Abstract
BACKGROUND:
Venous thromboembolism (VTE) is a serious and underestimated potentially fatal disease with an effective prophylactic antithrombotic therapy that is usually underused.
OBJECTIVES:
The primary study objective is to determine the percentage of VTE patients who received prophylactic antithrombotic therapy according to ACCP guidelines. Secondary study objectives are determining prevalence of confirmed VTE mortality among all cause hospital mortalities, measuring adherence to anticoagulation treatment after discharge and number of VTE events among those patients.
METHODS:
During the period from first of July 2008 till 30 of June 2009, we collected all hospital deaths, all patients with confirmed VTE diagnosis at King Fahd General Hospital, Jeddah, Kingdom of Saudi Arabia. Only patients with confirmed VTE diagnosis were included in the analysis.
RESULTS:
Five hundred cases with clinical diagnosis of VTE were identified. Out of them 178 were confirmed to be VTE. 36.5% of them received prophylactic antithrombotic therapy. Case fatality rate was 20.8% representing 1.9% of hospital deaths. Case fatality rate was 31% and 3.1% for patients who did not receive thromboprophylaxis and patients who received it, respectively (P < 0.0001). 66.3% and 33.7% of confirmed VTE cases occurred in surgical and medical patients respectively. Only 44.1% of surgical patients and 21.7% of medical patients received prophylaxis (P < 0.01). Case fatality rate is 11% for surgical patients and 40% for medical patients (P < 0.001). Of 141 survived cases, 118 (83.7%) were adherent to anticoagulation therapy after discharge.
CONCLUSIONS:
VTE prophylaxis guideline is not properly implemented and extremely underutilized. Mortality from VTE is significantly higher in patients who did not receive VTE prophylaxis. In the absence of regular post-mortem practice VTE related mortality rate would be difficult to estimate and likely will be underestimated. Health authorities should enforce VTE prophylaxis guideline within the healthcare system.
Keywords: Deep venous thrombosis, pulmonary embolism, thromboprophylaxis, venous thromboembolism
Venous thromboembolism (VTE) is a serious disease with a potentially effective preventive measures[1] and high rate of fatal outcome without treatment.[2] The incidence of VTE is 1-3 per 100 patients per year[3–6] while the incidence of massive pulmonary embolism (PE) is estimated to be 200 diagnosed cases per 1,000,000 inhabitants per year in western countries.[7]
Without prophylaxis the incidence of hospital-acquired deep venous thrombosis (DVT) with confirmed objective diagnosis is 10-40% among surgical and medical patients. However, this incidence rises to be 40-60% after major orthopedic surgery.[4,8]
The prevalence of PE among hospitalized patients is estimated to be about 1%.[7] Approximately, 0.2-10% of all hospital deaths are attributed to PE.[9,10] PE is fatal in 1.5% of treated patients[11] but this rate rise to be 25% in untreated patients.[2]
At least in one study the total VTE case fatality rate in hospitals found to be 12%[4] and 29-34% per year.[4,12] The DVT case fatality rate ranges from 1 to 10% which is mainly due to fatal PE and is highest in those with malignancies.[3–5]
There is a gap between guidelines and practice, in at least one large study ENDORSE study, they found that more than 50% of hospitalized patients should have received VTE thromboprophylaxis but in fact only half of them actually received it.[13]
In the current retrospective study we analyzed prophylactic antithrombotic therapy data of 178 patients with confirmed diagnosis of VTE over a period of 1 year. The primary study outcome was to determine the percentage of patients who received prophylactic antithrombotic therapy according to the ACCP guidelines. The secondary study outcomes were to determine the percentage of VTE mortality among all cause hospital mortalities, and to measure the adherence to anticoagulation treatment after discharge. Moreover, to measure the number of VTE events among those patients.
Methods
Study design
The study is a retrospective observational registry in a single hospital (King Fahd General Hospital, Jeddah, Kingdom of Saudi Arabia). It is a teaching hospital with 900 beds and affiliated with King Abdulaziz University. Through medical records, and computerized database of the hospital we collected our data to ensure that we did not miss any patient or file. It comprised a preliminary step of collecting all deaths and all patients with clinical diagnosis of VTE during the period from 1 July 2008 till 30 June 2009. In the next step, we identified all patients with confirmed VTE diagnosis at discharge whether dead or alive. Only those patients with confirmed diagnosis were included in the analysis.
