Abstract
BACKGROUND:
The protease-antiprotease hypothesis proposes that inflammatory cells and oxidative stress in chronic obstructive pulmonary disease (COPD) produce increased levels of proteolytic enzymes (neutrophil elastase, matrix metalloproteinases [MMP]) which contribute to destruction of parenchyma resulting in progressive decline in forced expiratory volume in one second. Doxycycline, a tetracycline analogue, possesses anti-inflammatory properties and inhibits MMP enzymes.
OBJECTIVES:
To assess the effect of 4 weeks doxycycline in a dose of 100 mg once a day in patients of moderate to severe COPD with stable symptoms.
METHODS:
In an interventional, randomized, observer-masked, parallel study design, the effect of doxycycline (100 mg once a day for 4 weeks) was assessed in patients of COPD having stable symptoms after a run-in period of 4 weeks. The study participants in reference group did not receive doxycycline. The parameters were pulmonary functions, systemic inflammation marker C-reactive protein (CRP), and medical research council (MRC) dyspnea scale. Use of systemic corticosteroids or antimicrobial agents was not allowed during the study period.
RESULTS:
A total of 61 patients completed the study (31 patients in doxycycline group and 30 patients in reference group). At 4 weeks, the pulmonary functions significantly improved in doxycycline group and the mean reduction in baseline serum CRP was significantly greater in doxycycline group as compared with reference group. There was no significant improvement in MRC dyspnea scale in both groups at 4 weeks.
CONCLUSION:
The anti-inflammatory and MMP-inhibiting property of doxycycline might have contributed to the improvement of parameters in this study.
Keywords: Anti-inflammatory, C-reactive protein, doxycycline, dyspnea, matrix metalloproteinase, respiratory function tests
The airflow limitation in chronic obstructive pulmonary disease (COPD) is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases.[1] Current management includes smoking cessation, bronchodilators, corticosteroids, and pulmonary rehabilitation. Smoking cessation is the only intervention in COPD that is associated with decreased progression of disease. The protease-antiprotease model proposes that inflammatory cells and oxidative stress in COPD result in increased levels of proteolytic enzymes (matrix metalloproteinases [MMP], neutrophil elastase) and decreased levels of antiproteases (α-1-antitrypsin, tissue inhibitors of metalloproteinases [TIMP]) which result in destruction of lung parenchyma leading to progressive decline of forced expiratory volume in one second (FEV1).[2,3] MMPs are zinc-dependent endopeptidases of which MMP-1,-2,- 8, and -9 have shown major role in pathogenesis of COPD. The concentrations of MMP-1,-8, and -9 are increased in bronchoalveolar lavage fluid from patients of COPD.[4,5]
Doxycycline, a tetracycline analogue, has shown to possess immunomodulatory properties in addition to its broad-spectrum antimicrobial activity. Doxycycline is a potent inhibitor of MMP enzymes, particularly MMP-8 and MMP-9.[6–9] Studies have demonstrated reduction of MMP-9 levels by doxycycline in lung tissues of rats.[10,11] Other immunomodulatory properties of doxycycline include suppression of neutrophil migration, antiapoptotic activity, decrease in monocyte chemoattractant protein-1, and increase in the expression/secretion of the natural inhibitor of MMP (TIMP-1).[12–14] Doxycycline has shown beneficial results in periodontitis, facial acne, recurrent oral ulceration, rheumatoid arthritis, and corneal erosions on account of its non-antimicrobial properties.[14–18] Long-term treatment with doxycycline is well tolerated. Adverse effects to doxycycline include photosensitivity and gastrointestinal disturbances.[19] It seemed reasonable to assume that drug possessing anti-inflammatory and MMP-inhibiting activity like doxycycline may have beneficial effect on lung function, systemic inflammation, and dyspnea in patients of COPD having stable symptoms. The present study was designed to study the effect of doxycycline in a dose of 100 mg once a day in patients of stable COPD.
