Abstract
Periodontal disease is a chronic microbial infection that triggers inflammation-mediated loss of the periodontal ligament and alveolar bone that supports the teeth. Because of the increasing prevalence and associated comorbidities, there is a need for the development of new diagnostic tests that can detect the presence of active disease, predict future disease progression, and evaluate the response to periodontal therapy, thereby improving the clinical management of periodontal patients. The diagnosis of active phases of periodontal disease and the identification of patients at risk for active disease represent challenges for clinical investigators and practitioners. Advances in diagnostic research are moving toward methods whereby the periodontal risk can be identified and quantified by objective measures using biomarkers. Patients with periodontitis may have elevated circulating levels of specific inflammatory markers that can be correlated to the severity of the disease. Advances in the use of oral fluids as possible biological samples for objective measures of the current disease state, treatment monitoring, and prognostic indicators have boosted saliva- and other oral-based fluids to the forefront of technology. Gingival crevicular fluid (GCF) is an inflammatory exudate that can be collected at the gingival margin or within the gingival crevice. This article highlights recent advances in the use of biomarker-based disease diagnostics that focus on the identification of active periodontal disease from plaque biofilms, GCF, and saliva.
Keywords: Biomarkers, C-telopeptide pyridinoline, gingival crevicular fluid, periodontal disease
INTRODUCTION
Diagnosis of periodontal disease has been primarily based upon clinical and radiographic measures of periodontal tissue destruction. These parameters provide a measure of past destruction and are of limited use in early diagnosis.[1] A goal of periodontal diagnostic procedures is to provide useful information to the clinician regarding the present periodontal disease, type, location, and severity which serves as a basis for treatment planning, and provide essential data during periodontal maintenance and disease monitoring phases of treatment. Biomarkers of disease play an important role in life sciences and have begun to assume a greater role in diagnosis, monitoring of therapy outcomes, and drug discovery.[2] For biomarkers to assume their rightful role in routine practice, it is essential that their relation to the mechanism of disease progression and therapeutic intervention be more fully understood.
On the basis of our current understanding of the complexity of periodontitis, the identification of one single diagnostic marker for all forms of periodontal disease seems illusionary.[3] Nevertheless, researchers have been searching actively for unequivocal markers of periodontitis in the gingival crevicular fluid (GCF) to develop a simple test, to be used chair-side, to determine whether a patient suffers from periodontitis and needs therapy, as opposed to another patient who needs no intervention even though he or she has gingivitis.[4]
BIOMARKERS
A biomarker is an objective measure that has been evaluated and confirmed either as an indicator of physiologic health, a pathogenic process, or a pharmacologic response to a therapeutic intervention.
NEED FOR A PERIODONTAL DIAGNOSTIC INDICATOR
A periodontal diagnostic tool provides pertinent information for differential diagnosis, localization of disease, and severity of infection. These diagnostics, in turn, serve as a basis for planning treatment and provide a means for assessing the effectiveness of periodontal therapy.[5]
Conventional disease diagnosis techniques lack the capacity to identify highly susceptible patients who are at risk for future breakdown. Both automated periodontal probes and subtraction radiography techniques are most often seen in the research setting and seldom in clinical practice. Researchers are confronted then with the need for an innovative diagnostic test that focuses on the early recognition of the microbial challenge to the host.[6] Optimal innovative approaches would correctly determine the presence of the current disease activity, predict sites vulnerable for future breakdown, and assess the response to periodontal interventions [Figures 1 and 2]. A new paradigm for periodontal diagnosis would ultimately affect the improved clinical management of periodontal patients.[7]
Figure 1.