Inclusion criteria include: Age 15 years and older, an established diagnosis of DVT and PE. DVT diagnosis was confirmed by leg veins Doppler US or lower vein cuts of pulmonary CT angiogram. PE diagnosis was confirmed by Spiral CT scan of the chest, or echocardiography in major PE and hemodynamic instability. Death was considered to be a result of VTE if the patient was diagnosed as VTE, VTE diagnosis was confirmed by the aforementioned investigations and there were no other possible clinical explanations for death. Any patient with a missing hospital chart is excluded.
Data collection
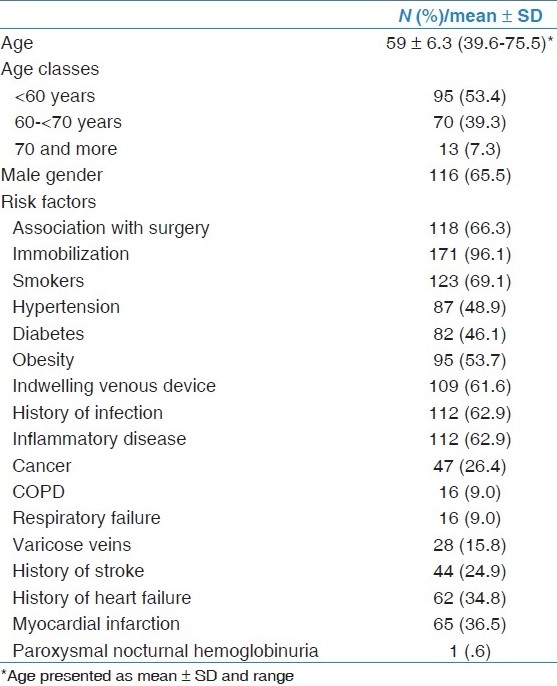
The following data were collected from the medical charts: Age, sex, risk factors for VTE [Table 1], investigations such as Chest X-ray, D-dimers, leg veins Doppler US, pulmonary CT angiogram (Spiral CT scan) of the chest, VTE prophylaxis compliance as per ACCP recommendations either type of prophylaxis (mechanical and/or pharmacological), the number of recurrent VTE events among those patients, adherent to anticoagulation therapy after discharge, and death.
Table 1.
Baseline characteristics of patients with confirmed diagnosis of venous thromboembolism
Statistical analysis
Statistical analysis was based on patients with confirmed diagnosis of VTE. Continuous variables were described as mean+SD for normally distributed variables and categorical variables as percentages. χ2 test was used to compare between categorical variables; whenever any of the expected values were less than 5, Fisher exact test was used instead. All used statistical tests were performed using two-tailed at a 5% level of significance.
Results
Total hospital mortality during the study period was 1968 cases and 500 cases whether dead or alive were identified with clinical diagnosis of VTE. One hundred and seventy-eight cases had confirmed VTE were included in the analysis. The baseline characteristics of them are shown in Table 1.
One hundred and twenty-nine patients (72.5%) of confirmed VTE patients had DVT, 46 patients (25.8%) had DVT progressed to PE and three (1.7%) had PE. The remaining 322 were excluded from analysis because of no confirmation of diagnosis. All of them (the 322 excluded patients) were dead with the cause of death mentioned “circulatory and respiratory collapse” without objective confirmation of the cause of death. Post-mortem autopsy is not the standard of practice in Kingdom of Saudi Arabia culture, so the reason of death could not be reached in these cases.
Risk categorization according to ACCP guidelines
One hundred and eighteen (66.3%) of patients were associated with surgery and 60 (33.7%) were associated with medical conditions.
According to ACCP risk categorization, surgical patients were classified into very high 98 (83.1%), high 12 (10.2%) and moderate 8 (6.8%). Medical patients were classified into high 51 (85.0%) and moderate 9 (15.0%).
Prophylactic treatment
All surgical and medical patients (178 cases) were eligible for prophylactic antithrombotic therapy based upon ACCP guideline; however, only 65 (36.5%) received it.
More surgical patients (44.1%) received prophylactic antithrombotic therapy compared to medical patients (21.7%) as shown in Table 2. This is also reflected in stratification of patients according to ACCP risk categorization for both surgical and medical patients. The surgical patients who were at very high (42.9%) and high (41.7%) risk for VTE received more prophylactic antithrombotic therapy compared to the medical patients who are at the high risk for VTE (21.6%). Similar findings were found for the moderate risks (62.5%) surgical patients and moderate risks (22.2%) medical patients as shown in Table 3.
