Abstract
OBJECTIVE:
The aim of this study is to evaluate the relationship between acute exacerbations and the mean platelet volume (MPV) trend in children with cystic fibrosis (CF), to predict the exacerbations.
METHODS:
A total of 46 children with CF and 37 healthy children were enrolled in the study. White blood cell count (WBC), hemoglobin level, platelet count, mean platelet volume (MPV), and mean corpuscular volume (MCV) were retrospectively recorded.
RESULTS:
Our study population consisted of 25 (54.3%) males and 21 (45.7%) females with CF and 20 (54.0%) males and 17 (46.0%) females in the healthy control group. The mean age of the CF patients was 6.32 ± 4.9 years and that of the healthy subjects was 7.02 ± 3.15 years. In the acute exacerbation period of CF, the MPV values were lower and WBC and platelet counts were higher than those in the healthy controls (P = 0.00, P = 0.00, P = 0.00, respectively). Besides, in acute exacerbation, the MPV values were lower and the WBC count was higher than the values in the non-exacerbation period (P 0= 0.01, P = 0.00, respectively). In the non-exacerbation period MPV was lower and platelet count was higher when compared to healthy subjects (P = 0.02, P = 0.04, respectively).
CONCLUSION:
This study suggests that MPV might be used as a simple, cost effective, diagnostic, predictive indicator for platelet activation in pediatric CF patients related to chronic inflammation, which might be helpful to discriminate or estimate exacerbations.
Keywords: Cystic fibrosis, inflammation, mean platelet volume, platelets
Cystic fibrosis (CF) is a common autosomal recessive disease, mostly encountered in the Caucasian population.[1] According to the limited number of studies, the prevalence is 1 in the 2500 in general population in our country, Turkey.[2] CF is caused by the mutations in the CF trans- membrane conductance regulator gene (CFTR).[3] It is a multi-systemic disease in which variabilities affecting individuals in all ages are characterized by acute or persistant respiratory symptoms, chronic sinopulmonary diseases, malnutrition, steatorrhea, meconium ileus, pseudobartter syndrome, nasal poliposis and male infertility.[1,4,5]
To our knowledge, this is the first study in literature with regard to platelet function by measuring mean platelet volume (MPV) levels in children with CF to anticipate exacerbations.
Therefore, the present study was designed to investigate the relationship between acute exacerbations and MPV trend in children with CF, to clarify its significance.
Methods
Case records of all patients diagnosed as CF and followed-up in the Pediatric Allergy Department between November 2006 and March 2011, were retrospectively reviewed. All patients included in the study were diagnosed as CF according to the established criteria of Cystic Fibrosis Foundation Consensus report. These criteria were characterized as phenotypic features consistent with CF (chronic sinopulmonary disease, gastrointestinal and nutritional abnormalities, salt loss syndromes) or a history of CF in a sibling, a positive newborn screening test accompanied with an increased sweat chloride concentration, two detected CF mutations, or an abnormal nasal epithelial ion transport.[5] Exclusion criteria included having non-classic CF phenotype and no recorded exacerbation in the follow- up period and pre-existing hematological, cardiovascular, and metabolic diseases to avoid unreliability in the MPV measurement.
Cystic fibrosis exacerbation was defined as the presence of the following signs or symptoms; increase in cough frequency, sputum production, changes in sputum quality, dyspnea, tachypnea, exercise intolerance, loss of appetite, weight loss, hemoptysis, fever above 38°C, and an increase in crepitations, and wheezing.[4,5]
Finally, a total of 46 CF patients were enrolled in the study. Thirty seven age and sex-matched healthy children constituted the control group. Demographic data, signs and symptoms of disease at the time of diagnosis, age of onset, age at the time of diagnosis, time interval between disease and diagnosis, CF gene mutation, bacterial colonization status, and exacerbation frequencies and treatments were recorded for each patient.
Laboratory data were obtained at the time of diagnosis and then in the exacerbation period from the computerized patient database: White blood cell count (WBC), hemoglobin level, platelet count, MPV, mean corpuscular volume (MCV). The same computerized database was used for evaluation of complete blood count parameters for healthy children.
Blood samples were aseptically drawn by vein puncture into sterile standard tubes with a constant amount of sodium citrate for anticoagulation, recommended for reliable estimations of MPV, in order to avoid platelet swelling, and maintained at 20°C. The complete blood count analyses were performed in the same Coulter analyzer model LH within one hour, which was routinely checked every month in the central laboratory of our institution. The reference value for MPV ranged between 7.0 and 11 fl.
