Abstract
This experiment was designed to investigate the extent of peroxidative changes and histological alterations in the myocardium of rats exposed to high fluoride for two generations, in addition to ameliorative role of selenium and vitamin E on the above indices. Adult albino Wistar rats were given fluoride through drinking water (200 ppm F) and maintained subsequently for two generations, while they were exposed to fluoride throughout the experiment. Fluoride treatment significantly increased the lipid peroxidation and decreased the activity of antioxidant enzymes, viz., catalase, superoxide dismutase, and glutathione level in auricle and ventricle regions of the heart. Decreased feed and water consumption, organ somatic index and marginal drop in body growth rate were observed. Decreased antioxidant enzymes and increased malondialdehyde levels might be related to oxidative damage that occurs variably in the myocardium of rats. Biochemical changes were supported by the histological observations, which also revealed that chronic exposure to fluoride causes damage to the myocardium. Results of this study can be taken as an index of cardio-toxicity in rats exposed to water fluoridation. Further, oral supplementation of selenium and vitamin E not only inhibited oxidative stress but also enhanced the activities of antioxidant enzymes. Administration of antioxidants during fluoride exposure significantly overcame cardiac fluoride toxicity and therefore may be a therapeutic strategy for fluorotic victims.
Keywords: Antioxidant supplementation, chronic fluorosis, oxidative stress, second generation
INTRODUCTION
Fluoride in drinking water is known to cause both beneficial and detrimental effects on health. When consumed in excess, besides inducing skeletal and dental fluorosis, it is also known to cause damage to major organs of the body including the heart.[1] In soft tissues, it interferes by inhibiting numerous enzymes, finally leading to the production of free radicals.[2] Its accumulation in soft tissues causes injury by reducing the potential of scavenging free radicals which critically injure the biological membranes. Intensified free radical production or disturbed antioxidant level leads to oxidative stress which is known to be a key etiopathological factor in a variety of cardiac diseases such as heart failure and ischemic heart disease. Therefore, antioxidant balance is very essential in protecting the heart to perform its normal functions. Studies of Cicek et al.[1] indicated a slow and altered development of metabolic, functional and structural damages in myocardium as a consequence of fluoride exposure. Okushi[3] found a higher incidence of myocardial damage followed by changes in the electrocardiogram and cardiac dilation (roentgen cardiac studies) in inhabitants of a high fluoride zone, where the drinking water fluoride levels were 6-13 ppm. Electrocardiogram studies by Takamori et al.[4] showed a direct relationship between increased myocardial damage and mottled dental enamel where the fluoride level ranged between 0.5 and 6.2 ppm in residents of a Japanese village. Changes in the electrocardiogram and heart enlargement in children were linked to fluoride in the drinking water, but there was less certainty about the nature of these effects reported. Pribilla[5] noticed fibrous necrosis, dissolution of nuclei, fibrillolysis, interstitial edema, minute hemorrhages, infiltration of histiocytes, lymphocytes and granulocytes in the myocardium of patients with acute silicofluoride intoxification. Okushi[3] reported cloudy swellings, infiltration with round cells, thickening of adventitia, diffuse hemorrhages, vacuolar and colloid degeneration of myocardium in rabbits fed with 10-100 mg of sodium fluoride (NaF) for 132 days. Although acute toxic effects of fluoride on the hard tissues are fairly known, information about the exact nature of these effects as well as chronic effects on heart tissue is still scanty.
Chinoy et al.[6] in their study found that supplementing the diet with antioxidants reversed the toxic effects of fluoride in the body. Selenium, at a certain concentration range, played a role in excreting high fluoride, adjusting the disorder of free radicals and lipid metabolism, thereby promoting the recovery of fluorosis in rats. Keeping in view the paucity of information in relation to high fluoride exposure in population residing in endemic areas and its impact on heart tissue, the present study was undertaken. The significance of this study is to evaluate the protective effects of selenium and vitamin E through their antioxidant properties as well as histological alterations in the myocardium of rats exposed to fluoride for two generations.
