Abstract
Current diagnostic tools to assess neurological injury after aneurysmal subarachnoid hemorrhage (aSAH) and traumatic brain injury (TBI) have poor discriminatory abilities. Free radicals are associated with the pathophysiology of secondary damage after brain trauma. We examined cerebrospinal fluid (CSF) lipid markers of oxidative stress, isofurans (IsoFs), F4-neuroprostanes (F4-NeuroPs), and F2-isoprostanes (F2-IsoPs), in two case-controlled studies in patients with aSAH or severe TBI. Patients with aSAH (n=18) or TBI (n=18) were age and gender matched with separate control groups. CSF samples were collected from patients within 24 h of the injury. CSF IsoFs and F4-NeuroPs were increased in aSAH patients compared with their controls. In TBI patients, IsoFs and F4-NeuroPs were increased compared with their controls. F2-IsoPs were increased in aSAH patients, but not in TBI patients, compared with their respective controls. CSF IsoFs and F4-NeuroPs are consistently increased after a catastrophic central nervous system injury. These results suggest their measurement may enhance the management of unconscious patients in neurological care. Antioxid. Redox Signal. 15, 2663–2667.
Brain Injury and Oxidative Stress
Aneurysmal subarachnoid hemorrhage (aSAH) and traumatic brain injury (TBI) are associated with devastating central nervous system (CNS) injury. aSAH is associated with a high mortality rate and substantial lifelong morbidity in survivors. TBI is a major health and socioeconomic problem more prevalent in young male adults. The most common secondary injury resulting from aSAH and TBI is attributable to vasospasm-induced microvascular injury and cerebral ischemia. Diagnostic tools use surrogate measures such as intracranial pressure changes and interval changes in neuroimaging, which have poor discriminatory abilities.
After acute brain injury, there is a disruption of calcium homeostasis, excitotoxicity from release of neurotransmitters such as glutamate, overproduction of reactive oxygen species (ROS), and opening of the mitochondrial permeability transition pore, all key factors in promoting mitochondrial dysfunction, with ensuing activation of either necrotic or apoptotic cell death pathways. The high oxygen requirements of the brain for metabolism and its high polyunsaturated fatty acid composition, in particular docosahexaenoic acid (DHA, 22:6 ω 3), make the brain vulnerable to oxidative insult.
Innovation
Aneurysmal subarachnoid hemorrhage (aSAH) and traumatic brain injury (TBI) associate with catastrophic central nervous system (CNS) injury. Diagnostic tools to assess neurological injury after aSAH and TBI currently have poor discriminatory capacity. Free radicals are likely to be involved in mediating secondary damage after brain trauma. In two case-controlled studies of patients with aSAH or severe TBI, we examined lipid markers of oxidative stress, isofurans (IsoFs), F4-neuroprostanes (F4-NeuroPs), and F2-isoprostanes (F2-IsoPs), in cerebrospinal fluid (CSF) samples. In aSAH and TBI patients, IsoFs and F4-NeuroPs were increased compared with their respective controls. F2-IsoPs were increased in aSAH patients, but not in TBI patients. Increased IsoFs and F4-NeuroPs in the CSF of these patients is a new and important finding that may have important clinical implications.
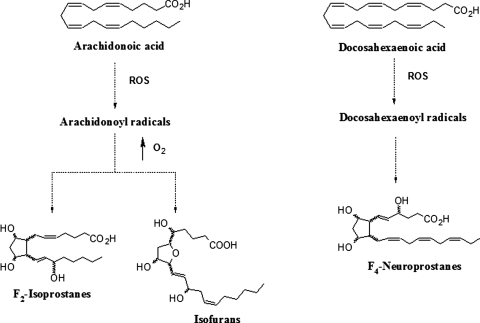
Free radicals are associated with the pathophysiology of secondary damage after brain trauma. Nonenzymatic free radical lipid markers of oxidative stress produced in vivo include F2-isoprostanes (F2-IsoPs) derived from arachidonic acid (AA), isofurans (IsoFs) formed from AA under high oxygen tension, and F4-neuroprostanes (F4-NeuroPs) derived from DHA (6) (Fig. 1). F2-IsoPs, such as 15-F2t-IsoP, affect vascular and platelet function, whereas the role of F4-NeuroPs and IsoFs on vascular homeostasis has not been fully characterized.