Data were analyzed using the Statistical Package for Social Sciences version 15.0 (SPSS for Windows 15.0, Inc., Chicago, IL, USA). Parametric and non-parametric tests were conducted with Student t test, paired- samples t test, and the Chi square test. Pearson correlation was used to examine the association between MPV and other laboratory data. A P value of < 0.05 was considered as statistically significant. Data were expressed as the mean ± standard deviation (SD).
Results
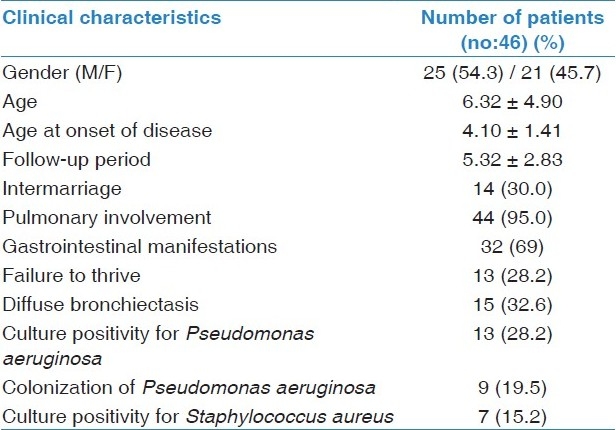
Our study population consisted of 25 (54.3%) males and 21 (45.7%) females with CF and 20 (54.0%) males and 17 (46.0%) females in the control group. Mean age of the CF patients was 6.32 ± 4.9 years and that of the healthy subjects was 9.02 ± 3.15 years. No significant difference considering age and gender was found between the CF patients and the control group. Mean diagnosis by age was 4.01 ± 1.41 and the mean follow- up period of the patients was 5.32 ± 2.83 years. In 16 patients (34.7%) the mutations were defined and 13 of them (81.2%) had mutations in ∆F 508, one patient (6%) had a mutation in M2183, and two patients (12.5%) in 1078 delT. The clinical characteristics of patients are given in Table 1.
Table 1.
Clinical characteristics of patients
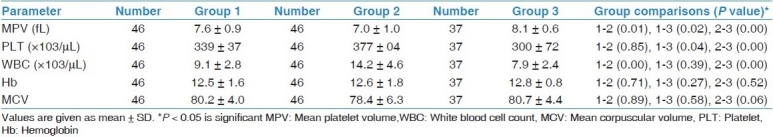
In the acute exacerbation period of CF, the MPV values were lower, but either the WBC or platelet counts were higher than in the healthy controls (P 0= 0.00, P = 0.00, P = 0.00, respectively). Besides, in acute exacerbation the MPV values were lower and the WBC count was higher than the values of the non-exacerbation period (P = 0.01, P = 0.00, respectively). In the non-exacerbation period MPV was lower and platelet count was higher when compared to the healthy subjects (P = 0.02, P = 0.04, respectively).
However, there was no significant difference between hemoglobin and MCV levels among the CF patients in the exacerbation period, exacerbation-free period, and the healthy controls. Comparison between laboratory parameters is given in Table 2.
Table 2.
Comparison of laboratory parameters between patients’ exacerbation-free period (group 1), during exacerbation (group 2), and healthy controls (group 3)
In seven CF patients (15.2%) sputum culture was positive for Staphylococcus aureus and in thirteen patients (28.6%) for Pseudomonas aeruginosa. Nine of thirteen patients (69.2%) had pulmonary colonization of Pseudomonas aeruginosa and were under nebulized antibiotic treatment. MPV values of CF patients in the exacerbation period were not significantly altered by either culture positivity or negativity (P = 0.20) for Pseudomonas aeruginosa and the results were also similar for Staphylococcus aureus infections (P = 0.82). Although in CF patients, with colonization of Pseudomonas aeruginosa WBC was significantly higher than non-colonized patients. There was no significant difference between MPV values in patients who were colonisated with either Pseudomonas aeruginosa or Staphylococcus aureus, when compared to non-colonized CF patients in the exacerbation period (P = 0.23, P = 0.20, respectively). Besides these, there was a positive correlation between WBC and platelet counts (P = 0.00, r = 0.494) in the non-exacerbation period for CF patients.