MATERIALS AND METHODS
Chemicals
5,5-Dithio-bis-2-nitrobenzoic acid (Ellman's reagent), epinephrine and glutathione were procured from Sigma-Aldrich Ltd. Bangalore, India and other chemicals (AR grade) were obtained from Merck Ltd. Mumbai, India.
Preparation of fluoride water
A stock of 1000 ppm sodium fluoride solution was prepared by dissolving 2.21 g of sodium fluoride in 1 l of tap water. To prepare 200 ppm fluoride water, 200 ml of the stock solution was taken and made up to 1 l with tap water.
Animals
The protocol of this study was approved by the Institutional Animal Ethics Committee (IAEC), Bangalore University, Bangalore, India. Healthy adult female Wistar albino rats of 3 months age, weighing 170-200 g, and male rats weighing 200-250 g were procured from Sri Raghavendra Enterprises, Bangalore, acclimated for a week, and maintained at room temperature of 25 ± 2°C with 12-hour dark-light cycle. Animals were fed with standard rodent diet (Amruth feeds, Sangli, India). There was no water and light restriction throughout the experimental period.
Experimental design
To evaluate the impact of fluoride on second generation rats, twelve adult Wistar strain (F0) were selected and maintained in four cages, each of which consisted of two females (weighing 170-200 g) and one male (weighing 200-250 g). The rats of each group were housed in a cage for a night, and then the following day, the vaginal plugs of the females of each group were examined to confirm pregnancy. Eight females were identified as pregnant, and these were selected to obtain the first generation rats (F1). These eight pregnant rats were then divided into two groups of four rats each: Group I (control) was provided with tap water ad libitum (<1 ppm F) and Group II was given 200 ppm fluoride in drinking water during the gestational period.
After the gestation period of 21 ± 2 days, there were thirty six pups in the litters of the two treatment groups. The mothers received fluoridated water during the lactation period (21 days). The pups born to F1 generation had free access to the fluoridated water until they became mature. Only certain numbers of adult rats from the first generation in each group were selected to be used further in the study on the basis of being healthy. Four females and two males from Group I, and eight females and four males from Group II were housed for mating.Six female rats were identified as pregnant and were subjected to the same experimental procedure as above. The pups so obtained were considered as the second generation rats (F2). There were fifty six pups altogether. The treatment groups were exposed to 200 ppm NaF through mother rat (during lactation through mother's milk and in post-suckling period directly exposed through fluoride water) for 3 months and divided into four groups of six rats each, i.e., one control and three experimental groups. Further, Group III and IV animals were exposed to antioxidants for 15 days with the doses indicated below:
Group I: Control
Group II: Experimental (200 ppm NaF)
Group III: Experimental (200 ppm NaF) + supplemented with selenium (diluted in water) for 15 days (5 mg/kg body weight/day)
Group IV: Experimental (200 ppm NaF) + supplemented with vitamin E (diluted in olive oil) for 15 days (10 mg/kg body weight /day)
Animals were sacrificed after the completion of chronic fluoride exposure and antioxidant supplementation, and heart tissues were quickly dissected, separated into auricle and ventricle regions, homogenized in phosphate buffer (pH 7.2) for biochemical estimations [Figures 1–4].
Figure 1.
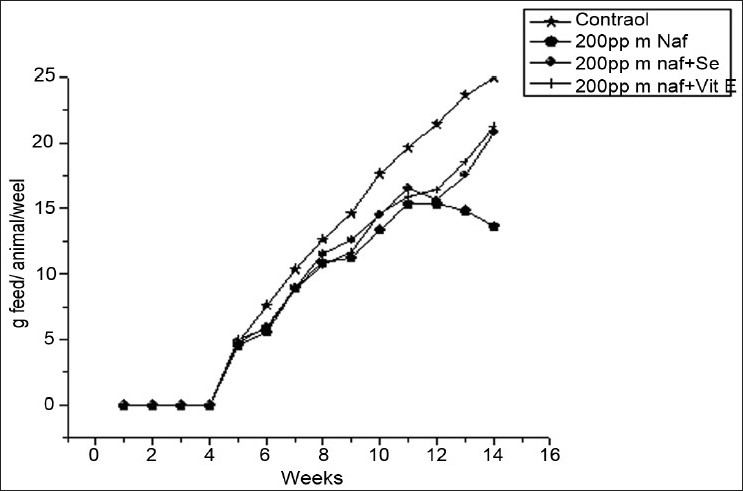
Average food consumption in rats on fluoride and antioxidant supplementation
Figure 4.
