary intake and arsenic methylation in a U.S. population. Environ Health Perspect. 2005;113:1153–9. doi: 10.1289/ehp.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arsenic in drinking water. Washington D C: National Academy Press; 2001. National Research Council (NRC) [Google Scholar]
- 4.Kinniburgh DG, Smedley PL. Arsenic contamination of ground water in Bangladesh: Volume 2: Final report. Keyworth, Nottinghamshire: British Geological Survey. 2001 [Google Scholar]
- 5.Alam MG, Allinson G, Stagnitti F, Tanaka A, Westbrooke M. Arsenic contamination in Bangladesh groundwater: a major environmental and social disaster. Int J Environ Health Res. 2002;12:235–53. doi: 10.1080/0960312021000000998. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Parvez F, Gamble M, Islam T, Ahmed A, Argos M, et al. Arsenic exposure at low-to-moderate levels and skin lesions, arsenic metabolism, neurological functions, and biomarkers for respiratory and cardiovascular diseases: review of recent findings from the Health Effects of Arsenic Longitudinal Study (HEALS) in Bangladesh. Toxicol Appl Pharmacol. 2009;239:184–92. doi: 10.1016/j.taap.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng CH. An overview on peripheral vascular disease in blackfoot disease-hyperendemic villages in Taiwan. Angiology. 2002;53:529–37. doi: 10.1177/000331970205300505. [DOI] [PubMed] [Google Scholar]
- 8.Domingo JL. Prevention by chelating agents of metal-induced developmental toxicity. Reprod Toxicol. 1995;9:105–13. doi: 10.1016/0890-6238(94)00060-3. [DOI] [PubMed] [Google Scholar]
- 9.Klaassen CD. Heavy metals and heavy metal antagonist. In: Gilman AG, Rall TW, Nies AS, Taylor P, editors. The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw Hill; 1996. pp. 1592–614. [Google Scholar]
- 10.Ratnaike RN. Acute and chronic arsenic toxicity. Postgraduate Med J. 2003;79:391–6. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindberg AL, Kumar R, Goessler W, Thirumaran R, Gurzau E, Koppova K, et al. Metabolism of low-dose inorganic arsenic in a central European population: influence of sex and genetic polymorphisms. Environ Health Perspect. 2007;115:1081–6. doi: 10.1289/ehp.10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen ES. International Commission for Protection against Environmental Mutagens and Carcinogens. ICPEMC Working Paper 7/1/2. Shared risk factors for cancer and atherosclerosis-a review of the epidemiological evidence. Mutat Res. 1990;239:163–79. doi: 10.1016/0165-1110(90)90004-u. [DOI] [PubMed] [Google Scholar]
- 13.Chen CJ, Hsueh YM, Lai MS, Shyu MP, Chen SY, Wu MM, et al. Increased prevalence of hypertension and long-term arsenic exposure. Hypertension. 1995;25:53–60. [PubMed] [Google Scholar]
- 14.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16:504–10. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 15.Rahman M, Axelson O. Diabetes mellitus and arsenic exposure: a second look at case-control data from a Swedish copper smelter. Occup Environ Med. 1995;52:773–4. doi: 10.1136/oem.52.11.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Liu Y, Goyer RA, Achanzar W, Waalkes MP. Metallothionein-I/II null mice are more sensitive than wild-type mice to the hepatotoxic and nephrotoxic effects of chronic oral or injected inorganic arsenicals. Toxicol Sci. 2000;55:460–7. doi: 10.1093/toxsci/55.2.460. [DOI] [PubMed] [Google Scholar]
- 17.Gurr JR, Yih LH, Samikkannu T, Bau DT, Lin SY, Jan KY. Nitric oxide production by arsenite. Mutat Res. 2003;533:173–82. doi: 10.1016/j.mrfmmm.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Ohnishi K, Yoshida H, Shigeno K, Nakamura S, Fujisawa S, Naito K, et al. Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Ann Intern Med. 2000;133:881–5. doi: 10.7326/0003-4819-133-11-200012050-00012. [DOI] [PubMed] [Google Scholar]
- 19.Barchowsky A, Klei LR, Dudek EJ, Swartz HM, James PE. Stimulation of reactive oxygen, but not reactive nitrogen species, in vascular endothelial cells exposed to low levels of arsenite. Free Radic Biol Med. 1999;27:1405–12. doi: 10.1016/s0891-5849(99)00186-0. [DOI] [PubMed] [Google Scholar]