Discussion
This is the first study evaluating the MPV values in CF patients. This study reveals that the MPV levels in CF patients decrease, particularly in the exacerbation period, when compared to healthy children and CF patients in the non-exacerbation period.
Pulmonary complications are the major cause of morbidity and mortality in children with CF.[4] In infants with CF, born with normal lung parenchyma, inflammation and chronic infection begin histologically when they are as young as four weeks. The deterioration develops over time despite the negative bacterial cultures.[4–6] Recurrent episodes of increasing pulmonary symptoms are defined as exacerbations.[7] Diagnostic tools that can be used for detection of exacerbation are radiographic changes, spirometric declines and identification of pathogens by bacterial cultures;[8] however, these have no quantitative or specific role to anticipate exacerbations.
Many studies showed that platelets play a major role in inflammation as in hemostasis and thrombosis.[9] There were a number of studies demonstrating that the patients with CF had an increased number of circulating platelets,[10] increased platelet activation as a response to gram negative infection,[11] and increased urinary tromboxane excretion correlated with decreased FEV1.[12] These studies suggested that in cystic fibrosis, the mediators secreted by platelets for leucocyte recruitment also activated themselves causing a positive feedback loop, which had a potential risk for lung disease exacerbation and progressive pulmonary damage.[13]
Also, it is demonstrated that the platelet volume is correlated with platelet function and activation.[14] Small platelets have lower functional capabilities than larger ones.[15] Therefore, the mean platelet volume (MPV) has been used as an indicator of platelet function for inflammatory diseases.[14–16]
Many recent studies demonstrated that increased MPV values have been associated with metabolic syndromes including myocardial infarction,[17] acute ischemic stroke,[18] diabetes,[19] obesity,[20] and bronchopulmonary dysplasia.[21]
The results of the present study are in agreement with the previous studies that have also reported a decrease in MPV values in chronic inflammatory diseases including, ulcerative colitis,[22] Henoch Schφnlein purpura (HSP),[23] ankylosing spondylitis[24], rheumatoid arthritis,[24] and familial Mediterranean fever (FMF).[25,26] In addition, the decrease in MPV values was mainly associated with disease activation. In literature, the results for infectious diseases seem to be conflicting. MPV decreased in sepsis suggesting a disturbance of platelet production, and altered platelet activity, which was probably affecting the mortality rate[27] whereas, MPV increased in acute urinary tract infections.[28]
In a previous study the authors speculated that lower MPV levels particularly in FMF attacks were probably related to interleukin-6 (IL-6) levels, which increased significantly during the attack periods.[25,26] Besides these, some other studies were suggested that decreased the MPV levels, which might be an indicator of a defect in the regulation of thrombopoiesis for inflammatory bowel disease (IBD).[29] Studies showed increased levels of the tumor necrosis factor-α (TNF-α), interferon-γ (IF-γ), IL-1, IL-6 in ulcerative colitis, and Crohn's disease.[30] Administration of IL-6 also presented an increase in platelet number and a decrease in MPV levels in cancer patients.[31] MPV was also influenced by inflammation.[18] Some authors put forward IL-6 from other cytokines as an important pro- inflammatory cytokine which could induce thrombocytosis and affect platelet volume.[32,33]
There is evidence that platelets have an important role in the inflammatory cascade of CF lung pathologies.[34,35] Platelets can potentiate an inflammatory process by enhancing recruitment of leucocytes and inhibiting the capability of apoptosis of neutrophils, monocytes, and eosinophils.[35,36] In CF, the overall pulmonary inflammation begins with or without infection mediated by pro-inflammatory cytokines like IL-6 and leukotrien B4 (LTB4) and the inflammation becomes more intense in the presence of bacterial colonization and chronic infection.[37] The decrease in platelet count and volume might be related to some cytokines like IL-6, which are detected higher during the inflammatory process of the overall CF disease process, particularly in exacerbations, as found in previous studies.
Conclusion
In conclusion, this study suggests that MPV might be a useful parameter to be used as an indicator for chronic CF inflammation and a predicting factor for the onset of pulmonary exacerbations. This parameter counted by clinical hematology analyzers is a simple, effortless diagnostic tool for platelet function and activation and adds no extra cost or technical effort.