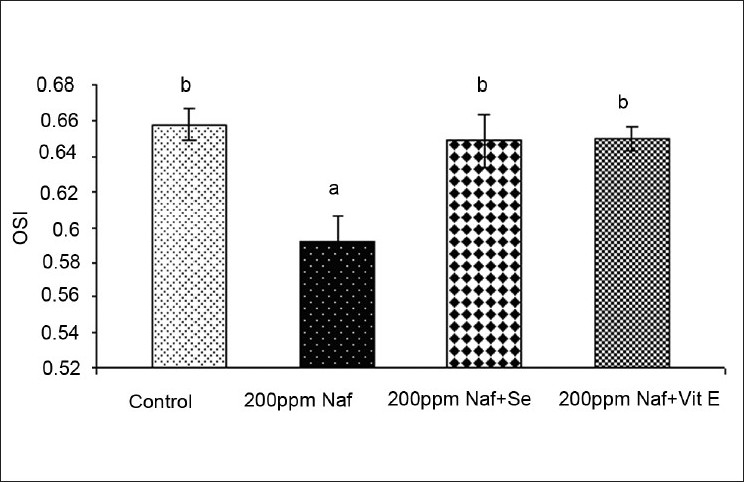
Changes in the organ somatic index in rats on fluoride and antioxidant supplementation
Figure 2.
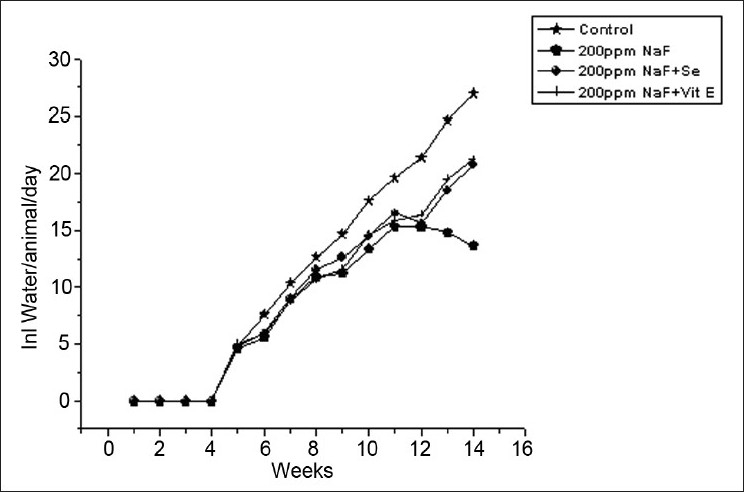
Average fluid consumption in rats on fluoride and antioxidant supplementation
Figure 3.
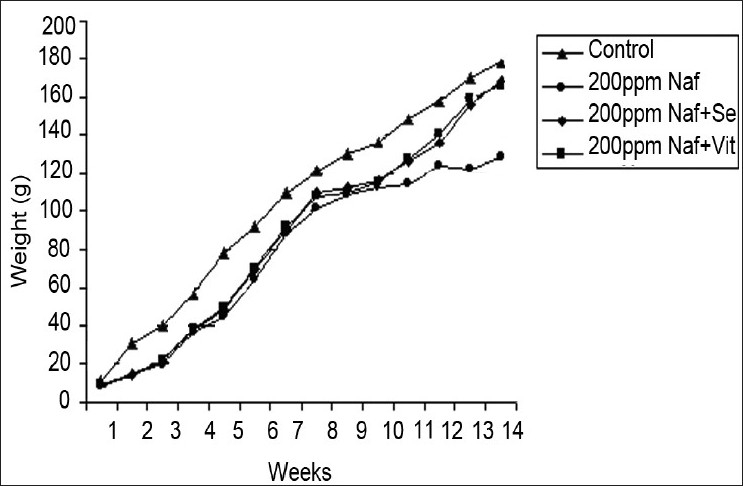
Changes in the body weight in rats as a consequence of fluoride and antioxidant supplementation
Histopathological analysis
The heart tissues harvested were immediately fixed in Bouin's fluid to prevent autolysis and putrefaction. Tissue processing was done by fixation, dehydration, embedding, sectioning and staining with hematoxylin and eosin. The micrographs of the relevant stained sections were subsequently taken with the aid of a light microscope (at magnification 40×).