Methods
The study was conducted in the outpatient department of pulmonary medicine of M. P. Shah medical college and Guru gobindsingh government hospital, Gujarat, India. The study protocol was approved by the institutional ethics committee and designed in accordance with good clinical practice. Written informed consent was obtained from all the participants included in the study. The study was carried out between July 2009 and February 2010.
In the present study, an interventional, randomized, observer-masked, comparative design was used to investigate the efficacy of doxycycline in addition to standard treatment in patients of stable COPD. Patients included had age >40 years, smoking history >10 pack years, and were classified as stage II or III on GOLD classification for severity. Patients presenting with acute exacerbation of COPD as defined by Anthonisen et al.,[20] and patients who had moderate/severe exacerbation in last 4 weeks as defined by severity scale proposed by Cazzola et al.,[21] were excluded. Patients with active respiratory disease other than COPD (tuberculosis, pneumonia, lung malignancies, bronchial asthma), acute systemic/local infection, cardiac and gastrointestinal disorders were excluded. Patients on systemic corticosteroids at the time of screening were also excluded. The primary endpoint was change in post-bronchodilator FEV 1 from baseline to end of 4 weeks, and secondary endpoints were changes in forced expiratory volume (FVC), serum C-reactive protein (CRP), and medical research council (MRC) dyspnea scale after 4 weeks.
At initial screening visit, patients were assessed by history, clinical examination, pulmonary function tests, chest X-ray, and sputum examination. Data regarding smoking history and exacerbations were recorded. Patients who qualified inclusion and exclusion criteria and gave written informed consent underwent a run-in period of 4 weeks. During run-in period, all the study participants received treatment with deriphyllin (100 mg thrice a day), inhaled salbutamol and ipratropium. A run-in period of 4 weeks was selected because physiological changes associated with acute exacerbation return to baseline values in 70 to 74% patients at 4 weeks.[22] Use of inhaled corticosteroids and long-acting β2 agonists was allowed during the run-in and study period.
After run-in period, at baseline visit, patients were assessed for values of pulmonary functions by spirometry. Assessment of dyspnea was done using MRC dyspnea scale and blood sample was collected for serum CRP estimation. Subsequently, the study participants were randomized using blocked randomization method with the help of computer software into the following two groups: Group 1 (Doxycycline group): Capsule doxycycline 100 mg once a day daily for 4 weeks in addition to run-in period treatment; Group 2 (Reference group): Continued to receive treatment received in run-in period. Systemic corticosteroids or antibiotics were not allowed during the study period until clinical necessity arouse in which the patient was then excluded from the study.
After study intervention period of 4 weeks, patients were followed up and assessed for lung functions, MRC dyspnea scale, and serum CRP. Post-bronchodilator FEV1 and FVC were recorded after administration of 200 μg of salbutamol, using metered dose inhaler. Each subject performed a minimum three acceptable FVC measures. The greatest FEV1 and corresponding FVC value were used in subsequent analysis. Serum CRP was estimated using immunoturbidimetry on the same day of collection of sample.
Statistical analysis
A total of 36 patients were required in each group with the power of the study as 80% to detect a difference of 20% between the two groups in post-bronchodilator FEV1 at 4 weeks. Baseline variables were compared using chi-square or Fisher's exact test for categorical variables and unpaired t test or Mann-Whitney test for continuous variables. Statistical evaluations were accomplished with paired t tests for before and after values of pulmonary functions for both groups and unpaired t test for difference in change among both groups. Change in serum CRP was expressed in percent change from baseline and compared using unpaired t test among both groups. Pre- and post-MRC dyspnea scores were compared using Wilcoxon signed rank test. P value <0.05 was considered as significant.