The major limitations of this study are its retrospective design and small number of patient enrollment. Therefore, further prospective studies investigating the factors affecting the platelet size are required, to determine whether MPV has a clinical implication and role in this disease.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Walters S, Mehta A. Epidemiology of cystic fibrosis. In: Hodson M, Geddes D, Bush A, editors. Cystic Fibrosis. 3rd ed. London: Hodder Arnold Publishers; 2007. pp. 21–80. [Google Scholar]
- 2.Kiper N, Yalçιn E. Kistik fibrozis. Sürekli Tıp Eğitimi Dergisi. 2003;12:131–3. [Google Scholar]
- 3.Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: Genetic analysis. Science. 1989;245:1073–80. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- 4.Cutting GR, Zeitlin PL. Cystic fibrosis. In: Chernick V, Boat T, Wilmott RW, Bush A, editors. Kendig's Disorders of Respiratory Tract in Children. 7th ed. Philadelphia: Saunders Publishers; 2006. pp. 848–900. [Google Scholar]
- 5.Lloyd-Still JD. Pulmonary manifestations. In: Lloyd-Still JD, editor. Textbook of cystic fibrosis. Boston: John Wright Publishers; 1983. pp. 165–98. [Google Scholar]
- 6.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–82. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 7.Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr. 2006;148:259–64. doi: 10.1016/j.jpeds.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax. 2007;62:360–7. doi: 10.1136/thx.2006.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 10.Mikhailidis DP, Stead RJ, Barradas MA, Hodson ME, Batten JC, Dandona P. Platelet abnormalities in patients with cystic fibrosis and obligate heterozygotes. Haematologica. 1990;75:137–40. [PubMed] [Google Scholar]
- 11.Permin H, Skov PS, Norn S, Hoiby N, Schiøtz PO. Platelet 3H- serotonin releasing immune complexes induced by Pseudomonas aeruginosa in cystic fibrosis. Allergy. 1982;37:93–100. doi: 10.1111/j.1398-9995.1982.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 12.Falco A, Romano M, Lapichino L, Collura M, Davì G. Increased soluble CD40 ligand levels in cystic fibrosis. J Thromb Haemost. 2004;2:557–60. doi: 10.1111/j.1538-7836.2004.00683.x. [DOI] [PubMed] [Google Scholar]
- 13.O’Sullivan BP, Michelson AD. The inflammatory role of platelets in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:483–90. doi: 10.1164/rccm.200508-1243PP. [DOI] [PubMed] [Google Scholar]
- 14.Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: Its relationship to bleeding time, thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983;32:443–60. doi: 10.1016/0049-3848(83)90255-4. [DOI] [PubMed] [Google Scholar]
- 15.Thompson CB, Eaton KA, Princiotta SM, Rushin CA, Valeri CR. Size dependent platelet subpopulations: Relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50:509–19. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 16.Dastjerdi MS, Emami T, Najafian A, Amini M. Mean platelet volume measurement, EDTA or citrate? Hematology. 2006;11:317–9. doi: 10.1080/10245330600954163. [DOI] [PubMed] [Google Scholar]
- 17.Varol E, Icli A, Ozaydιn M, Erdogan D, Arslan A. Mean platelet volume is elevated in patients with myocardial infarction with normal coronary arteries, as in patients with myocardial infarction with obstructive coronary artery disease. Scand J Clin Lab Invest. 2009;69:570–4. doi: 10.1080/00365510902829354. [DOI] [PubMed] [Google Scholar]
- 18.Bath PM, Butterworth RJ. Platelet size: Measurement, physiology and vascular disease. Blood Coagul Fibrinolysis. 1996;7:157–61. [PubMed] [Google Scholar]
- 19.Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K, et al. Platelet indices in diabetes mellitus: Indicators of diabetic microvascular complications. Hematology. 2011;16:86–9. doi: 10.1179/102453311X12902908412110. [DOI] [PubMed] [Google Scholar]
- 20.Coban E, Ozdogan M, Yazicioglu G, Akcit F. The mean platelet volume in patients with obesity. Int J Clin Pract. 2005;59:981–2. doi: 10.1111/j.1742-1241.2005.00500.x. [DOI] [PubMed] [Google Scholar]