In vivo biochemical assays
Lipid peroxidation (LPO): LPO product was estimated by measurement of thiobarbituric acid reactive substances (TBARS) using the method of Niehaus and Samuelsson.[7] The pink chromogen produced by the reaction of thiobarbituric acid with malondialdehyde (MDA), a secondary product of LPO, was estimated at 535 nm.
Catalase (CAT, EC 1.11.1.6): CAT activity was measured as described by Aebi.[8] The decomposition of hydrogen peroxide was monitored by measuring the decrease in absorbance at 240 nm.
Superoxide dismutase (SOD EC 1.15.1.1.): SOD activity was assayed by measuring the inhibition of epinephrine auto-oxidation as described by Misra and Fridovich.[9]
Reduced glutathione (GSH): Reduced glutathione content was determined by the method of Ellman[10] based on the development of a yellow color while adding DTNB to compounds containing sulfhydryl groups.
Protein assay: Protein content was estimated by the method of Lowry et al.,[11] using bovine serum albumin as standard.
Statistical analysis
Values are expressed as Mean ± SE. Statistical analysis was done by one-way analysis of variance (ANOVA) with Duncan's multiple range test (DMRT) post hoc at P < 0.05 level of significance by using SPSS software (17.0 version).
RESULTS
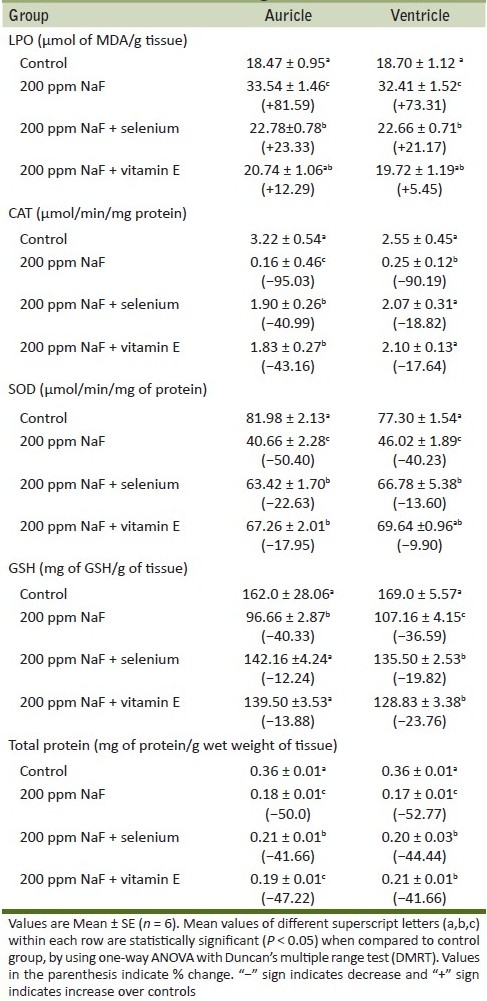
The feed and water consumption in fluoride treated pregnant rats decreased considerably as a result of 200 ppm fluoride exposure. Although there was no much difference in the litter size between the two generations on fluoride exposure, a slight decrease in body weight of pups at parturition was observed when compared to controls. Considerable decrease in feed and water intake was observed in the fluoride exposed group. The alterations in oxidative enzyme activities in auricle and ventricle regions of rat due to NaF exposure and antioxidant supplementation are shown in Table 1. NaF treatment resulted in significant (P < 0.05) enhanced level of MDA (1.81 and 1.72 fold), while decreased activities of CAT (20.12 and 10.20 fold), SOD (2.01 and 1.67 fold) and GSH (1.67 and 1.57 fold) were noticed in auricle and ventricle regions, respectively. The supplementation of selenium and vitamin E to fluoride intoxicated animals reversed pro and antioxidant systems toward near normal.