Table 2.
Prophylactic antithrombotic therapy and mortality of surgical and medical patients
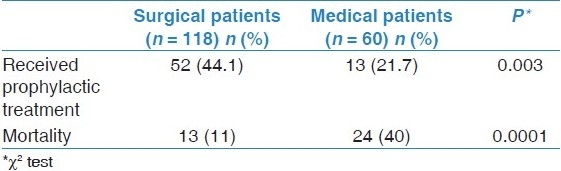
Table 3.
Prophylactic antithrombotic therapy and mortality of patients according to risk stratification

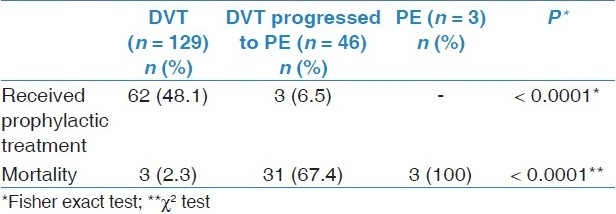
Another finding is that patients with DVT received prophylactic antithrombotic therapy more than other diagnoses (P < 0.0001) as illustrated in Table 4. Nevertheless, 65 (37.1%) of 175 patients with DVT and only three (6.1%) of patients with PE received prophylactic antithrombotic treatment.
Table 4.
Prophylactic antithrombotic therapy and mortality of patients by diagnosis
Mortality
Irrespective of receiving thromboprophylaxis the case fatality rate is 37 out of 178 patients (20.8%); making confirmed VTE accounts for 1.9% of the all 1968 hospital deaths. Case fatality rate in patients who received prophylactic antithrombotic therapy (3.1%) is significantly (P < 0.0001) less than those who have not received it (31%) as shown in Figure 1.
Figure 1.
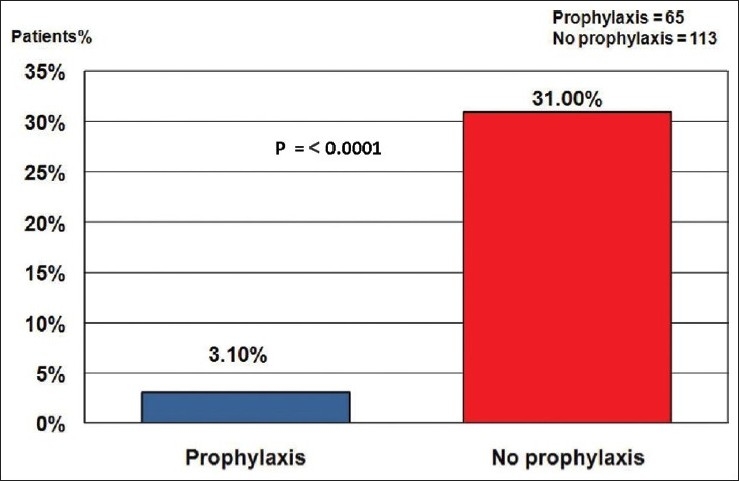
Mortality according to receiving prophylaxis
Case fatality rate in medical patients (40%) is significantly (P = 0.0001) higher than surgical patients (11%), respectively, as shown in Table 2.
Among surgical patients based upon receiving prophylactic antithrombotic therapy shows that mortality was 1 (1.9%) in the subgroup of patients who received it compared to 12 (18.2%) in patients who did not receive it. The difference was highly significant (P = 00.005). For medical patients based upon receiving prophylactic antithrombotic therapy showed that mortality was 1 (7.7%) in patients who received it compared to 23 (48.9%) in patients who did not receive it. The difference was highly significant (P = 0.007).
In regards to the ACCP risk stratifications of surgical patients it showed that the mortality rate was 13 (13.3%) in the subgroup of very high-risk patients and none of the high and moderate risk groups. The difference was not statistically significant (P = 0.399). For subgroup analysis of medical patients based upon ACCP risk stratifications showed that the mortality rate was 6 (66.7%) in the subgroup of moderate risk patients compared to 18 (35.3%) in the subgroup of high-risk patients. The difference was not statistically significant (P = 0.137).
