Abstract
Pulmonary alveolar microlithiasis (PAM) is an uncommon lung disease characterized by accumulation of intraalveolar calcifications. The disease can be diagnosed based on the radiological findings. We present a 27-year-old women with five-year history of shortness of breath. She was diagnosed with PAM due to the presence of the characteristic chest X-ray and thorax computed tomography (CT) findings. We performed 18F-fluorodeoxyglucose (FDG)-PET/CT imaging in order to detect any evidence of inflamation in the lung before deciding an anti-inflammatory treatment. The lung regions with dense calcifications revealed low FDG uptakes (SUVmax: 2.7) and the lung regions without calcifications showed lower FDG uptakes. No further treatment modality was planned besides inhaler salbutamol. Herein, we discuss this rare entity with literature search.
Keywords: FDG, inflammation, PET/CT, pulmonary alveolar microlithiasis
Pulmonary alveolar microlithiasis (PAM) is a rare idiopathic disease characterized by diffuse presence of microliths in the alveoli, and was first described by Friedrich in 1856 and then was more detailed by Harbitz.[1,2] Approximately 600 cases have been reported to date worldwide, mainly from Turkey.[3,4]
There are several studies indicating CT findings of PAM.[5,6] However, we could find only one study demonstrating abnormal accumulation of fluorodeoxyglucose (FDG) in both the lung-sparing calcification.[3] In this paper, we reported the first case of 27-year-old female with PAM presenting with low FDG uptake in FDG-Positron Emission Tomography (PET)/CT.
Case Report
A 27-year-old female patient came to our clinic with five-year history of exertional dyspnea. The patient was a clerk for 8 years. She was smoker with history of 8 pack-year. Her medical history and family history were unremarkable, except 3-year history of PAM. In her physical examination, pulmonary and cardiac system examinations were unremarkable, except the presence of the chest wall deformity (pectus excavatum). The posteroanterior chest X-ray revealed bilateral innumerable, diffuse micronodular opacities predominantly in the middle and lower lung fields [Figure 1]. The pulmonary function test showed a restrictive pattern with results as follows: Forced Vital Capacity (FVC): 1.65 l (44% of predicted), Forced Expiratory Volume in 1st second (FEV1): 1.46 l (52% of predicted), FEV1/FVC: 88%, Diffusing capacity of the lung for carbon monoxide (DLCO): 47 mmol/kPa.min, and DLCO/Alveolar Volume (VA): 80 mmol/kPa.min/l. The laboratory tests, including complete blood count, erythrocyte sedimentation rate, C-reactive protein, and serum calcium level, were in normal limits. The thorax computed tomography (CT) revealed bilateral diffuse interstitial pattern (sandstorm appearance), ground-glass opacities, bilateral apical fibrotic changes, and surrounding pleural calcifications mimicing cardiac constriction [Figure 2]. The whole-body bone scintigraphy showed diffuse Tc-99m methylene diphosphonate (MDP) uptake in the both lungs [Figure 3]. The echocardiography revealed mitral and tricuspit valve calcifications. The pulmonary arterial pressure and the right and left heart functions were measured in normal ranges. The 18F-FDG-PET/CT imaging was performed to evaluate any evidence of inflamation in the lung before starting an anti-inflamatory therapy. The images of FDG-PET/CT revealed low metabolic activity in areas of micronodular opacities [Maximum tandardized uptake value(SUVmax): 2.7]. Other lung regions without calcifications revealed lower FDG uptakes [Figure 4].
Figure 1.
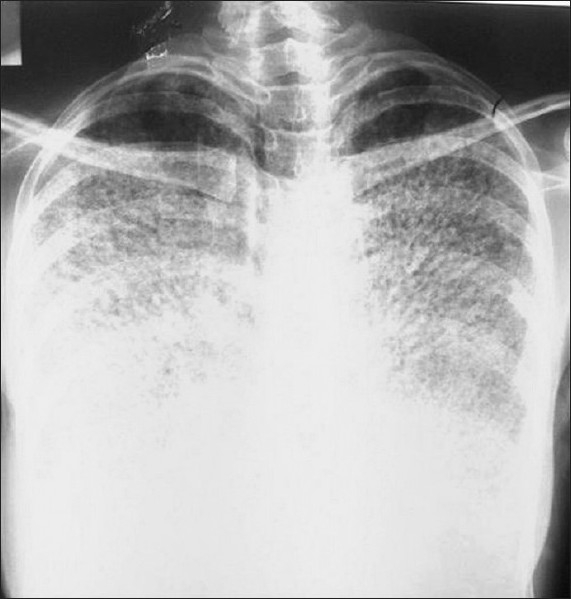
PA chest X-ray of the patient showing bilateral symmetrical micronodular alveolar opacities obscuring cardiac and diaphragmatic shadows
Figure 2.