Table 1.
Changes in oxidative stress indices on fluoride and antioxidant supplementation in auricle and ventricle regions of heart
Hematoxylin-eosin stained sections were evaluated under light microscope. Figures 5 and 6 depict the normal histology of auricle and ventricle regions of heart. Disorganization of cells in the successive layers of auricle and ventricle was seen on 200 ppm fluoride exposure. Changes like cloudy swelling, sarcoplasmic vacuolization, small hemorrhages, interstitial edema, fibrous necrosis and dissolution of nuclei, fibrillolysis, edematous fluid in the interstitial spaces and extensive vacuolization of both auricle and ventricle regions were observed on fluoride exposure, while selenium and vitamin E treatment to experimental fluoride fed animals showed considerable favor in reducing the above alterations.
Figure 5.
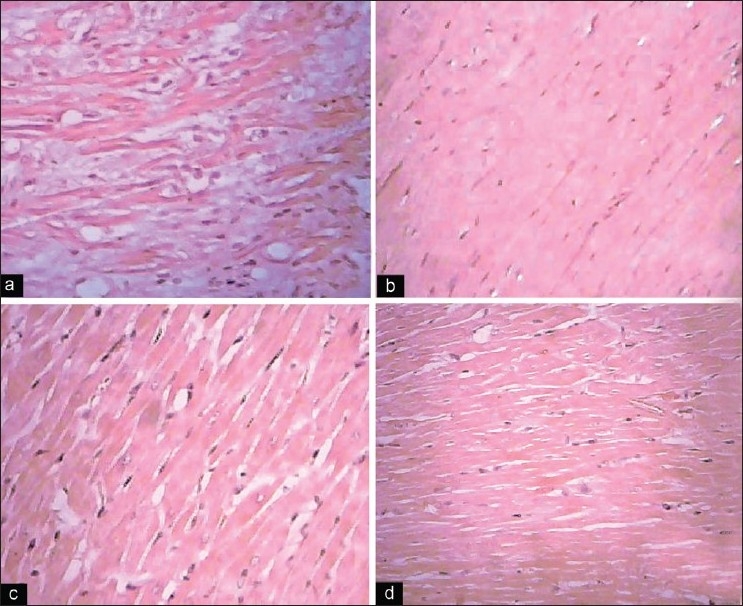
Photomicrographs showing histopathological changes in auricle region of the heart in control and experimental animals (H and E, ×40). (a) Control showing normal architecture; (b) 200 ppm NaF exposed rats; (c) 200 ppm NaF exposed rats supplemented with selenium for 15 days; (d) 200 ppm NaF exposed rats supplemented with vitamin E for 15 days
Figure 6.