Results
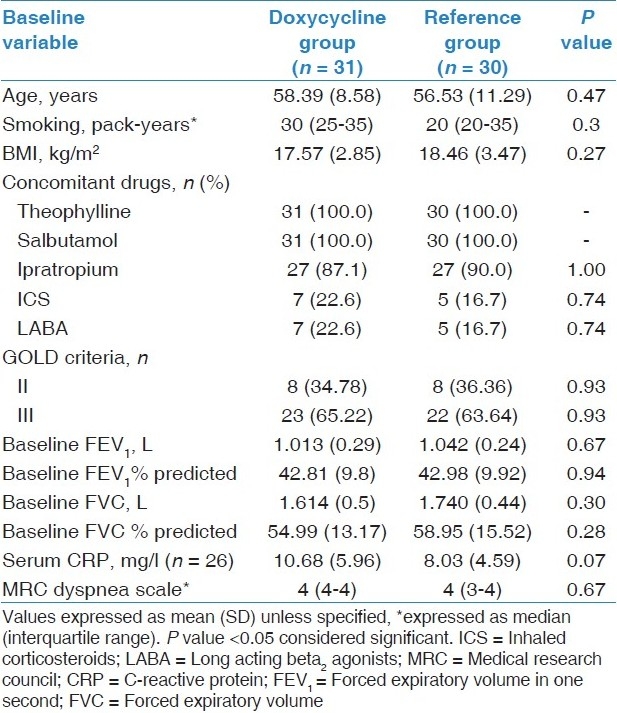
At baseline visit, 72 patients were randomized equally to two groups. During the study period, six patients were lost to follow-up and five received antibiotics/systemic corticosteroids on account of moderate to severe exacerbations. Thus, these 11 patients were excluded and data of remaining 61 patients were analyzed [Figure 1]. There was no significant difference in baseline variables of both groups at the end of run-in period [Table 1].
Figure 1.
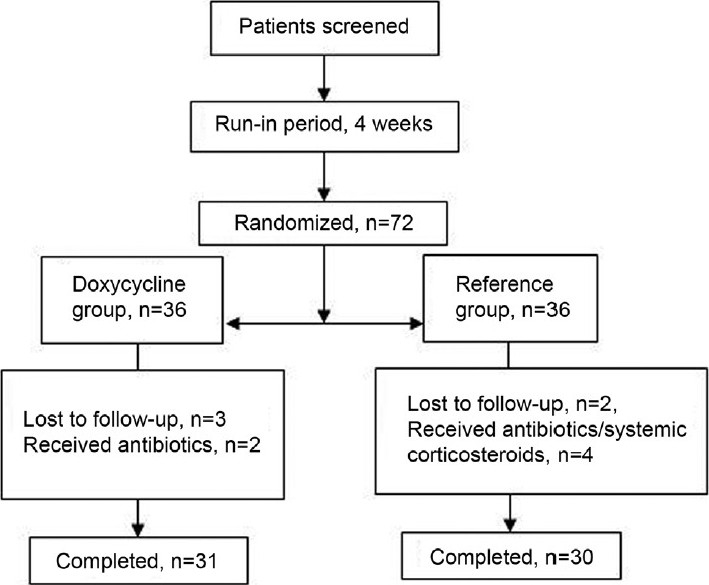
Trial profile
Table 1.
Baseline characteristics of study participants
At baseline, there was a difference of 29 ml in the mean FEV1 among two groups. At 4 weeks, mean FEV 1 increased significantly by 153 ml in doxycycline group (P < 0.001, 95% CI = -0.190 to -0.116) and decreased by 33 ml in reference group [Table 2,Figure 2]. There was significant difference in the mean change in FEV1 (ΔFEV1) in both groups (P < 0.001, 95% CI = 0.140 to 0.234). There was significant increase in FEV1 % predicted, FVC, and FVC % predicted from baseline values at 4 weeks in doxycycline group. Lung function parameters did not improve in reference group. There was significant difference in mean change in FEV1 % predicted, FVC, and FVC % predicted among both groups. The mean percent reduction in baseline serum CRP was 45.59 ± 4.6 and 15.78 ± 5.8 in doxycycline and standard group, respectively. There was significant difference in reduction of serum CR P values between both groups. MRC dyspnea did not significantly decrease from baseline in both the groups at 4 weeks [Table 3].