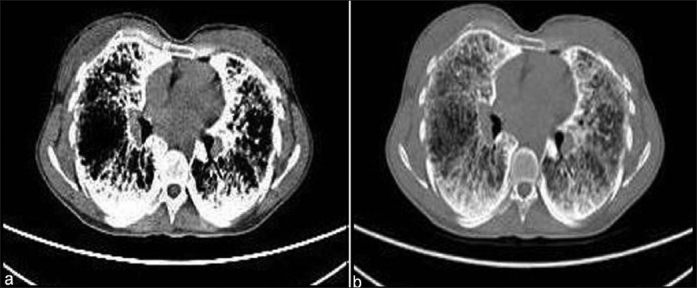
Thorax CT sections of the patient demonstrating bilateral parenchymal micronodular opacities, ground-glass apearance, and mediastinal pleural calcifications on mediastinal (a) and lung (b) windows. Opacities dominantly located at the lower lung regions
Figure 3.
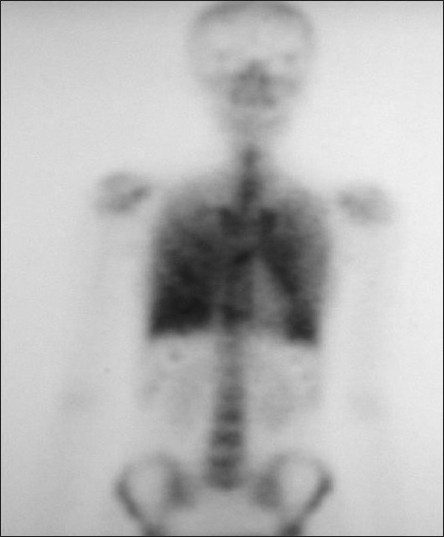
The 99mTc bone scintigraphy of the patient showing diffuse uptake in both the lungs
Figure 4.
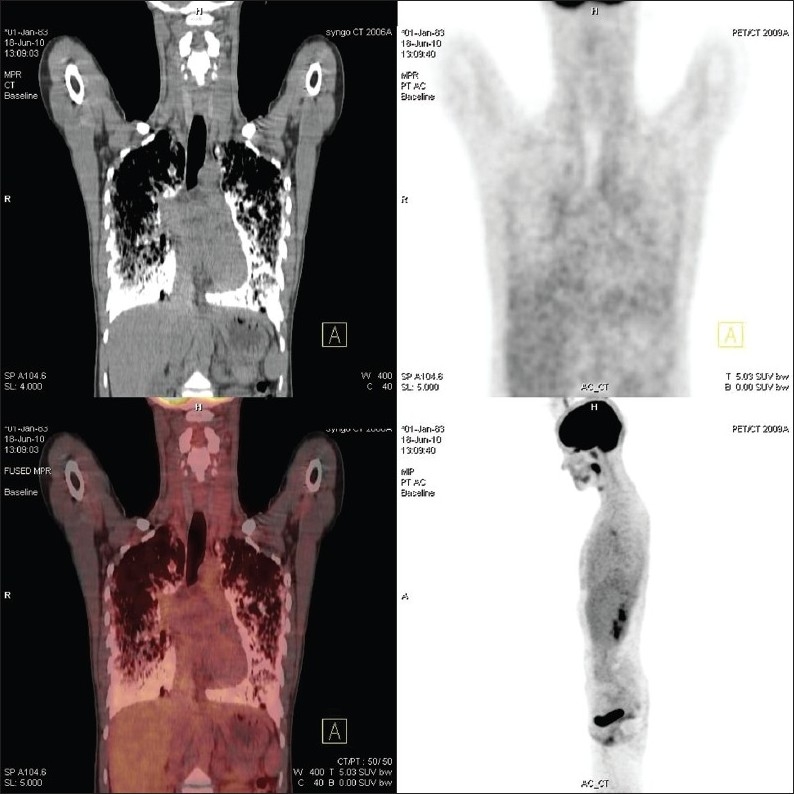
18FDG-PET/CT showing low FDG uptakes (SUVmax: 2.7) in the lower lung areas with oppacities and lower FDG uptakes in the normal lung areas
Only the palliative treatment consisting inhaler salbutamol (max. 800 mg/day, as needed) was prescribed. Patient was got under regular follow-up.