FIG. 1.
Formation of F2-isoprostanes (F2-IsoPs) and Isofurans (IsoFs) from arachidonic acid, and F4-Neuroprostanes (F4-NeuroPs) from docosahexaenoic acid.
Previous studies have shown that cerebrospinal fluid (CSF) but not plasma concentrations of F2-IsoPs (4) and CSF F4-NeuroPs (3) are significantly elevated in patients after an aSAH. To our knowledge, no study has examined whether IsoFs are altered in the CSF of patients after aSAH. Increased CSF F2-IsoPs have been reported in TBI patients (2, 7–9). To date, no study has examined IsoFs and F4-NeuroPs in patients with TBI.
The aim of this study was to examine CSF IsoFs, F4-NeuroPs, and F2-IsoPs in two case-controlled studies in patients after an aSAH or severe TBI. The study also aimed to determine whether free radical production in CSF is different between aSAH and TBI patients. These data could provide important information on the reliability of these markers of oxidative stress in CNS injury and assist in the neurocritical care of these patients.
Measures of Lipid Oxidation in SAH and TBI
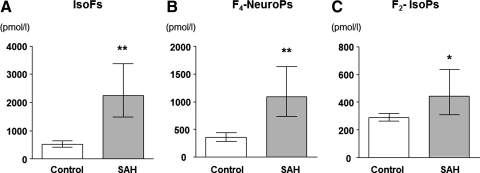
aSAH and TBI patients and their respective controls were comparable (Table 1). However, aSAH patients were significantly older and had a greater prevalence of pre-existing hypertension and hypercholesterolemia than TBI patients (p<0.05). At admission, 12 of the 18 aSAH patients and all 18 of the TBI patients had a Glasgow Coma Score less than 8, indicating severe brain injury. Figure 2 shows IsoFs, F4-NeuroPs, and F2-IsoP in the CSF of aSAH patients and their respective controls. IsoFs and F4-NeuroPs were significantly increased in aSAH patients compared with controls and remained significant after adjusting for age and gender (p<0.001 and p<0.001, respectively). Age- and gender-adjusted F2-IsoP in CSF of aSAH patients were increased compared with controls (p=0.011).
Table 1.
Characteristics of the Participants
| aSAH (n=18) | Controls (n=18) | TBI (n=18) | Controls (n=18) | |
|---|---|---|---|---|
| Age (years)a | 49.1±3.9 | 50.1±3.8 | 31.3±3.1 | 33.4±3.2 |
| Gender ratio M/F (n) | 7/11 | 7/11 | 15/3 | 15/3 |
| Smokers (n) | 0 | 0 | 1 | 0 |
| Pre-existing conditions (n) | ||||
| Hypertensiona | 7 | 0 | 0 | 0 |
| Diabetes | 1 | 0 | 1 | 0 |
| Hypercholesterolemiaa | 8 | 0 | 0 | 0 |
| COPD | 1 | 0 | 0 | 0 |
Values are mean±SD.
p<0.05 for aSAH compared with TBI patients.
aSAH, aneurysmal subarachnoid hemorrhage; COPD, chronic obstructive pulmonary disease; TBI, traumatic brain injury.
FIG. 2.
Cerebrospinal fluid (CSF) IsoFs (A), F4-NeuroPs (B), and F2-IsoPs (C) in subarachnoid hemorrhage (SAH) patients and in controls. Values are geometric mean and 95% CI, *p≤0.05, **p≤0.001.
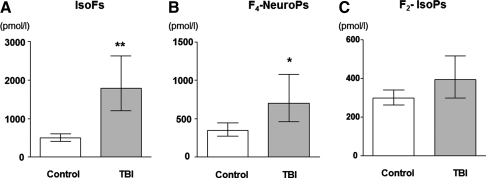
Figure 3 shows CSF IsoFs, F4-NeuroPs, and F2-IsoP in TBI patients and their respective controls. IsoFs and F4-NeuroPs in TBI patients were significantly increased compared with controls and remained significant after adjusting for age and gender (p<0.001 and p=0.013, respectively). CSF F2-IsoPs were not significantly different between the groups (p=0.119).