Table 2.
Change in pulmonary functions and serum CRP
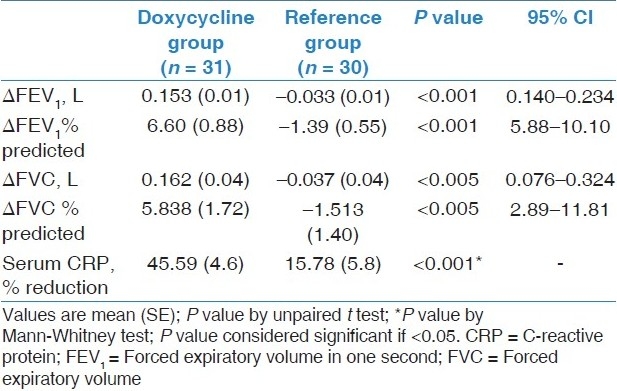
Figure 2.
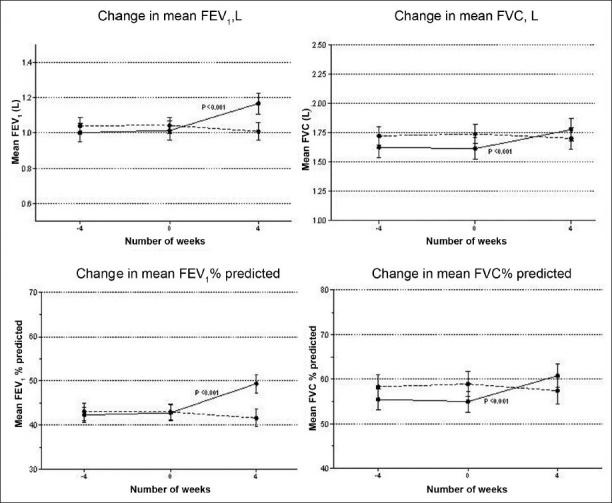
Change in mean pulmonary functions. Error bars represent SE. P value by paired t test for pre (baseline) and post (after 4 weeks) values. Straight line - doxycycline group, dotted line - reference group. Screening: -4 weeks, baseline: 0 week, post-intervention: 4 weeks
Table 3.
Change in MRC dyspnea scale
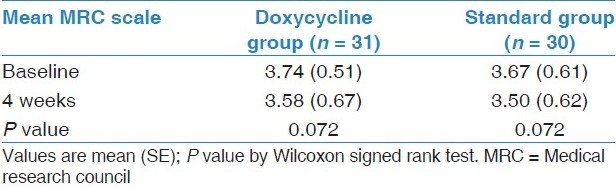
Discussion
Doxycycline in a dose of 100 mg once a day for duration of 7 to 10 days has been used as an antimicrobial agent to treat acute exacerbations in COPD. The present study is first of its kind in which efficacy of 4 weeks doxycycline was assessed in patients of stable COPD. All the study participants were male patients because of inclusion of only those patients who had smoking history of more than 10 pack-years and the fact that smoking is much less common in females in India. Both the groups were comparable in terms of drop-outs and withdrawals during the study period.
Doxycycline demonstrated improvement in lung function parameters for which antimicrobial action of doxycycline is unlikely to be responsible. The present study excluded patients with acute exacerbation, as defined by Anthonisen et al.[20] This classification indicates likelihood of bacterial infection as cause of an exacerbation. The study also excluded patients who had moderate to severe exacerbations in last 4 weeks according to event-based definition given by Cazzola et al.[21] Patients having infective pathology were also excluded. A randomized controlled trial has provided evidence for significant beneficial effect of antibiotics only in those COPD patients who present with increase in all of the following cardinal symptoms: Dyspnea, increased sputum volume and sputum purulence[20] or who present with sputum purulence, and at least one of other two cardinal symptoms.[23] In conclusion, there is less possibility that antimicrobial action might have contributed to beneficial effect of doxycycline in improving lung function parameters in the present study.