Discussion
Pulmonary alveolar microlithiasis is a rare lung disease characterized by accumulation of numerous, intraalveolar calcified bodies. Microliths range from 0.01 to 3 mm in diameter. The chemical analysis and energy dispersion X-ray microanalysis of the calcified bodies showed that calcium phosphate was the main compenent.[7,8] The etiology and pathogenesis of PAM are unknown. The proposed mechanisms include an inborn error of metabolism, an unusual response to an unspecified pulmonary insult, an immune reaction to various irrritants and infections, and acquired abnormality of calcium and phosphorus metabolism.[9]
Asymptomatic causes, even with extensive radiographic involvement, are often discovered incidentally. Cough and progressive dyspnea are the most common symptoms. Normal or mild restrictive type of pulmonary function tests may be present in the asympthomatic cases.[10] With the progression of the disease, severe lung restriction may ensue with impairement of the diffusion capacity.[11] In our case, dyspnea with exertion was the main complaint. The pulmonary funtion test revealed restrictive type of pulmonary disease with decreased diffusion capacity.
The characterictic chest X-ray findings in adults in PEM are ground-glass or fine sand-like opacities and nodular pattern opacities that may obscure the margins of the mediastinum, heart, and diaphragm, depending on the amount of calcium deposition. The most common chest X-ray findings in children is ground-glass opacities.[4,6] This finding represents the initial stage of the microscopic calcifications that are later followed by macroscopic calcific depositions.[4] Other less common findings are including bullae in the lung apices, black pleural lines, and pleural calcifications.[4,6,12] The black pleural line as a zone of increased translucency between the lung parenchyma and the ribs are first described by Felson.[12] These lines are often an early radiological finding in patients (especially in children) with PAM. The chest X-rays of our patient revealed diffuse micronodular oppacities suggesting PAM.
The use of high-resolution CT (HRCT) allows the identification of minimal structural changes in the lung parenchyma.[4,6] HRCT scans show that there is a gradient of distribution of the calcifications in which lung bases, especially posterior, are more effected than the middle of upper zones.[13] High attenuation of lung parenchyma caused by calcipherites even smaller than 1 mm is visible on HRCT. CT scans demonstrate these micronodular calcific dansities with a greater concentration in the subpleural parenchyma and along the bronchovascular bundles, whereas HRCT scans show thickening of the lobular septae with a distribution of microliths along the septae and around the centrilobular distal bronchioles.[6,14–17] It is believed that chest X-ray and HRCT findings are pathognomonic for the PAM and an open lung biopsy may be avoided in the presence of these characteristic findings.[3,6,18,19] The thorax CT of our patient showed bilateral diffuse interstitial calcifications with a sandstorm appearance, especially on the middle and lower lung fields, bilateral ground-glass opacities, surrounding mediastinal pleural calcifications mimicing cardiac constriction.
Bone scintigraphy can also trace the pulmonary uptake of 99mTc diphosphonate in PAM patients.[4] The bone scan in our patient revealed diffuse accumulation of 99mTc MDP in both lung, suggesting the diagnosis of PAM as well.
To the best of our knowledge, Ito et al. reported the first case with high FDG uptake in PAM.[3] In their case report, high FDG uptake (SUVmax: 7.3) was observed in the lung parenchyma sparing calcifications on CT, whereas lower FDG uptake (SUVmax: 2.6) was observed in the radiodense areas. They discussed the inflammatory changes in PAM and the role of FDG-PET/CT. They could not determinate the mechanism of the enhanced FDG uptake patterns, even though they examined the lung tissue in the postmortem period, and they could not establish a viable answer.[3] In this paper, we present the first case showing low FDG uptake in the calcification of PAM and lower FDG accumulation in the lung parenchyma without micronodular opacities. We believed that FDG-PET/CT revealed low SUVmax levels due the normal levels of inflamatory markers. We postulated that high FDG uptake may be a poor prognosis predictor for PAM because of the death of the patient within one month period in the case report of Ito et al.[3]
Although there is no effective treatment for PAM, treatment with systemic corticosteroids, whole lung lavage, diphosphonates, and lung transplantation (including single lung and bilateral lung transplantaion) were discussed in the literature.[4,17,20] Therefore, we planned to follow up our patient with palliative treatment of inhaler salbutamol without anti-inflammatory treatment.