FIG. 3.
CSF IsoFs (A), F4-NeuroPs (B), and F2-IsoPs (C) in traumatic brain injury (TBI) patients and in controls. Values are geometric mean and 95% CI, *p≤0.05, **p≤0.001.
There were no significant differences between the aSAH and TBI patients in CSF IsoFs, F2-IsoPs, or F4-NeuroPs, after adjusting for age and gender.
Do IsoFs, F4-NeuroPs, and F2-IsoP Have a Role in SAH and TBI?
This is the first study to comprehensively examine IsoFs, F4-NeuroPs, and F2-IsoPs in the CSF of patients with two catastrophic CNS injuries, namely, aSAH and TBI. In two case-controlled studies we have shown a significant increase in IsoFs in aSAH and TBI patients compared with their respective age- and gender-matched controls. aSAH patients also had significantly increased levels of F4-NeuroPs and F2-IsoPs. Patients with TBI had significantly increased F4-NeuroPs, but F2-IsoPs were not different from their controls. These data show that CNS injury as a result of aSAH or TBI results in increased oxidative stress.
Increased concentrations of IsoFs in CSF after an aSAH or TBI are a new and important finding that may have clinical implications. IsoFs are influenced by oxygen tension (6) and altering oxygen concentration is an easily modifiable core component of clinical care in these patients. In contrast to F2-IsoPs, production of IsoFs continues to rise with increasing ambient oxygen concentrations (6). Previous studies have shown that increased CSF F2-IsoPs (4) and F4-NeuroPs (3) correlated with poor neurological outcome in aSAH patients. Whether increased IsoF levels associate with adverse outcomes and whether their concentration can be varied by altering inspired oxygen concentration remain to be determined.
We have shown for the first time that F4-NeuroPs are significantly increased in the CSF of patients with TBI. Our data also confirm a previous report that F4-NeuroPs are significantly elevated in the CSF of aSAH patients (3). Since DHA is the major polyunsaturated fatty acid in the brain, F4-NeuroP levels in CSF are likely a more specific indicator of possible neurological dysfunction than F2-IsoPs. The F2-IsoPs derive from oxidative damage to both neural and non-neural cells in the brain, including vascular cells and blood cells, since AA is present in all cells. In this regard, Hsieh et al. (3) showed that increased F4-NeuroPs in CSF of patients with aSAH correlated with poor neurological outcome and suggested that F4-NeuroPs might be more useful than F2-IsoPs in CSF to predict outcome and interpret the role of hemorrhage in aSAH.
Our study has shown that CSF F2-IsoPs in aSAH patients were significantly increased. These data accord with previous work showing that CSF F2-IsoPs obtained within 24 h of surgery are significantly higher in aSAH patients compared with control patients (4). F2-IsoPs were also significantly higher in aSAH patients who developed delayed onset cerebral vasospasm (1). Asaeda et al. (1) showed that F2-IsoPs increased during the period of peak vasospasm and decreased gradually thereafter. The authors suggested that increased CSF F2-IsoPs derived from the subarachnoid clot could be one of the causes of delayed cerebral vasospasm after aSAH. Since F2-IsoPs promote vasoconstriction and platelet aggregation (6), they may be either mediators or markers of increased vasospasm risk. This might also explain their association with poor outcome (4). It remains to be seen whether therapeutic interventions (cerebral angioplasty) can alter the concentrations of these compounds.
Increased CSF F2-IsoPs have in been shown in adults (7, 9) and infants and children (2) following a TBI compared with controls. Varma et al. (8) showed that increased CSF F2-IsoPs in infants and children with TBI correlated with neuron-specific enolase, a marker of neuronal damage. In contrast, we have shown that CSF F2-IsoPs were not different between TBI and controls. Differences between previous studies and our findings may relate to methodological differences in the measurement of F2-IsoPs and/or patient characteristics. The measurement of F2-IsoPs using mass spectrometry as used by us is considered the gold standard, whereas previous studies (2, 9) have used an enzyme-linked immunoassay kit. We have shown poor agreement between F2-IsoPs measured using an enzyme-linked immunoassay kit and mass spectrometry (5).