Improvement in lung function parameters in present study might be the result of anti-inflammatory and MMP-inhibiting activity of doxycycline. A study demonstrated that the collagenase activity in tracheal aspirates from horses suffering from COPD was sensitive to doxycycline inhibition.[24] Study by Nordstrom et al.[14] tested clinical response to 3 months doxycycline in concert with collagenase activity in patients of rheumatoid arthritis. Significant reduction in joint score and pain visual analogue scale was seen as early as 6 weeks. In the same study, saliva samples showed significant reduction in collagenase (MMP-8) activity at 12 weeks. Doxycycline has shown improvement in pulmonary disorders in which dysregulated MMP activity is held responsible. Maugban et al.[25] studied the effect of addition of doxycycline to immunosuppressive therapy in lung transplantation patients with recurrent acute rejections or obliterative bronchiolitis (OB/BOS). This was associated with improved lung functions in serial pulmonary function tests. OB/BOS is associated with elevated MMP- 9levels and the immunomodulatory effect of doxycycline might have been responsible for the beneficial effect. Long treatment with doxycycline in patient of idiopathic pulmonary fibrosis was associated with improvement in symptoms, physiological and radiological parameters.[26] Doxycycline inhibits neutrophil collagenase (MMP-8) and MMP-9 at doses readily attainable by therapeutic doses. It achieves similar concentration in lungs and plasma.[27,28]
Airway limitation and airway inflammation are separate and independent factors in pathophysiology of COPD. CRP reflects the total systemic burden of inflammation in patients of COPD.[29] Eight-year follow-up study of large cohort with airway obstruction showed that the increased CRP levels are strong predictor of COPD hospitalization and deaths[30] Increased serum CRP levels have been associated with all-cause mortality in patients with mild to moderate COPD, reduced lung function, and greater FEV 1 decline.[31] The present study demonstrated significant difference in reduction of baseline serum CRP levels in doxycycline and standard group. The baseline serum CRP was higher in doxycycline group, though this was not significant. Anti-inflammatory agents like corticosteroids (inhaled fluticasone) for 2 weeks reduced baseline serum CRP levels by 50% in COPD patients who had stable symptoms in previous 3 months in a study.[32] In MIDAS trial, subantimicrobial doses of doxycycline significantly reduced serum CRP level by 47% in patients with coronary artery disease.[27] The study also demonstrated significant reduction in serum IL-6. The authors concluded that reduction in CRP due to doxycycline might be due to upstream inhibition of IL-6 or direct inhibitory effect on CRP synthesis in liver or both. IL-6 is a major signaling cytokine stimulant and induces CRP production and release by liver. Similar mechanism might be responsible for reduction in CRP in the present study.
Studies have demonstrated that dyspnea scores and lung function are distinct and separate in terms of their influence on health outcome.[33] The lack of improvement in MRC dyspnea scale despite significant increase in FEV1 might be due to following reasons. First of all, the study intervention period might not be sufficiently long enough to change the perceived respiratory disability in patients of stable COPD. Second, association of FEV1 and MRC dyspnea scale has not been demonstrated.[34] Lastly, there could be factors other than COPD which might have contributed to dyspnea in the study participants.
Limitations of the study include short study intervention period and lack of data on effect of doxycycline on long-term clinical outcomes in COPD like symptom scores, health status, exercise tolerance, and exacerbation rates. Alternate explanation for serum CRP reduction in doxycycline group could be resolution of mild or occult infection which was not recognized prior to study. The study did not address the problem of antimicrobial resistance.
In conclusion, the study demonstrates beneficial effect of short-term doxycycline in lung function parameters and systemic inflammatory marker, CRP in patients of stable COPD.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Global Initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2009. [Last accessed on 2010 Oct 15]. Available from: http:/www.goldcopd.com .