As a result, PAM is a rare idiopathic lung disease with no effective treatment modality. Chest X-ray and HRCT findings are pathognomonic for diagnosis of PAM. However, FDG accumulation in PET/CT is not helpful for differential diagnosis, but may be useful for evaluation of inflamation or prediction of prognosis. Nevertheless, further studies for investigating the sensitivity of FDG-PET/CT for detecting inflammatory changes and predicting prognosis of PAM are warranted.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
References
- 1.Friedrich N. Corpora amylacea in den lungen. Arch Pathol Anat. 1856;9:613–8. [Google Scholar]
- 2.Harbitz F. Extensive calcifications of the lungs as distinct disease. Arch Intern Med. 1918;21:139–46. [Google Scholar]
- 3.Ito K, Kubota K, Yukihiro M, Izumi S, Miyano S, Kudo K, et al. FDG-PET/CT finding of high uptake in pulmonary alveolar microlithiasis. Ann Nucl Med. 2007;21:415–8. doi: 10.1007/s12149-007-0039-6. [DOI] [PubMed] [Google Scholar]
- 4.Castellana G, Lamorgese V. Pulmonary alveolar microlithiasis world causes and review of the literature. Respiration. 2003;70:549–55. doi: 10.1159/000074218. [DOI] [PubMed] [Google Scholar]
- 5.Deniz O, Ors F, Tozkoparan E, Ozcan A, Gumus S, Bozlar U, et al. High resolution computed tomographic features of pulmonary alveolar microlithiasis. Eur J Radiol. 2005;55:452–60. doi: 10.1016/j.ejrad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Tanrikulu AC, Eren Dagli C, Senygit A, Nazaroglu H, Babayigit C. Pulmonary alveolar microlithiasis: Radiological findings of eight cases in Turkey. Turkiye Klinikleri J Med Sci. 2010;30:713–20. [Google Scholar]
- 7.Mariotta S, Ricci A, Papale M, De Clementi F, Sposato B, Guidi L, et al. Pulmonary alveolar microlithiasis: Report on 576 cases published in the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2004;21:173–81. [PubMed] [Google Scholar]
- 8.Kanra G, Tanyol E, Göçmen A, Yurdakök M, Seçmeer G, Oran O, et al. Pulmonary alveolar microlithiasis: A case report. Turk J Pediatr. 1988;30:61–7. [PubMed] [Google Scholar]
- 9.Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med. 2002;165:1654–69. doi: 10.1164/rccm.2108054. [DOI] [PubMed] [Google Scholar]
- 10.Shah MS, Nanavati KJ, Airon A, Shah RR, Joshi BD. Case report-pulmonary alveolar microlithiasis. Indian J Radiol Imag. 2003;13:277–9. [Google Scholar]
- 11.Barbolini G, Rossi G. Pulmonary alveolar microlithiasis. N Eng J Med. 2002;347:69–70. doi: 10.1056/NEJM200207043470118. [DOI] [PubMed] [Google Scholar]
- 12.Felson B. The reontgen diagnosis of disseminated pulmonary alveolar diseases. Semin Roentgenol. 1967;2:3–6. [Google Scholar]
- 13.Chalmer AG, Wyatt J, Robinson PJ. Computed tomographic and pathological findings in pulmonary alveolar microlithiasis. Br J Radiol. 1986;59:408–11. doi: 10.1259/0007-1285-59-700-408. [DOI] [PubMed] [Google Scholar]
- 14.Abu-Ghazzeh YM. Pulmonary alveolar microlithiasis: High-resolution CT scan. Ann Saudi Med. 2000;20:47–8. doi: 10.5144/0256-4947.2000.47. [DOI] [PubMed] [Google Scholar]
- 15.Khan AN, Al-Jahdali HH, Allen CM, Irion KL, Al Ghanem S, Koteyar SS. The calcified lung nodule: What does it mean? Ann Thorac Med. 2010;5:67–79. doi: 10.4103/1817-1737.62469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachibana T, Hagiwara K, Johkoh T. Pulmonary alveolar microlithiasis review and management. Curr Opin Pulm Med. 2009;15:486–90. doi: 10.1097/MCP.0b013e32832d03bb. [DOI] [PubMed] [Google Scholar]
- 17.Samano MN, Waisberg DR, Canzian M, Campos SV, Pêgo-Fernandes PM, Jatene FB. Lung transplantation for pulmonary alveolar microlithiasis: A case report. Clinics. 2010;65:233–6. doi: 10.1590/S1807-59322010000200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gasparetto EL, Tazoniero P, Escuissato DL, Marchiori E, Frare E Silva RL, et al. Pulmonary alveolar microlithiasis presenting with crazy-paving pattern on high resolution CT. Br J Radiol. 2004;77:974–6. doi: 10.1259/bjr/96331922. [DOI] [PubMed] [Google Scholar]
- 19.Kirova G, Sergieva S, Palaeev K. Pulmonary alveolar microlithiasis associated with hypertrophic osteoarthropathy-CT and scintigraphic presentaions in two sblings. Eur J Radiol Extra. 2004;51:31–5. [Google Scholar]
- 20.Gocmen A, Toppare MF, Kiper N, Büyükpamukcu N. Treatment of pulmonar alveolar microlithiasis with diphosphanate: Preliminary results of a case. Respiration. 1992;59:250–2. doi: 10.1159/000196068. [DOI] [PubMed] [Google Scholar]