Concluding Remarks and Future Directions
There is an urgent need to identify sensitive and specific markers that will enable the assessment of ongoing CNS injury in deeply comatose patients. CSF IsoFs and F4-NeuroPs are consistently increased after a catastrophic CNS injury and their measurement may enhance the management of unconscious patients in neurological care. The increase in CSF IsoFs and F4-NeuroPs in aSAH and TBI patients will require further investigation to establish their association with outcomes and whether they can indicate the efficacy of therapeutic interventions. In particular, increased IsoFs in the CSF of patients are a new and important finding that may have important clinical implications. IsoFs are altered by oxygen tension, which is an easily modifiable during the clinical care of these patients (a fully referenced discussion may be viewed as Supplementary Data; available online at www.liebertonline.com/ars).
Notes
Patients
In two case-controlled studies, 18 comatose patients with severe aSAH and 18 comatose patients with severe TBI were recruited and matched for age and gender with healthy controls. Controls were adults undergoing subarachnoid anesthesia for elective lower limb surgery. The age difference between aSAH and TBI patients necessitated recruitment of two separate control groups matched for age with the patients. The studies were approved by the human ethics committee of Royal Perth Hospital. Informed written consent was obtained from controls and next-of-kin provided consent for the comatose patients.
CSF sampling
CSF samples were collected from patients within 24 h of the injury from an external ventricular drain inserted, to permit monitoring of intracranial pressures and drainage of CSF. Samples of CSF from control patients were obtained from the subarachnoid space using a spinal needle. CSF was collected into EDTA, reduced glutathione, and butylated hydroxytoluene to prevent ex vivo oxidation. Samples were centrifuged at 4°C and stored at −80°C until analysis.
Measurement of CSF IsoFs, F4-NeuroPs, and F2-IsoPs
IsoFs, F4-NeuroPs, and F2-IsoPs were measured using a modification of our previously reported method (5). Briefly, samples were spiked with 5 ng internal standard 15-F2t-IsoP-d4 (8-iso-PGF2α-d4) purchased from Cayman Chemicals (Ann Arbor, MI), then hydrolyzed with 1 M potassium hydroxide in methanol, acidified, and applied to prewashed Certify II cartridges (Varian, Lake Forrest, CA). After washing with methanol/water (1:1) and hexane/ethyl acetate (75:25), the metabolites were eluted with ethyl acetate/methanol (90:10) and dried under vacuum. Samples were derivatized using pentafluorobenzylbromide and N,N-diisopropylethylamine (Sigma Chemicals, St. Louis, MO), dried under nitrogen, and then treated with the silylating agent N,O-bis-(trimethylsilyl)trifluoroacetamide with 1% trimethylchlorosilane (Pierce Chemicals, Rockford, IL). IsoFs, F2-IsoPs, and F4-NeuroPs were quantitated by gas chromatography–mass spectrometry using electron capture negative ionization and selected ion monitoring. Ions monitored were m/z 569, 573, 585, and 593 for F2-IsoP, 15 F2t-IsoP-d4, IsoFs, and F4-NeuroPs, respectively. IsoFs and 4(RS)-F4t-NeuroP were synthesized in our laboratory as previously described.
Statistical analysis
Analyses used the STATA (version 11) statistical package (STATA Corp, College Station, TX). CSF IsoFs, F2-IsoPs, and F4-NeuroPs were transformed using the natural log before analysis. Differences in CSF IsoFs, F2-IsoPs, and F4-NeuroPs between aSAH and controls, and TBI and controls, were analyzed using a general linear model (GLM) with bootstrap estimation of the standard error to accommodate the matched clusters. Differences in CSF IsoF, F2-IsoP, and F4-NeuroPs between aSAH and TBI patients were examined using GLM adjusting for age and gender. A p-value less than 0.05 was regarded as statistically significant. A power analysis indicated that the sample size of the study would have power in excess of 80% to detect an effect size of 0.5.