- 20.Smith KR, Klei LR, Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L442–9. doi: 10.1152/ajplung.2001.280.3.L442. [DOI] [PubMed] [Google Scholar]
- 21.Bunderson M, Coffin JD, Beall HD. Arsenic induces peroxynitrite generation and cyclooxygenase-2 protein expression in aortic endothelial cells: possible role in atherosclerosis. Toxicol Appl Pharmacol. 2002;184:11–8. [PubMed] [Google Scholar]
- 22.Lee PC, Ho IC, Lee TC. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol Sci. 2005;85:541–50. doi: 10.1093/toxsci/kfi101. [DOI] [PubMed] [Google Scholar]
- 23.Pysher MD, Chen QM, Vaillancourt RR. Arsenic alters vascular smooth muscle cell focal adhesion complexes leading to activation of FAK-src mediated pathways. Toxicol Appl Pharmacol. 2008;231:135–41. doi: 10.1016/j.taap.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol. 2004;201:32–9. doi: 10.1016/j.taap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Tsai SH, Hsieh MS, Chen L, Liang YC, Lin JK, Lin SY. Suppression of Fas ligand expression on endothelial cells by arsenite through reactive oxygen species. Toxicol Lett. 2001;123:11–9. doi: 10.1016/s0378-4274(01)00373-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen SC, Tsai MH, Wang HJ, Yu HS, Chang LW. Involvement of substance P and neurogenic inflammation in arsenic-induced early vascular dysfunction. Toxicol Sci. 2007;95:82–8. doi: 10.1093/toxsci/kfl136. [DOI] [PubMed] [Google Scholar]
- 27.Pereira FE, Coffin JD, Beall HD. Activation of protein kinase C and disruption of endothelial monolayer integrity by sodium arsenite-Potential mechanism in the development of atherosclerosis. Toxicol Appl Pharmacol. 2007;220:164–77. doi: 10.1016/j.taap.2006.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsou TC, Tsai FY, Hsieh YW, Li LA, Yeh SC, Chang LW. Arsenite induces endothelial cytotoxicity by down-regulation of vascular endothelial nitric oxide synthase. Toxicol Appl Pharmacol. 2005;208:277–84. doi: 10.1016/j.taap.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Balakumar P, Kaur J. Arsenic exposure and cardiovascular disorders: an overview. Cardiovasc Toxicol. 2009;9:169–76. doi: 10.1007/s12012-009-9050-6. [DOI] [PubMed] [Google Scholar]
- 30.Lee MY, Lee YH, Lim KM, Chung SM, Bae ON, Kim H, et al. Inorganic arsenite potentiates vasoconstriction through calcium sensitization in vascular smooth muscle. Environ Health Perspect. 2005;113:1330–5. doi: 10.1289/ehp.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cifuentes F, Bravo J, Norambuena M, Stegen S, Ayavire A, Palacios J. Chronic exposure to arsenic in tap water reduces acetylcholine-induced relaxation in the aorta and increases oxidative stress in female rats. Int J Toxicol. 2009;28:534–41. doi: 10.1177/1091581809345924. [DOI] [PubMed] [Google Scholar]
- 32.Raghu KG, Yadav GK, Singh R, Prathapan A, Sharma S, Bhadauria S. Evaluation of adverse cardiac effects induced by arsenic trioxide, a potent anti-APL drug. J Environ Pathol Toxicol Oncol. 2009;28:241–52. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i3.60. [DOI] [PubMed] [Google Scholar]
- 33.Jindal S, Singh M, Balakumar P. Effect of bis (maltolato) oxovanadium (BMOV) in uric acid and sodium arsenite-induced vascular endothelial dysfunction in rats. Int J Cardiol. 2008;128:383–91. doi: 10.1016/j.ijcard.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Kaur T, Goel RK, Balakumar P. Effect of rosiglitazone in sodium arsenite-induced experimental vascular endothelial dysfunction. Arch Pharm Res. 2010;33:611–8. doi: 10.1007/s12272-010-0416-x. [DOI] [PubMed] [Google Scholar]
- 35.Wauson EM, Langan AS, Vorce RL. Sodium arsenite inhibits and reverses expression of adipogenic and fat cell-specific genes during in vitro adipogenesis. Toxicol Sci. 2002;65:211–9. doi: 10.1093/toxsci/65.2.211. [DOI] [PubMed] [Google Scholar]