A case fatality of PE was (100%) and DVT progressed to PE (67.4%) were significantly (P < 0.0001) more than DVT (2.3%) as shown in Table 4.
Adherence to anticoagulation treatment after discharge
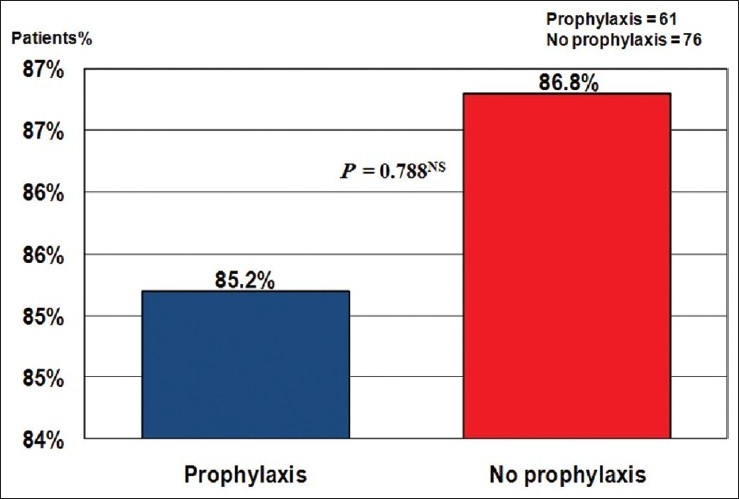
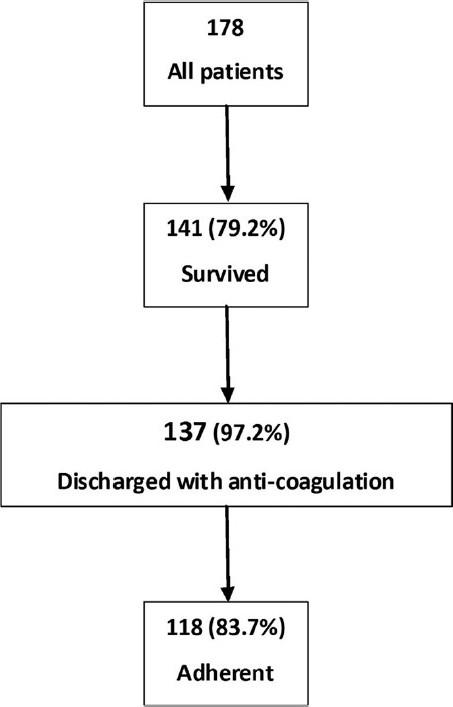
Figure 2 illustrates that 118 patients were adherent to anticoagulation therapy after discharge. There was no difference between patients who previously received prophylactic antithrombotic therapy compared to those who did not receive it as regards further adherence to anticoagulation therapy after discharge as shown in Figure 3.
Figure 2.
Patients adherence to anticoagulation therapy after discharge
Figure 3.
Patients adherence to anticoagulation therapy according to previous prophylaxis
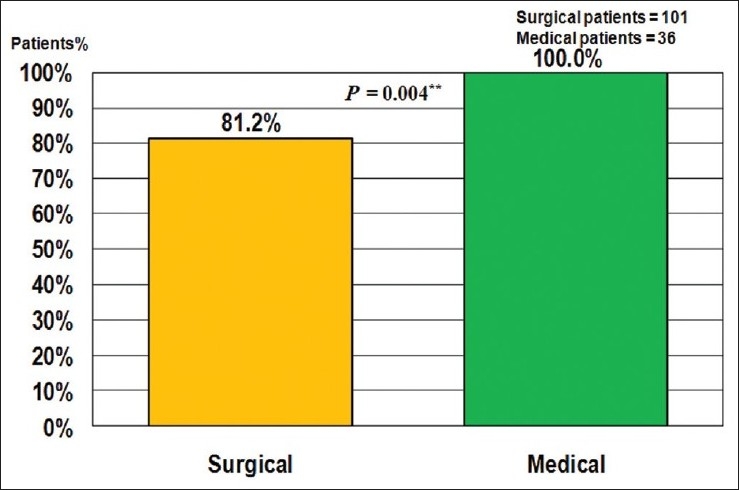
However, surgical patients were less adherent to anticoagulation therapy after discharge compared to medical patients (P = 0.004) as illustrated in Figure 4.
Figure 4.