Supplementary Material
Abbreviations Used
- AA
arachidonic acid
- aSAH
aneurysmal subarachnoid hemorrhage
- CNS
central nervous system
- COPD
chronic obstructive pulmonary disease
- CSF
cerebrospinal fluid
- DHA
docosahexaenoic acid
- EDTA
ethylenediaminetetraacetic acid
- F2-IsoPs
F2-isoprostanes
- F4-NeuroPs
F4-neuroprostanes
- GLM
general linear model
- IsoFs
isofurans
- ROS
reactive oxygen species
- TBI
traumatic brain injury
Acknowledgments
We acknowledge Lynette McCahon for technical assistance. The study was supported by a grant from the Neurotrauma Research Program of the West Australian Institute of Medical Research and the University of Western Australia. The synthesis of the isofurans (Prof Roberts II L.J.) was supported by a National Institutes of Health Grant GM42056. The synthesis of the neuroprostanes (Dr. Durand T.) was supported by a University Montpellier 1 Grant BQR-2008.
References
- 1.Asaeda M. Sakamoto M. Kurosaki M. Tabuchi S. Kamitani H. Yokota M. Watanabe T. A non-enzymatic derived arachidonyl peroxide, 8-iso-prostaglandin F2 alpha, in cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage participates in the pathogenesis of delayed cerebral vasospasm. Neurosci Lett. 2005;373:222–225. doi: 10.1016/j.neulet.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Bayir H. Kagan VE. Tyurina YY. Tyurin V. Ruppel RA. Adelson PD. Graham SH. Janesko K. Clark RS. Kochanek PM. Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res. 2002;51:571–578. doi: 10.1203/00006450-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Hsieh YP. Lin CL. Shiue AL. Yin H. Morrow JD. Hsu JC. Hsieh TC. Wei HJ. Yen HC. Correlation of F4-neuroprostanes levels in cerebrospinal fluid with outcome of aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2009;47:814–824. doi: 10.1016/j.freeradbiomed.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 4.Lin CL. Hsu YT. Lin TK. Morrow JD. Hsu JC. Hsu YH. Hsieh TC. Tsay PK. Yen HC. Increased levels of F2-isoprostanes following aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2006;40:1466–1473. doi: 10.1016/j.freeradbiomed.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Proudfoot J. Barden A. Mori TA. Burke V. Croft KD. Beilin LJ. Puddey IB. Measurement of urinary F(2)-isoprostanes as markers of in vivo lipid peroxidation-A comparison of enzyme immunoassay with gas chromatography/mass spectrometry. Anal Biochem. 1999;272:209–215. doi: 10.1006/abio.1999.4187. [DOI] [PubMed] [Google Scholar]
- 6.Roberts LJ. Fessel JP. The biochemistry of the isoprostane, neuroprostane, and isofuran pathways of lipid peroxidation. Chem Phys Lipids. 2004;128:173–186. doi: 10.1016/j.chemphyslip.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Seifman MA. Adamides AA. Nguyen PN. Vallance SA. Cooper DJ. Kossmann T. Rosenfeld JV. Morganti-Kossmann MC. Endogenous melatonin increases in cerebrospinal fluid of patients after severe traumatic brain injury and correlates with oxidative stress and metabolic disarray. J Cereb Blood Flow Metab. 2008;28:684–696. doi: 10.1038/sj.jcbfm.9600603. [DOI] [PubMed] [Google Scholar]
- 8.Varma S. Janesko KL. Wisniewski SR. Bayir H. Adelson PD. Thomas NJ. Kochanek PM. F2-isoprostane and neuron-specific enolase in cerebrospinal fluid after severe traumatic brain injury in infants and children. J Neurotrauma. 2003;20:781–786. doi: 10.1089/089771503767870005. [DOI] [PubMed] [Google Scholar]
- 9.Wagner AK. Bayir H. Ren D. Puccio A. Zafonte RD. Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI: the impact of gender, age, and hypothermia. J Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.