- 36.Tseng CH. The potential biological mechanisms of arsenic-induced diabetes mellitus. Toxicol Appl Pharmacol. 2004;197:67–83. doi: 10.1016/j.taap.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Bodwell JE, Kingsley LA, Hamilton JW. Arsenic at very low concentrations alters glucocorticoid receptor (GR)-mediated gene activation but not GR-mediated gene repression: complex dose-response effects are closely correlated with levels of activated GR and require a functional GR DNA binding domain. Chem Res Toxicol. 2004;17:1064–76. doi: 10.1021/tx0499113. [DOI] [PubMed] [Google Scholar]
- 38.Pal S, Chatterjee AK. Prospective protective role of melatonin against arsenic-induced metabolic toxicity in wistar rats. Toxicology. 2005;208:25–33. doi: 10.1016/j.tox.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Díaz-Villaseñor A, Sánchez-Soto MC, Cebrián ME, Ostrosky-Wegman P, Hiriart M. Sodium arsenite impairs insulin secretion and transcription in pancreatic beta-cells. Toxicol Appl Pharmacol. 2006;214:30–4. doi: 10.1016/j.taap.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 40.Izquierdo-Vega JA, Soto CA, Sanchez-Peña LC, De Vizcaya-Ruiz A, Del Razo LM. Diabetogenic effects and pancreatic oxidative damage in rats subchronically exposed to arsenite. Toxicol Lett. 2006;160:135–42. doi: 10.1016/j.toxlet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Paul DS, Harmon AW, Devesa V, Thomas DJ, Stýblo M. Molecular mechanisms of the diabetogenic effects of arsenic: inhibition of insulin signaling by arsenite and methylarsonous acid. Environ Health Perspect. 2007;115:734–42. doi: 10.1289/ehp.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav RS, Shukla RK, Sankhwar ML, Patel DK, Ansari RW, Pant AB, et al. Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in rats. Neurotoxicology. 2010;31:533–9. doi: 10.1016/j.neuro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Brinkel J, Khan MH, Kraemer A. A systematic review of arsenic exposure and its social and mental health effects with special reference to Bangladesh. Int J Environ Res Public Health. 2009;6:1609–19. doi: 10.3390/ijerph6051609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piao F, Ma N, Hiraku Y, Murata M, Oikawa S, Cheng F, et al. Oxidative DNA damage in relation to neurotoxicity in the brain of mice exposed to arsenic at environmentally relevant levels. J Occup Health. 2005;47:445–9. doi: 10.1539/joh.47.445. [DOI] [PubMed] [Google Scholar]
- 45.Felix K, Manna SK, Wise K, Barr J, Ramesh GT. Low levels of arsenite activates nuclear factor-kappaB and activator protein-1 in immortalized mesencephalic cells. J Biochem Mol Toxicol. 2005;19:67–77. doi: 10.1002/jbt.20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yip SF, Yeung YM, Tsui EY. Severe neurotoxicity following arsenic therapy for acute promyelocytic leukemia: potentiation by thiamine deficiency. Blood. 2002;99:3481–2. doi: 10.1182/blood-2001-12-0325. [DOI] [PubMed] [Google Scholar]
- 47.Gopalkrishnan A, Rao MV. Amelioration by vitamin A upon arsenic induced metabolic and neurotoxic effects. J Health Sci. 2006;52:568–77. [Google Scholar]
- 48.Vahidnia A, Romijn F, Tiller M, van der Voet GB, de Wolff FA. Arsenic-induced toxicity: effect on protein composition in sciatic nerve. Hum Exp Toxicol. 2006;25:667–74. doi: 10.1177/0960327106070671. [DOI] [PubMed] [Google Scholar]
- 49.Bardullas U, Limón-Pacheco JH, Giordano M, Carrizales L, Mendoza-Trejo MS, Rodríguez VM. Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in mice. Toxicol Appl Pharmacol. 2009;239:169–77. doi: 10.1016/j.taap.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Dwivedi N, Flora SJ. Concomitant exposure to arsenic and organophosphates on tissue oxidative stress in rats. Food Chem Toxicol. 2011;49:1152–9. doi: 10.1016/j.fct.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Rodríguez VM, Carrizales L, Mendoza MS, Fajardo OR, Giordano M. Effects of sodium arsenite exposure on development and behavior in the rat. Neurotoxicol Teratol. 2002;24:743–50. doi: 10.1016/s0892-0362(02)00313-6. [DOI] [PubMed] [Google Scholar]