- 2.Belvisi MG, Bottomley KM. The role of matrix metalloproteinases (MMPs) in the pathophysiology of chronic obstructive pulmonary disease (COPD): A therapeutic role for inhibitors of MMPs? Inflamm Res. 2003;52:95–100. doi: 10.1007/s000110300020. [DOI] [PubMed] [Google Scholar]
- 3.Reilly JJ, Jr, Silverman EK, Shapiro SD. Chronic Obstructive Pulmonary Disease. In: Casper DL, Braunwald E, editors. Harrison's Principles of Internal Medicine. New York: McGraw-Hill; 2006. pp. 1547–54. [Google Scholar]
- 4.Betsuyaku T, Nishimura M, Takeyabu K, Tanino M, Venge P, Xu S, et al. Neutrophil granule proteins in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Am J Respir Crit Care Med. 1999;159:1985–91. doi: 10.1164/ajrccm.159.6.9809043. [DOI] [PubMed] [Google Scholar]
- 5.Finlay GA, Russell KJ, McMahon KJ, D’arcy EM, Masterson JB, FitzGerald MX, et al. Elevated levels of matrix metalloproteinases in bronchoalveolar lavage fluid of emphysematous patients. Thorax. 1997;52:502–6. doi: 10.1136/thx.52.6.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golub LM, Suomalainen K, Sorsa T. Host modulation with tetracyclines and their chemically modified analogues. Curr Opin Dent. 1992;2:80–90. [PubMed] [Google Scholar]
- 7.Suomalainen K, Sorsa T, Golub LM, Ramamurthy N, Lee HM, Uitto VJ, et al. Specificity of the anticollagenase action of tetracyclines: Relevance to their anti-inflammatory potential. Antimicrob Agents Chemother. 1992;36:227–9. doi: 10.1128/aac.36.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiotti N, Altamura N, Moretti M, Wassermann S, Zacchigna S, Farra R, et al. Short term effects of doxycycline on matrix metalloproteinases 2 and 9. Cardiovasc Drugs Ther. 2009;23:153–9. doi: 10.1007/s10557-008-6150-7. [DOI] [PubMed] [Google Scholar]
- 9.Zhao S, Choksuchat C, Zhao Y, Ballagh SA, Kovalevsky GA, Archer DF. Effects of doxycycline on serum and endometrial levels of MMP-2, MMP-9 and TIMP-1 in women using a levonorgestrel-releasing subcutaneous implant. Contraception. 2009;79:469–78. doi: 10.1016/j.contraception.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Sochor M, Richter S, Schmidt A, Hempel S, Hopt UT, Keck T. Inhibition of matrix metalloproteinase-9 with doxycycline reduces pancreatitis-associated lung injury. Digestion. 2009;80:65–73. doi: 10.1159/000212080. [DOI] [PubMed] [Google Scholar]
- 11.Doroszko A, Hurst TS, Polewicz D, Sawicka J, Fert-Bober J, Johnson DH, et al. Effects of MMP-9 inhibition by doxycycline on proteome of lungs in high tidal volume mechanical ventilation-induced acute lung injury. Proteome Sci. 2010;8:3. doi: 10.1186/1477-5956-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raza M, Ballering JG, Hayden JM, Robbins RA, Hoyt JC. Doxycycline decreases monocyte chemoattractant protein-1 in human lung epithelial cells. Exp Lung Res. 2006;32:15–26. doi: 10.1080/01902140600691399. [DOI] [PubMed] [Google Scholar]
- 13.Sapadin AN, Fleischmajer R. Tetracyclines: Nonantibiotic properties and their clinical implication. J Am Acad Dermatol. 2006;54:258–64. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Nordström D, Lindy O, Lauhio A, Sorsa T, Santavirta S, Konttinen YT. Anti-collagenolytic mechanism of action of doxycycline treatment in rheumatoid arthritis. Rheumatol Int. 1998;17:175–80. doi: 10.1007/s002960050030. [DOI] [PubMed] [Google Scholar]
- 15.Ciancio S, Ashley R. Safety and efficacy of sub-antimicrobial dose doxycycline therapy in patients with adult periodontitis. Adv Dent Res. 1998;12:27–37. doi: 10.1177/08959374980120011501. [DOI] [PubMed] [Google Scholar]