- 21.Dani C, Poggi C, Barp J, Berti E, Fontanelli G. Mean platelet volume and risk of bronchopulmonary dysplasia and intraventricular hemorrhage in extremely preterm infants. Am J Perinatol. 2011 doi: 10.1055/s-0031-1274503. [In Press] [DOI] [PubMed] [Google Scholar]
- 22.Yüksel O, Helvaci K, Basar O, Köklü S, Caner S, Helvaci N, et al. An overlooked indicator of disease activity in ulcerative colitis: Mean platelet volume. Platelets. 2009;20:277–81. doi: 10.1080/09537100902856781. [DOI] [PubMed] [Google Scholar]
- 23.Makay B, Türkyilmaz Z, Duman M, Unsal E. Mean platelet volume in Henoch-Schönlein purpura: Relationship to gastrointestinal bleeding. Clin Rheumatol. 2009;28:1225–8. doi: 10.1007/s10067-009-1219-7. [DOI] [PubMed] [Google Scholar]
- 24.Kisacik B, Tufan A, Kalyoncu U, Karadag O, Akdogan A, Ozturk MA, et al. Mean platelet volume (MPV) as an inflammatory marker in ankylosing spondylitis and rheumatoid arthritis. Joint Bone Spine. 2008;75:291–4. doi: 10.1016/j.jbspin.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Baykal Y, Saglam K, Yilmaz MI, Taslipinar A, Akinci SB, Inal A. Serum sIL-2r, IL-6, IL-10 and TNF-alpha level in familial Mediterranean fever patients. Clin Rheumatol. 2003;22:99–101. doi: 10.1007/s10067-002-0682-1. [DOI] [PubMed] [Google Scholar]
- 26.Gang N, Drenth JP, Langevitz P, Zemer D, Brezniak N, Pras M, et al. Activation of the cytokine network in familial Mediterranean fever. J Rheumatol. 1999;26:890–7. [PubMed] [Google Scholar]
- 27.Becchi C, Al Malyan M, Fabbri LP, Marsili M, Boddi V, Boncinelli S. Mean platelet volume trend in sepsis: Is it a useful parameter? Minerva Anestesiol. 2006;72:749–56. [PubMed] [Google Scholar]
- 28.Catal F, Bavbek N, Bayrak O, Uz E, Isik B, Karabel M, et al. Platelet parameters in children with upper urinary tract infection: Is there a specific response? Renal Fail. 2008;30:377–81. doi: 10.1080/08860220801947389. [DOI] [PubMed] [Google Scholar]
- 29.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, Potamianos SP, Kosmadaki MG, Koutroubakis IE, et al. Mean platelet volume: A useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96:776–81. doi: 10.1111/j.1572-0241.2001.03621.x. [DOI] [PubMed] [Google Scholar]
- 30.Fantini MC, Monteleone G, Macdonald TT. New players in the cytokine orchestra of inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:1419–23. doi: 10.1002/ibd.20212. [DOI] [PubMed] [Google Scholar]
- 31.van Gameren MM, Willemse PH, Mulder NH, Limburg PC, Groen HJ, Vellenga E, et al. Effects of recombinant human interleukin-6 in cancer patients: a phase I-II study. Blood. 1994;84:1434–41. [PubMed] [Google Scholar]
- 32.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, et al. Interleukin- 6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–5. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 33.Scheid P, Kempster L, Griesenbach U, Davies JC, Dewar A, Weber PP, et al. Inflammation in cystic fibrosis airways: Relationship to increased bacterial adherence. Eur Respir J. 2001;17:27–35. doi: 10.1183/09031936.01.17100270. [DOI] [PubMed] [Google Scholar]
- 34.Weyrich AS, Lindemann S, Zimmerman GA. The evolving role of platelets in inflammation. J Thromb Haemost. 2003;1:1897–905. doi: 10.1046/j.1538-7836.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- 35.Brunetti M, Martelli N, Manarini S, Mascetra N, Musiani P, Cerletti C, et al. Polymorphonuclear leukocyte apoptosis is inhibited by platelet-released mediators: Role of TGFb- 1. Thromb Haemost. 2000;84:478–83. [PubMed] [Google Scholar]
- 36.O’Sullivan BP, Michelson AD. The inflammatory role of platelets in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:483–90. doi: 10.1164/rccm.200508-1243PP. [DOI] [PubMed] [Google Scholar]
- 37.Carpagnano GE, Barnes PJ, Geddes DM, Hodson ME, Kharitonov SA. Increased leukotriene B4 and interleukin-6 in exhaled breath condensate in cystic fibrosis. Am J Respir Crit Care Med. 2003;167:1109–12. doi: 10.1164/rccm.200203-179OC. [DOI] [PubMed] [Google Scholar]