- 52.Namgung U, Xia Z. Arsenic induces apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinases. Toxicol Appl Pharmacol. 2001;174:130–8. doi: 10.1006/taap.2001.9200. [DOI] [PubMed] [Google Scholar]
- 53.Vahidnia A, Romijn F, van der Voet GB, de Wolff FA. Arsenic-induced neurotoxicity in relation to toxicokinetics: effects on sciatic nerve proteins. Chem Biol Interact. 2008;176:188–95. doi: 10.1016/j.cbi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Patlolla AK, Tchounwou PB. Serum acetyl cholinesterase as a biomarker of arsenic induced neurotoxicity in sprague-dawley rats. Int J Environ Res Public Health. 2005;2:80–3. doi: 10.3390/ijerph2005010080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo JH, Qiu ZQ, Shu WQ, Zhang YY, Zhang L, Chen JA. Effects of arsenic exposure from drinking water on spatial memory, ultra-structures and NMDAR gene expression of hippocampus in rats. Toxicol Lett. 2009;184:121–5. doi: 10.1016/j.toxlet.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 56.Krüger K, Straub H, Hirner AV, Hippler J, Binding N, Musshoff U. Effects of monomethylarsonic and monomethylarsonous acid on evoked synaptic potentials in hippocampal slices of adult and young rats. Toxicol Appl Pharmacol. 2009;236:115–23. doi: 10.1016/j.taap.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 57.Ríos R, Zarazúa S, Santoyo ME, Sepúlveda-Saavedra J, Romero-Díaz V, Jiménez V, et al. Decreased nitric oxide markers and morphological changes in the brain of arsenic-exposed rats. Toxicology. 2009;261:68–75. doi: 10.1016/j.tox.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 58.Burton CA, Hatlelid K, Divine K, Carter DE, Fernando Q, Brendel K, et al. Glutathione effects on toxicity and uptake of mercuric chloride and sodium arsenite in rabbit renal cortical slices. Environ Health Perspect. 1995;103:81–4. doi: 10.1289/ehp.95103s181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parrish AR, Zheng XH, Turney KD, Younis HS, Gandolfi AJ. Enhanced transcription factor DNA binding and gene expression induced by arsenite or arsenate in renal slices. Toxicol Sci. 1999;50:98–105. doi: 10.1093/toxsci/50.1.98. [DOI] [PubMed] [Google Scholar]
- 60.Sasaki A, Oshima Y, Fujimura A. An approach to elucidate potential mechanism of renal toxicity of arsenic trioxide. Exp Hematol. 2007;35:252–62. doi: 10.1016/j.exphem.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Kimura A, Ishida Y, Hayashi T, Wada T, Yokoyama H, Sugaya T, et al. Interferon-gamma plays protective roles in sodium arsenite-induced renal injury by up-regulating intrarenal multidrug resistance-associated protein 1 expression. Am J Pathol. 2006;169:1118–28. doi: 10.2353/ajpath.2006.060024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saxena PN, Anand S, Saxena N, Bajaj P. Effect of arsenic trioxide on renal functions and its modulation by Curcuma aromatica leaf extract in albino rat. J Environ Biol. 2009;30:527–31. [PubMed] [Google Scholar]
- 63.Das AK, Bag S, Sahu R, Dua TK, Sinha MK, Gangopadhyay M, et al. Protective effect of Corchorus olitorius leaves on sodium arsenite-induced toxicity in experimental rats. Food Chem Toxicol. 2010;48:326–35. doi: 10.1016/j.fct.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 64.Nandi D, Patra RC, Swarup D. Oxidative stress indices and plasma biochemical parameters during oral exposure to arsenic in rats. Food Chem Toxicol. 2006;44:1579–84. doi: 10.1016/j.fct.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 65.Kokilavani V, Devi MA, Sivarajan K, Panneerselvam C. Combined efficacies of DL-alpha-lipoic acid and meso 2, 3 dimercaptosuccinic acid against arsenic induced toxicity in antioxidant systems of rats. Toxicol Lett. 2005;160:1–7. doi: 10.1016/j.toxlet.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 66.Bashir S, Sharma Y, Irshad M, Nag TC, Tiwari M, Kabra M, et al. Arsenic induced apoptosis in rat liver following repeated 60 days exposure. Toxicology. 2006;217:63–70. doi: 10.1016/j.tox.