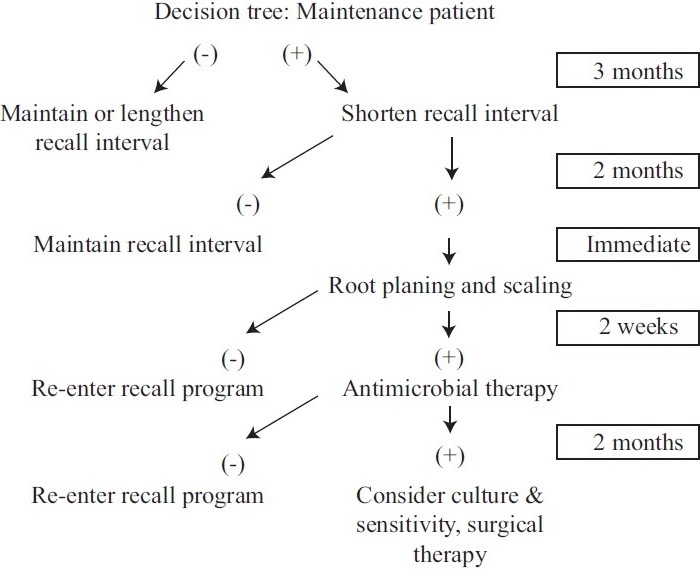
A hypothetical clinical decision tree for patients who have been treated for chronic adult periodontitis and are on a 3-month recall schedule. Diagnostic testing occurs once per year. If the test is negative, the recall program does not have to be altered. If the test is positive, the recall interval can be shortened and the patient would be tested at the next recall, but before any treatment is provided. If the test is negative, the patient can reenter the regular, but shortened, recall schedule. If the test is positive, more aggressive therapy (i.e., root planning and scaling) could be provided and the patient is tested again 2 weeks after therapy. At this point, a negative test would return the patient to the recall schedule, but a positive test would suggest an increased risk for PAL, and more aggressive therapy is indicated
Figure 2.
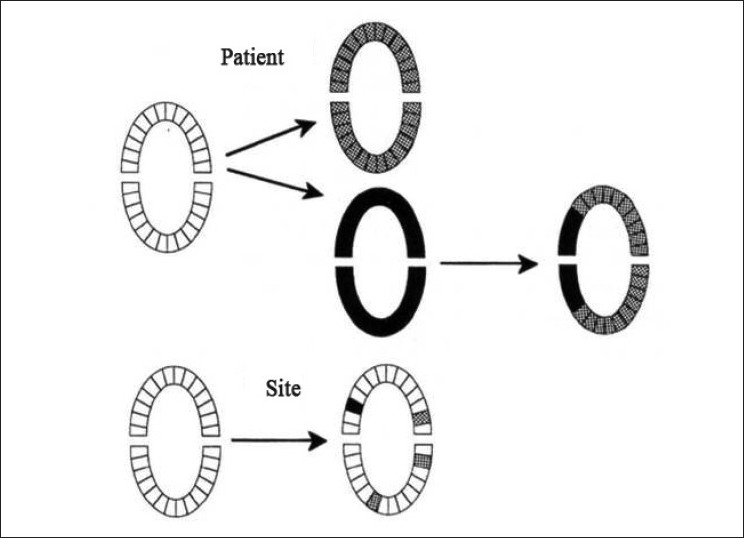
Approaches to patient sampling for diagnostic tests for periodontal disease that do not involve measurements at all sites in a mouth. In the configuration at the top, one sample is collected from each tooth. The patient is identified as positive (at risk) or negative (not at risk) based on the data from two collection times (before and after therapy). Additional information concerning the regions of the mouth that may be at risk (arch, sextant) can be determined. The specific sites experiencing PAL could not always be identified. In the example at the bottom, individual sites are sampled, and the risk for PAL at those sites is monitored. Sites or regions in the mouth experiencing PAL that are not sampled would not be identified.  = patient, region, or site that is sampled and identified as positive. ■ = patient, region, or site that is sampled and identified as negative
= patient, region, or site that is sampled and identified as positive. ■ = patient, region, or site that is sampled and identified as negative
BIOMARKERS OF PERIODONTAL DISEASE
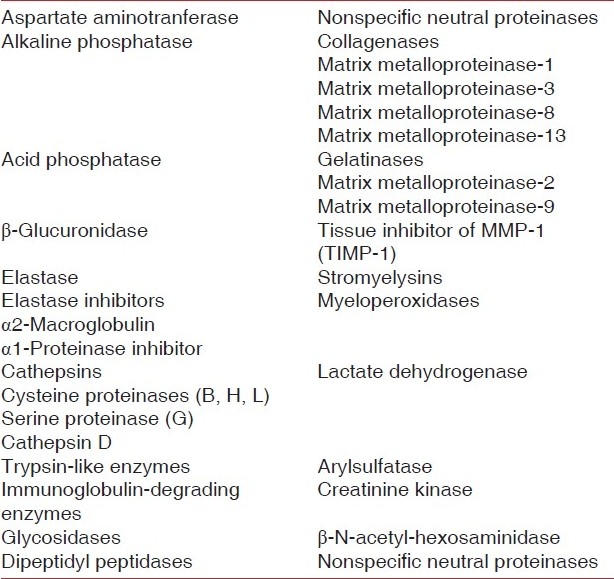
In the disease process, potential biomarkers of the disease activity would need to be involved in some way in the disease process or released as a consequence of tissue damage during disease progression [Figure 3 and Tables 1 & 2].