- 16.Skidmore R, Kovach R, Walker C, Thomas J, Bradshaw M, Leyden J, et al. Effects of subantimicrobial-dose doxycycline in the treatment of moderate acne. Arch Dermatol. 2003;139:459–64. doi: 10.1001/archderm.139.4.459. [DOI] [PubMed] [Google Scholar]
- 17.Skulason S, Holbrook WP, Kristmundsdottir T. Clinical assessment of the effect of a matrix metalloproteinase inhibitor on aphthous ulcers. Acta Odontol Scand. 2009;67:25–9. doi: 10.1080/00016350802526559. [DOI] [PubMed] [Google Scholar]
- 18.Dursun D, Kim MC, Soloman A, Pflugfelder SC. Treatment of recalcitrant corneal erosions inhibitors of matrix metalloproteinase-9, doxycycline and corticosteroids. Am J Ophthalmol. 2001;132:8–13. doi: 10.1016/s0002-9394(01)00913-8. [DOI] [PubMed] [Google Scholar]
- 19.Smieja M, MacPherson DW, Kean W, Schmuck ML, Goldsmith CH, Buchanan W, et al. Randomised, blinded, placebo controlled trial of doxycycline for chronic seronegative arthritis. Ann Rheum Dis. 2001;60:1088–94. doi: 10.1136/ard.60.12.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 1987;106:196–204. doi: 10.7326/0003-4819-106-2-196. [DOI] [PubMed] [Google Scholar]
- 21.Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: From lung function to biomarkers. Eur Respir J. 2008;31:416–69. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 22.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–13. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 23.Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117:1638–45. doi: 10.1378/chest.117.6.1638. [DOI] [PubMed] [Google Scholar]
- 24.Koivunen AL, Maisi P, Konttinen YT, Prikk K, Sandholm M. Collagenolytic activity and its sensitivity to doxycycline inhibition in tracheal aspirates of horses with chronic obstructive pulmonary disease. Acta Vet Scand. 1997;38:9–16. doi: 10.1186/BF03548503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maugban T, Peterson-Short K, Truax C, Lee S, Kenyon N, Liou TG. Doxycycline improves decline in pulmonary function in OB/BOS. J Heart Lung Transplant. 2009;28:S281–2. [Google Scholar]
- 26.Bhattacharyya P, Nag S, Ghosh D, Roy-Chowdhury S, Bardhan S, Mukherjee A. Treatment of probable idiopathic pulmonary fibrosis with long term doxycycline, a matrix metalloproteinase inhibitor. Indian J Chest Dis Allied Sci. 2007;49:180. [Google Scholar]
- 27.Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733–8. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- 28.Michel G, Mosser J, Olle J. Pharmacokinetics and tissue localization of doxycycline polyphosphate and doxycycline hydrochloride in the rat. Eur J Drug Metab Pharmacokinet. 1984;9:149–53. doi: 10.1007/BF03189618. [DOI] [PubMed] [Google Scholar]
- 29.Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175:250–5. doi: 10.1164/rccm.200605-713OC. [DOI] [PubMed] [Google Scholar]
- 31.Man P, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax. 2006;61:849–53. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sin DD, Lacy P, York E, Man SF. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:760–5. doi: 10.1164/rccm.200404-543OC. [DOI] [PubMed] [Google Scholar]
- 33.Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:1185–9. doi: 10.1164/ajrccm.158.4.9802091. [DOI] [PubMed] [Google Scholar]
- 34.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–6. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]