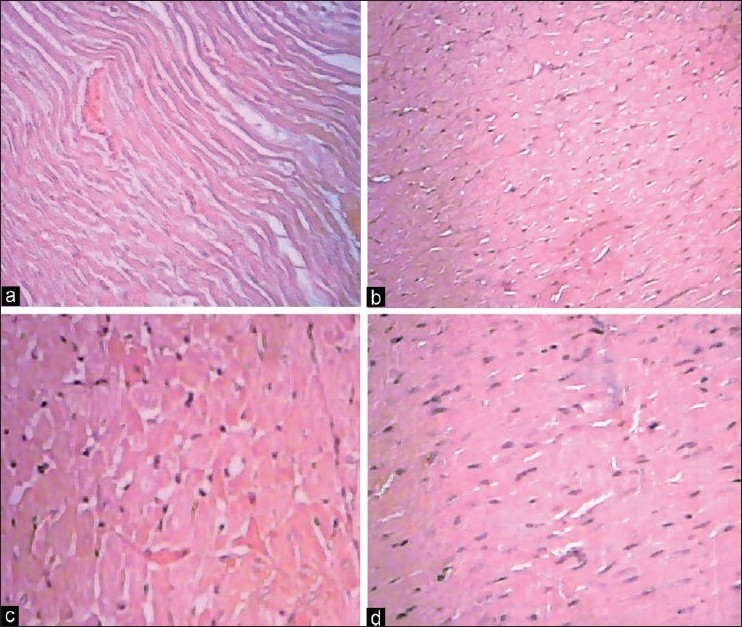
Photomicrographs showing histopathological changes in ventricle region of the heart in control and experimental animals (H and E, ×40). (a) Control showing normal architecture; (b) 200 ppm NaF exposed rats; (c) 200 ppm NaF exposed rats supplemented with selenium for 15 days; (d) 200 ppm NaF exposed rats supplemented with vitamin E for 15 days
DISCUSSION
In this study, excessive ingestion of fluoride considerably decreased the animal growth symptomized by decreased appetite leading to poor growth rate. This decrease in the body weight can be attributed to decreased consumption of feed and water, which ultimately lead to reduced growth rate, whereas administration of vitamin E and selenium to NaF exposed rats caused no marked changes in the body weight. On the contrary, Collins et al.,[12] while assessing the effects of NaF exposure in multigenerational rats, showed no alterations in body weight, whereas studies of Verma and Guna-Sherlin[13] found significant amelioration in body weight and feed consumption in rats on administration of vitamin E. The decrease found in body weight in the present investigation on fluoride ingestion could be due to primary malnutrition caused by fluoride by displacing other nutrients present in the diet (minerals/elements) and secondary malnutrition too results either from mal-digestion or mal-absorption of nutrients. In addition, gastrointestinal complications could have altered nutritional abilities and deficiencies virtually on all the nutrients.
SOD offers first line of defense against the superoxide radicals. It is one of the most important intracellular antioxidant enzymes, which has an antitoxic effect against superoxide anion that causes impairment to macromolecules like proteins, lipids, carbohydrates and nucleotides. Similarly, CAT, a ferroporphyrin located mainly in peroxisomes, lysosomes and mitochondria, helps to protect the biological tissues by avoiding the accumulation of hydrogen peroxide by dismutating it to form water and oxygen. In general, a decreased SOD and CAT level suggests an increased demand of these enzymes than the amount being produced on toxic exposure. Available literature indicates that fluoride has a direct effect on the antioxidant enzymes in the soft tissue functioning and creates an imbalance in pro-oxidant and antioxidants levels. Consumption of high doses of fluoride has been found to interfere with the cardiac system of animals, causing irregularities and low blood pressure due to increase in oxidative stress.[4,14] In the present study, activities of CAT and SOD and GSH content were decreased in both auricle and ventricle regions on fluoride exposure. This decrease can be attributed to direct action of fluoride on the enzyme rather than to increased generation of free radicals induced by fluoride intoxication and the changes in the antioxidant levels might be due to the adaptive response.
LPO as well as oxidation of functional proteins has been suggested to affect cardiac and endothelial cell function.[15] The enhanced cardiac LPO observed on fluoride administration could play an important role in the impairment of myocardial function. Further, injury caused to heart muscle appears to be increased on persistent oxidative stress caused by chronic fluoride exposure,[16] probably by inhibiting the oxidation of fatty acids thus increasing the level of MDA. Oxygen radicals might have also caused the LPO through a chain reaction which ultimately leads to tissue damage.