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki T, Tsukamoto I. Arsenite induces apoptosis in hepatocytes through an enhancement of the activation of Jun N-terminal kinase and p38 mitogen-activated protein kinase caused by partial hepatectomy. Toxicol Lett. 2006;165:257–64. doi: 10.1016/j.toxlet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Jain A, Yadav A, Bozhkov AI, Padalko VI, Flora SJ. Therapeutic efficacy of silymarin and naringenin in reducing arsenic-induced hepatic damage in young rats. Ecotoxicol Environ Saf. 2011;74:607–14. doi: 10.1016/j.ecoenv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 69.Flora SJ, Mehta A, Gupta R. Prevention of arsenic-induced hepatic apoptosis by concomitant administration of garlic extracts in mice. Chem Biol Interact. 2009;177:227–33. doi: 10.1016/j.cbi.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 70.Xu Y, Wang H, Wang Y, Zheng Y, Sun G. Effects of folate on arsenic toxicity in Chang human hepatocytes: Involvement of folate antioxidant properties. Toxicol Lett. 2010;195:44–50. doi: 10.1016/j.toxlet.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 71.Li GX, Pei QL, Gao Y, Liu KM, Nie JS, Han G, et al. Protective effects of hepatocellular canalicular conjugate export pump (Mrp2) on sodium arsenite-induced hepatic dysfunction in rats. Exp Toxicol Pathol. 2007;58:447–53. doi: 10.1016/j.etp.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Dopp E, Hartmann LM, Florea AM, von Recklinghausen U, Pieper R, Shokouhi B, et al. Uptake of inorganic and organic derivatives of arsenic associated with induced cytotoxic and genotoxic effects in Chinese hamster ovary (CHO) cells. Toxicol Appl Pharmacol. 2004;201:156–65. doi: 10.1016/j.taap.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 73.Yang C, Frenkel K. Arsenic-mediated cellular signal transduction, transcription factor activation, and aberrant gene expression: implications in carcinogenesis. J Environ Pathol Toxicol Oncol. 2002;21:331–42. [PubMed] [Google Scholar]
- 74.Liu J, Waalkes M. Focal adhesion kinase as a potential target in arsenic toxicity. Toxicol Sci. 2005;84:212–3. doi: 10.1093/toxsci/kfi111. [DOI] [PubMed] [Google Scholar]
- 75.Kinoshita A, Wanibuchi H, Wei M, Yunoki T, Fukushima S. Elevation of 8-hydroxydeoxyguanosine and cell proliferation via generation of oxidative stress by organic arsenicals contributes to their carcinogenicity in the rat liver and bladder. Toxicol Appl Pharmacol. 2007;221:295–305. doi: 10.1016/j.taap.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki S, Arnold LL, Pennington KL, Kakiuchi-Kiyota S, Cohen SM. Effects of co-administration of dietary sodium arsenite and an NADPH oxidase inhibitor on the rat bladder epithelium. Toxicology. 2009;261:41–6. doi: 10.1016/j.tox.2009.04.042. [DOI] [PubMed] [Google Scholar]
- 77.Simeonova PP, Wang S, Toriuma W, Kommineni V, Matheson J, Unimye N, et al. Arsenic mediates cell proliferation and gene expression in the bladder epithelium: association with activating protein-1 transactivation. Cancer Res. 2000;60:3445–53. [PubMed] [Google Scholar]
- 78.Bates MN, Smith AH, Cantor KP. Case-control study of bladder cancer and arsenic in drinking water. Am J Epidemiol. 1995;141:523–30. doi: 10.1093/oxfordjournals.aje.a117467. [DOI] [PubMed] [Google Scholar]
- 79.Hays AM, Srinivasan D, Witten ML, Carter DE, Lantz RC. Arsenic and cigarette smoke synergistically increase DNA oxidation in the lung. Toxicol Pathol. 2006;34:396–404. doi: 10.1080/01926230600824926. [DOI] [PubMed] [Google Scholar]
- 80.Klein CB, Leszczynska J, Hickey C, Rossman TG. Further evidence against a direct genotoxic mode of action for arsenic-induced cancer. Toxicol Appl Pharmacol. 2007;222:289–97. doi: 10.1016/j.taap.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tapio S, Grosche B. Arsenic in the aetiology of cancer. Mutat Res. 2006;612:215–46. doi: 10.1016/j.mrrev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Biswas J, Sinha D, Mukherjee S, Roy S, Siddiqi M, Roy M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum Exp Toxicol. 2010;29:513–24. doi: 10.1177/0960327109359020. [DOI] [PubMed] [Google Scholar]