Figure 3.
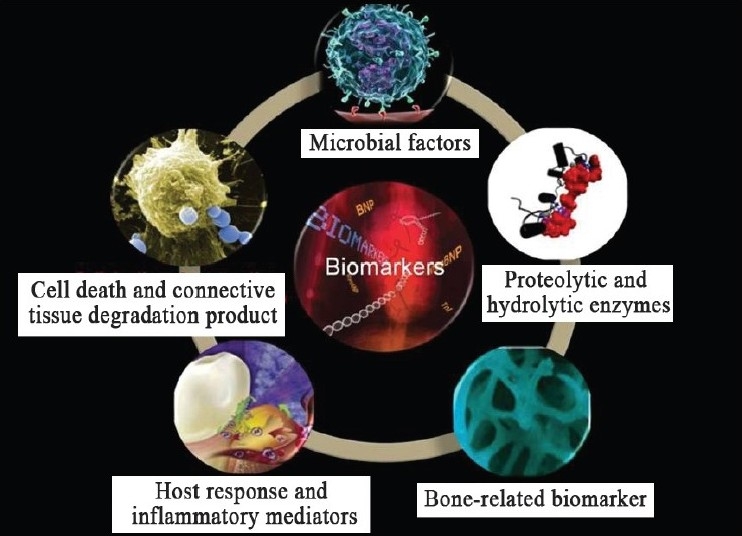
Biomarkers in periodontal disease
Table 1.
Diagnostic tools to measure periodontal disease at molecular, cellular, and clinical levels
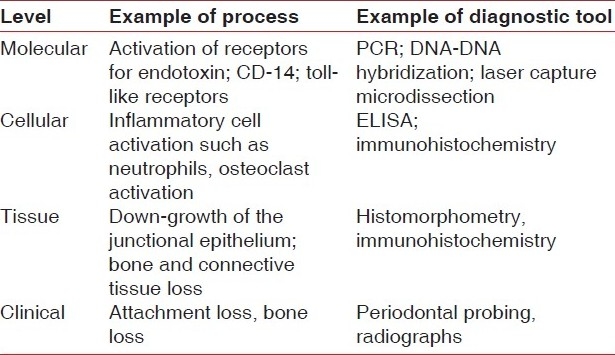
Table 2.
Biomarkers of periodontal disease identified from plaque biofilm, gingival crevicular fluid, or saliva

MICROBIAL FACTORS FOR THE DIAGNOSIS OF PERIODONTAL DISEASES
A number of specific periodontal pathogens have been implicated in periodontal diseases, including Tanerella forsythensis, Porphyromonas gingivalis, and Treponema denticola. These three organisms are members of the “red complex” of bacteria (and exhibit the benzoyl-DL-arginine-naphthylamide, or BANA, activity) that are highly implicated in the progression of periodontal diseases. Aggregatibacter actinomycetemcomitans has been linked with early onset forms of periodontal disease and aggressive periodontitis, whereas red complex bacteria are associated with chronic periodontitis.
The rationale for the use of microbial analysis for periodontitis monitoring is to target pathogens implicated in disease to:
Identify specific periodontal diseases,
Identify antibiotic susceptibility of infecting organisms colonizing diseased sites, and
Predict disease activity.
In patients with periodontal diseases, does microbial identification influence patient management compared with treatment prescribed without this information?
Critical analysis
The goal of microbiologic monitoring is twofold (disease monitoring and disease treatment guidance); however, microbial tests (e.g., BANA test, DNA probe analysis, or culturing) have failed to predict future disease progression.