Dietary supplementation of selenium and vitamin E increased the oxidative stability of cardiac tissues by increasing the endogenous antioxidants. Altered serum enzyme activities and lipids were observed in fluorosis and their recovery was pronounced on selenium supplementation.[17] It appears that the interference of selenium in balancing the redox state and controlling the activation of kinases and transcription factors might have helped in bringing amelioration in the level of affected antioxidant enzyme status. It can be drawn from this study that selenium plays a critical role in the maintenance of proper functioning and acts as a potent protective agent for cardiac tissue through the expression of selenoproteins as they involve in regulation of redox status under physiological conditions. Similar results were observed in earlier studies conducted in discrete regions of brain samples of fluoride treated rats.[18]
Vitamin E, the most effective chain-breaking antioxidant within the cell membrane, offers protection to membrane fatty acids from LPO.[19] Studies of Crewe et al.[20] reported beneficial role of vitamin E against toxicity induced by environmental toxicants. In comparison to vitamin E, less amelioration was shown in selenium-treated, fluoride-exposed groups. The present study also shows convincing results indicating inhibition of peroxidation of membrane lipids by vitamin E and scavenging of lipid peroxyl radicals, which later gets converted into a tocopheroxyl radical.[21] Results of this study corroborate the findings of earlier studies which indicated involvement of vitamin E in reducing the level of free radicals induced by fluoride in hepatic and neuronal cells.[22] The principal role of selenium has been associated with the control of LPO, as selenium supplementation alleviates LPO in fluoride exposed animals.[23] Administration of antioxidants is beneficial in promoting the recovery from fluoride induced toxicity, perhaps by augmentation of glutathione system; its involvement in detoxification process might help to delay LPO rate.[24]
Evidence has been accumulated indicating that chronic fluoride consumption leads to direct or indirect changes in the viability of soft tissue functioning via oxidative stress. The results of histological sections in the present study showed signs of severe cloudy swelling, sarcoplasmic vacuolization, small hemorrhages, interstitial edema, fibrous necrosis, dissolution of nuclei, fibrillolysis, edematous fluid in the interstitial spaces and extensive vacuolization of both auricle and ventricle regions on exposure to chronic fluoride. These results are in line with the earlier reports of Takamori,[4] who reported the presence of cloudy swellings, vacuolar degeneration, round-cell infiltration and hemorrhages in rats exposed to fluoride for 1 month. Similarly, in rabbits, regressive degeneration, cellular infiltration, hyper-anemia, hemorrhages and thickening of the vessel walls in the heart muscles were reported. In this study, treatment with selenium and vitamin E reduced the sensitivity to oxidative stress by scavenging the free radicals, thereby minimizing the cardiac damage. The aforementioned observations suggest that dietary supplementation of antioxidants might be beneficial for counteracting the toxic effects of fluoride. Thus, it can be drawn that vitamin E and selenium are significantly important in reducing chronic fluorosis and detoxification of heart tissue against the toxic effects of fluoride by preventing the oxidative stress caused due to fluoride.
ACKNOWLEDGEMENTS
University Grants Commission, New Delhi, India for research grants (F.No. 31-220/ 2005 (SR) dated 31-03-2006 & MRP(S)/139/08-09/KABA027/UGC/SWRO).
Footnotes
Source of Support: University Grants Commission, New Delhi, India (F.No. 31-220/ 2005 (SR) dated 31-03-2006 & MRP(S)/139/08-09/KABA027/UGC/SWRO).
Conflict of Interest: The authors report no declarations of interest.