Adherence to anticoagulation therapy after discharge
For the discharge surgical patients and based upon ACCP risk stratifications showed that in patients who were recommended anticoagulation therapy at discharge 65 (80.2%) in the subgroup of very high risk patients (81 patients), 9 (75.0%) in the high-risk patients (12 patients) and 8 (100%) of moderate risk patients were adherent to it. The difference was not statistically significant (P = 0.333). On the other hand, all of medical patients (33 and 3 of the high and moderate risk groups respectively) were adherent.
When analyzing adherence to anticoagulation therapy after discharge from diagnosis perspective, we found that 104 (85.2%) of 122 DVT patients who were recommended anticoagulation treatment at discharge were adherent to it compared to 14 (93.3%) of the 15 DVT progressed to PE patients. The difference was statistically not significant (P = 0.39).
Number of VTE events
Finding and describing number of VTE events was one of our study objectives; however, such objective could not be actualized because of lack of documentation denoting a serious need for creating a followup system for VTE patients.
Discussion
In the multinational IMPROVE study,[14] it showed that only 60% of medical patients who are eligible for prophylactic antithrombotic therapy indeed received it. However, this percentage is much lower 16% in acutely ill hospitalized medical patients in Canada.[15]
In our study, we found that only 36.5% of the patients who where eligible for prophylactic antithrombotic therapy received it. This is very close to the finding of AVAIL ME study which included 200 patients from Saudi Arabia,[16] and found that VTE prophylaxis and guidelines applications ranging from (24-84%) among different subgroup.
The surgical patients in our study received prophylactic antithrombotic therapy significantly more than medical patients (P > 0.01); 44.1% and 21.7%, respectively. Use of prophylactic antithrombotic therapy in both medical and surgical patients, is found to be only 35-42% of patients in the highest risk groups.[17–19] However, results of the ENDORSE study which included 467 patients from the Middle East region (Kingdom of Saudi Arabia and the United Arab of Emirates) showed a wide range for surgical patients receiving prophylactic antithrombotic therapy; such that 0.2-92% of surgical patients received appropriate care compared to 3-7% of medical patients.[13] It is to be noted that medical patients were termed as having “double trouble”, because they have higher proportions of VTE risk factors and prophylaxis for them is omitted more commonly therefore they would experience higher rate of VTE.[20]
Although our Figures for thromboprophylaxis are in general lower than the reported numbers in the literature outside the Middle East region, the low incidence stays in accordance with many studies conducted at different countries, indicating the extreme underuse of thromboprophylaxis. Our study raises the necessities of improving the awareness of VTE risk and deploying the guidelines with special attention to medical patients.
We found that confirmed VTE accounts for 1.9% of hospital mortality within a period of 1 year. Some studies pointed to the under estimation of mortality rates caused by PE[21,22] due to methodological reasons i.e., evaluating patients survival few hours after the onset of symptoms not to mention the possibility of missing clinical diagnosis as evidenced by the high incidence of VTE in autopsy studies.[7,23] In comparison, our study is limited by unavoidable inability to perform autopsy in at least 320 cases with clinical diagnosis of VTE which would increase mortality share due to VTE within total hospital mortality.
Patients who received prophylactic antithrombotic therapy have mortality rate as low as 3.1% compared to those who have not received it (31%). The rational for the use of prophylactic antithrombotic therapy is based on solid principles and scientific evidence[24] with evident benefits.[1] The lower mortality of surgical patients in our study than medical patients is attributed to the higher rates of surgical patients receiving prophylactic antithrombotic therapy compared to medical patients such issue can be applied to the subgroup analysis based upon risk stratification.
The mortality of patients with DVT alone is lower than the patients with DVT who progressed to PE or patients who presented with PE only. This is thought to be related to the VTE prophylactic treatment. We may conclude that prophylactic antithrombotic therapies improve survival and would prevent DVT patients from progressly to PE.