Future studies are needed in this area to justify the use of microbial testing to predict the progression of periodontal diseases. New strategies that combine microbial identification with the host response or tissue breakdown factors using discriminant analysis may better improve the ability of microbial analysis to predict future periodontal disease around teeth and dental implants.
HOST RESPONSE AND INFLAMMATORY MEDIATORS
GCF has been extensively investigated for the release of host response factors [Table 3]. It includes a mixture of molecules from blood, host tissue, and plaque biofilms, such as electrolytes, small molecules, proteins, cytokines, antibodies, bacterial antigens, and enzymes.[8] Host cell-derived enzymes such as matrix metalloproteinases (MMPs) are an important group of neutral proteinases implicated in the destructive process of periodontal disease that can be measured in GCF. The neutrophils are the major cells responsible for the MMP release at the infected site, specifically MMP-8 (collagenase-2) and MMP-9 (gelatinase-B). Although MMP-8 is able to potently degrade interstitial collagens, MMP-9 degrades several extracellular matrix proteins. Studies showed the use of a rapid chair-side test based on the immunologic detection of elevated MMP-8 in GCF to diagnose and monitor the course and treatment of periodontitis.[9] With a threshold of 1 mg/L MMP-8 activity, the test provided a sensitivity of 0.83 and a specificity of 0.96, demonstrating the value as a potential tool to differentiate periodontitis from gingivitis and healthy sites and to monitor treatment of periodontitis. Macrophages and polymorphonuclear leukocytes, in response to the chemoattractant effect of bacterial lipopolysaccharide, are activated to produce important inflammatory mediators - notably, TNF-α, IL-1, IL-6, and other cytokines related to the host response and tissue destruction. Bone resorption activity and IL-1α, IL-1β, and IL-1 receptor antagonist levels in GCF in sites having no signs of periodontal disease and in sites having horizontal or angular periodontal bone loss were investigated.[10] The amounts of IL-1α, IL-1β, and IL-1 receptor antagonist from GCF were quantified by ELISA. It was observed that bone resorption activity and levels of IL-1α, IL-1β, and IL-1 receptor antagonists were significantly higher in GCF from diseased sites compared with healthy sites but did not relate to defect morphology. Elevated levels of aspartate aminotransferase (AST), however, were present at sites that did not subsequently exhibit disease progression.[11] Therefore, the biomarker does not discriminate between progressive sites and sites that are stable but inflamed.
Table 3.
Host response and inflammatory mediators

In summary, GCF carries multiple molecular factors derived from the host response and is considered a significant protective mechanism in periodontal infection. These host response factors represent important mediators that can aid in the development of periodontal diagnostics.
Advanced stages of periodontal lesions are populated by a large proportion of B lymphocytes and plasma cells and increased levels of immunoglobulins in GCF. Compared with healthy patients, the GCF of periodontitis patients contained significantly higher levels of IgA and IgG antibodies to the four micro-organisms tested.
PROTEOLYTIC AND HYDROLYTIC ENZYMES AS MARKERS OF THE DISEASE ACTIVITY
Critical analysis of biomarkers
Aspartate aminotransferase
Oringer in 2001 suggested that AST, a tissue destruction biomarker released from necrotic cells in GCF, is associated with periodontitis severity [Table 4].[12]
Table 4.