REFERENCES
- 1.Cicek E, Aydin G, Akdogan M, Okutan H. Effects of chronic ingestion of sodium fluoride on myocardium in a second generation of rats. Hum Exp Toxicol. 2005;24:79–87. doi: 10.1191/0960327105ht505oa. [DOI] [PubMed] [Google Scholar]
- 2.Waldbott GL, Burgstahler AW, McKinney HL, editors. Fluoridation: the great dilemma. Lawrence, Kansas: Coronado Press; 1978. [Google Scholar]
- 3.Okushi I. Changes of the heart muscle due to chronic fluorosis Part I.Electrocardiogram and cardiac X-rays in inhabitants of high fluoride zone. Shikoku Acta Med. 1954;5:159–65. [Google Scholar]
- 4.Takamori T, editor. The toxicology of fluorine symposium. Bern, Switzerland, Basel/Stuttgart: Schabe; 1962. Oct 15-17, The heart changes of growing albino rats fed on varied contents of fluorine. [Google Scholar]
- 5.Pribilla O. Four cases of acute silicofluoride intoxication, Clinical and pathological findings. Fluoride. 1968;1:102–9. [Google Scholar]
- 6.Chinoy NJ, Tripathi G, editors. Modern trends in environmental biology. New Delhi: 2002. Studies on fluoride, aluminium and arsenic toxicity in mammals and amelioration by some antidotes. [Google Scholar]
- 7.Niehaus WG, Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 8.Aebi H. Catalase in vitro. Met Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 9.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 10.Ellman GL. Tissue sulfhydrl groups. Arch Biochem Biophysics. 1952;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 11.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 12.Collins TF, Sprando RL, Black TN, Shackelford ME, Olejnik N, Ames MJ, et al. Developmental toxicity of sodium fluoride measured during multiple generations. Food Chem Toxicol. 2001;39:867–76. doi: 10.1016/s0278-6915(01)00033-3. [DOI] [PubMed] [Google Scholar]
- 13.Verma RJ, Guna-Sherlin DM. Sodium fluoride-induced hypoproteinemia and hypoglycemia in parental and F(1)-generation rats and amelioration by vitamin. Food Chem Toxicol. 2002;40:1781–8. doi: 10.1016/s0278-6915(02)00170-9. [DOI] [PubMed] [Google Scholar]
- 14.Leone NC, Geever EF, Moran NC. Acute and subacute toxicity studies of sodium fluoride in animals. Public Health Rep. 1956;71:459–67. [PMC free article] [PubMed] [Google Scholar]
- 15.Gutteridge JM, Halliwell B, editors. Antioxidants in nutrition, health and diseases. 1st ed. New York: Oxford University Press; 1994. [Google Scholar]
- 16.Halliwell B, Gutteridge JM. Free radicals, antioxidants, and human disease: where are we now. J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 17.Zofia M, Mokrzynska AM, Juzyszyn Z. Effects of selenium on serum lipids and enzyme activities in fluorideintoxicated rats. Fluoride. 2002;35:168–75. [Google Scholar]
- 18.Basha PM, Madhusudhan N. Pre and post natal exposure of fluoride induced oxidative macromolecular alterations in developing central nervous system of rat and amelioration by antioxidants. Neurochem Res. 2010;35:1017–28. doi: 10.1007/s11064-010-0150-2. [DOI] [PubMed] [Google Scholar]
- 19.El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH. Cadmium-induced changes in lipid peroxidation, blood hematology,biochemical parameters and semen quality of male rats protective role of vitamin E and B-carotene. Food Chem Toxicol. 2004;42:1563–71. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Crewe HK, Nothey LM, Wunach RM. Metabolism of tamoxifen by recombinant human cytochrome p 450 enzymes formation of the 4- hydroxy and N-desmethyl metabolites and isomerization of trans-4- hydroxy-tamoxifen. Drug Metab Dispos. 2002;30:869–74. doi: 10.1124/dmd.30.8.869. [DOI] [PubMed] [Google Scholar]
- 21.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Guney M, Oral B, Take G, Giray SG, Mungan T. Effect of fluoride intoxication on endometrial apoptosis and lipid peroxidation in rats: Role of vitamins E and C. Toxicology. 2007;231:215–23. doi: 10.1016/j.tox.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Li G, Du X, Cheng X, Bi H. The effects in fluorosis of selenium on the biochemical elements in the blood of rats and the urinary fluoride of patients. Fifth conference of the Chinese society for fluoride research (Chinese) Kuenming. 1991 [Google Scholar]
- 24.Li YY, Sun GF, Li FJ, Liang G, Jia XP. Proceedings of the PAN-Asia Pacific Conference on fluoride and arsenic research. Shenyang, China: 1999. Aug 16-20, Effect of Se and GSH on lipid peroxidation induced by fluoride: an experimental study; p. 111. [Google Scholar]