It is of great concern in our study to find that not all of patients who survived their episode of VTE were discharged with anticoagulation therapy (97.2%). Of them, 83.7% only were adherent to it after discharge. Usage of anticoagulation therapy for 3 months or longer after DVT reduces recurrence from 25% to 4% or less.[25] Patients with PE are very likely to die from recurrent PE than DVT. During the first 3 months of anticoagulation therapy, the risk of fatal subsequent PE is 1.5 in patients with previous PE versus 0.4 in patients with previous DVT.[11] According to ACCP guideline, patients should be treated with anticoagulation therapy for at least 3 months if they had reversible risk factors and at least for 6 months if they had idiopathic PE.[26] So comparing guideline with practice, there is a gap in prescription and a gap in adherence. Physicians should be directed towards implementing ACCP guideline. Moreover, they should instruct and educate their patients in collaboration with other healthcare providers to be adherent to prescribed anticoagulation therapy. The benefits of using and adhering to anticoagulation therapy in prevention of subsequent recurrence of VTE events should be an inseparable part within the management alliance between patients and healthcare providers.
King Fahad General Hospital is one of the biggest Ministry of Health hospitals in the Kingdom of Saudi Arabia. The elicited underuse of thromboprophylaxis is likely to be prevalent in all hospitals of Ministry of Health and represent the gap between guideline and application. In the AVAIL ME study, the authors suggested the use of several active strategies such as “regularly reminding clinicians to treat patients at VTE risk, electronic alerts for physicians about patients at risk, and assisting the selection of appropriate prophylaxis, are likely to result in the achievement of optimal outcomes” in addition to include those who are in position to assess VTE risk factors, namely clinical pharmacists and nurses and then direct patients to ask for prophylaxis. Based upon its prospective nature, most hospitals involved in the AVAIL ME study, start initiatives of carful planning to control the problem. Hospitals begin to implement educational sessions targeting practicing physicians and staff and start efforts toward changing guidelines to hospital policy.
From our perspective, all active strategies and methods used should be implemented within a system to be applied in all hospitals of Ministry of Health and its application should be enforced through health authorities.
We are aware of the limitations in our study, being a retrospective in a single hospital and our inability to perform autopsy to reach a more accurate mortality rate but we do not think that such limitation would impact the results. Nevertheless, such limitation in combination with the results and recommendations ought to emphasize the role of health authorities to consider enforcing VTE prophylaxis guideline application.
Conclusions
We found improper implementation of the ACCP VTE Prophylaxis guideline which is more prominent in medical patients. Such gap in practice could be attributed to under estimation of risk, and absence of formal policy for VTE prophylaxis.
We also found inaccurate data base documentation regarding the prevalence of PE-related death with the phrase “circulatory and respiratory collapse” mentioned in the filling system of the hospital as the cause of death in addition to inability to perform autopsy which can detect more cases. Such factors make the actual mortality rate difficult to estimate.
We need to enforce the VTE prophylaxis guideline through health authorities and all the healthcare personnel should be aware of and implement it. Also, patients involvement should be considered during the prophylaxis period and if VTE occurred, during treatment period and after discharge.
Acknowledgments
Analysis and medical writing were done by Amygate Healthcare, Cairo Egypt.
Footnotes
Source of Support: The authors have received unrestricted grant from Sanofi Avantis, Jeddah, KSA,
Conflict of Interest: Sharif Galal is an employee at Sanofi Avantis, Jeddah, KSA
References
- 1.Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: The seventh ACCP conference on antithrombotic and anti thrombotic therapy. Chest. 2004;126:338S–400S. doi: 10.1378/chest.126.3_suppl.338S. [DOI] [PubMed] [Google Scholar]
- 2.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 3.Nordströom M, Lindblad B, Bergqvist D, Kjellströom T. A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Intern Med. 1992;232:155–60. doi: 10.1111/j.1365-2796.1992.tb00565.x. [DOI] [PubMed] [Google Scholar]
- 4.Anderson FA, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT study. Arch Intern Med. 1991;151:933–8. [PubMed] [Google Scholar]
- 5.Oger E. Incidence of venous thromboembolism: A community-based study in western France. Thromb Haemost. 2000;83:657–60. [PubMed] [Google Scholar]
- 6.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: The longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Stein PD, Henry JW. Prevalence of acute pulmonary embolism in a general hospital. Chest. 1995;108:978–81. doi: 10.1378/chest.108.4.978. [DOI] [PubMed] [Google Scholar]
- 8.Scottish Intercollegiate Guidelines Network (SIGN) Prophylaxis of venous thromboembolism: A national clinical guideline. 2002. [Last accessed on 2010 Sept. 20]. SIGN Publication No. 62. Available from: http://www.sign.ac.uk .
- 9.Lindblad B, Eriksson A, Bergqvist D. Autopsy-verified pulmonary embolism in a surgical department: Analysis of the period from 1951 to 1968. Br J Surg. 1991;78:849–52. doi: 10.1002/bjs.1800780725. [DOI] [PubMed] [Google Scholar]
- 10.Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: Are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82:203–5. doi: 10.1177/014107688908200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douketis JD, Kearon C, Bates S, Duku EK, Ginsberg JS. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA. 1998;279:458–62. doi: 10.1001/jama.279.6.458. [DOI] [PubMed] [Google Scholar]
- 12.Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Predictors of survival after deep vein thrombosis and pulmonary embolism: A population-based, cohort study. Arch Intern Med. 1999;159:445–53. doi: 10.1001/archinte.159.5.445. [DOI] [PubMed] [Google Scholar]
- 13.Cohen AT, Tapson VF, Bergmann JF, Goldhaber SZ, Kakkar AK, Deslandes B, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): A multinational cross-sectional study. Lancet. 2008;371:387–94. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 14.Tapson VF, Decousus H, Pini M, Chong BH, Froehlich JB, Monreal M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: Findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132:936–45. doi: 10.1378/chest.06-2993. [DOI] [PubMed] [Google Scholar]
- 15.Kahn SR, Panju A, Geerts W, Pineo GF, Desjardins L, Turpie AG, et al. Multicenter evaluation of the use of venous thromboembolism prophylaxis in acutely ill medical patients in Canada. Thromb Res. 2007;119:145–55. doi: 10.1016/j.thromres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Taher AT, Aoun J, Salameh P. The AVAIL ME study: A multinational survey of VTE risk and prophylaxis. J Thromb Thrombolysis. 2011;31:47–56. doi: 10.1007/s11239-010-0492-2. [DOI] [PubMed] [Google Scholar]
- 17.Deheinzelin D, Braga AL, Martins LC, Martins MA, Hernandez A, Yoshida WB, et al. Incorrect use of thromboprophylaxis for venous thromboembolism in medical and surgical patients: Results of a multicentric, observational and cross-sectional study in Brazil. J Thromb Haemost. 2006;4:1266–70. doi: 10.1111/j.1538-7836.2006.01981.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad HA, Geissler A, MacLellan DG. Deep venous thrombosis prophylaxis: Are guidelines being followed? ANZ J Surg. 2002;72:331–4. doi: 10.1046/j.1445-2197.2002.02402.x. [DOI] [PubMed] [Google Scholar]
- 19.Learhinan ER, Alderman CP. Venous thromboembolism prophylaxis in a South Australian teaching hospital. Ann Pharmacother. 2003;37:1398–402. doi: 10.1345/aph.1D100. [DOI] [PubMed] [Google Scholar]
- 20.Piazza G, Seddighzadeh A, Goldhaber SZ. Double trouble for 2, 609 hospitalized medical patients who developed deep vein thrombosis: Prophylaxis omitted more often and pulmonary embolism more frequent. Chest. 2007;132:554–61. doi: 10.1378/chest.07-0430. [DOI] [PubMed] [Google Scholar]
- 21.Piazza G, Goldhaber SZ. Acute pulmonary embolism: Part I: Epidemiology and diagnosis. Circulation. 2006;114:e28–32. doi: 10.1161/CIRCULATIONAHA.106.620872. [DOI] [PubMed] [Google Scholar]
- 22.Piazza G, Goldhaber SZ. Acute pulmonary embolism: Part II: Treatment and prophylaxis. Circulation. 2006;114:e42–7. doi: 10.1161/CIRCULATIONAHA.106.620880. [DOI] [PubMed] [Google Scholar]
- 23.Ryu JH, Olson EJ, Pellikka PA. Clinical recognition of pulmonary embolism: Problem of unrecognized an asymptomatic cases. Mayo Clin Proc. 1998;73:873–9. doi: 10.4065/73.9.873. [DOI] [PubMed] [Google Scholar]
- 24.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8 th Edition) Chest. 2008;133:381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 25.van Dongen CJ, van der Belt AG, Prins MH, Lensing AW. Fixed dose subcutaneous low-molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database Syst Rev. 2004;4:CD001100. doi: 10.1002/14651858.CD001100.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Büuller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: The Seventh ACCP Conference on Antithrombotic and Anti thrombotic Therapy. Chest. 2004;126:401S–28S. doi: 10.1378/chest.126.3_suppl.401S. [DOI] [PubMed] [Google Scholar]