Proteolytic and hydrolytic enzymes
Alkaline phosphatase
Gibert in 2003 predicted alkaline phosphatase (ALP) as an indicator for the future periodontal breakdown. ALP has not been supported by research findings and therefore may best serve as a marker in periodontal treatment planning and monitoring.[13]
Lactate dehydrogenase
Wong in 2006 reported that the lactate dehydrogenase (LDH) activity is higher in patients with increased probing depth than individuals with healthy periodontium.[14]
Collagenase-2 (MMP-8)
Rai in 2008 stated that elevated levels of salivary MMP-8 were observed in the periodontitis and gingivitis subjects compared to control groups. The crevicular levels of MMP-9 in periodontitis and gingivitis were higher than those in healthy subjects. The crevicular levels were lower in gingivitis and periodontitis as compared to healthy controls.[15]
Cathepsin B
Eley and Cox in 1996 studied cathepsin B and evaluated its use as a predictor of attachment loss. Cathepsin B may have a potential use in distinguishing periodontitis from gingivitis and in planning treatment and monitoring treatment outcomes.[16]
Gelatinase (MMP-9)
Teng in 1992 showed a twofold increase in mean MMP-9 levels in patients with recurrent attachment loss. Thus, the use of MMP-9 in oral diagnostics may best serve as a guide in periodontal treatment monitoring.[17]
Collagenase-3 (MMP-13)
Ma in 2000 suggested that MMP-13 is expressed during bone formation and gingival wound healing. MMP-13 may be useful for diagnosing and monitoring the course of periodontal disease as well as tracking the efficacy of therapy.[18]
BONE-RELATED BIOMARKERS FROM ORAL FLUIDS ASSOCIATED WITH PERIODONTAL DISEASES
Osteocalcin
Serum osteocalcin is a specific marker of bone formation when formation and resorption are uncoupled. Nakashima et al. reported that osteocalcin levels were also significantly correlated with pocket depth, gingival index scores, and GCF levels of ALP and prostaglandin E2.[19]
Critical analysis
Increased osteocalcin levels in oral fluids are seen during an increased periodontal disease activity.
Calprotectin
Kido in 1999 suggested that calprotectin plays a role in immune regulation through its ability to inhibit immunoglobulin production and, of particular interest, its role as a proinflammatory protein for neutrophil recruitment and activation.[20]
Critical analysis
Calprotectin GCF levels in patients with periodontal disease were higher than those in healthy subjects.
Osteonectin
Bowers 1989 suggested that osteonectin is a single-chain polypeptide that binds strongly to hydroxyapatite and other extracellular matrix proteins including collagens.[21]
Critical analysis
Osteonectin appeared to be the more sensitive marker for detection of periodontal disease status.
Osteopontin
Sharma reported that GCF osteopontin (OPN) concentrations increased proportionally with the progression of disease and when nonsurgical periodontal treatment was provided, GCF OPN levels were significantly reduced.[22]
Critical analysis
OPN appears to hold great promise as a possible biomarker of periodontal disease progression.
PYRIDINOLINE CROSS-LINKED CARBOXYTERMINAL TELOPEPTIDE OF TYPE I COLLAGEN
Giannobile 1999 reported that pyridinoline cross-linked carboxyterminal telopeptide of type I collagen (ICTP) was a good predictor of future alveolar bone and attachment loss, was strongly correlated with clinical parameters and putative periodontal pathogens, and demonstrated significant reductions after periodontal therapy.[23]
Critical analysis
GCF ICTP levels can be used as a diagnostic marker of the periodontal disease activity.
MARKERS OF CELL DEATH AND CONNECTIVE TISSUE DEGRADATION PRODUCTS
Fibronectin
Huynh in 2002 suggested that fibronectin (FN) plays a role in a variety of cellular activities, including cell–cell and cell–ECM adhesion. FN and its fragments have also been thought to have a role in inflammation [Table 5].[9]
Table 5.
Markers of cell death and connective tissue degradation

Critical analysis
Specific FN fragments are markers for the periodontal disease status and support the role of FN fragments as potential components in the pathogenesis of periodontal disease.
Glycoaminoglycans
Giannobile in 1999 suggested that the sulcular fluid appears to be rich in metabolic and degradative products of the proteoglycans found in various periodontal tissues. The presence of raised levels of sulfated glycoaminoglycans (GAGs) in GCF reflects the active destruction of periodontal tissue, most likely, the alveolar bone.[23]
Critical analysis
Elevated GCF GAG levels increase the severity of disease and GAG analysis in GCF may be used to detect early preclinical changes in periodontal tissues.
SALIVARY BIOMARKERS
The challenge of salivary diagnostics is to discover its potential and optimize engineering technologies for use with this biofluid. Researchers envision that some of human health and disease states will be reflected diagnostically in saliva via proteomic or genomic information.
GCF AS A DIAGNOSTIC MARKER
GCF contains elevated levels of a vast array of biochemical factors which offer us proper diagnosis of disease activity. The future method of GCF analysis is shown in Figure 4.
Figure 4.
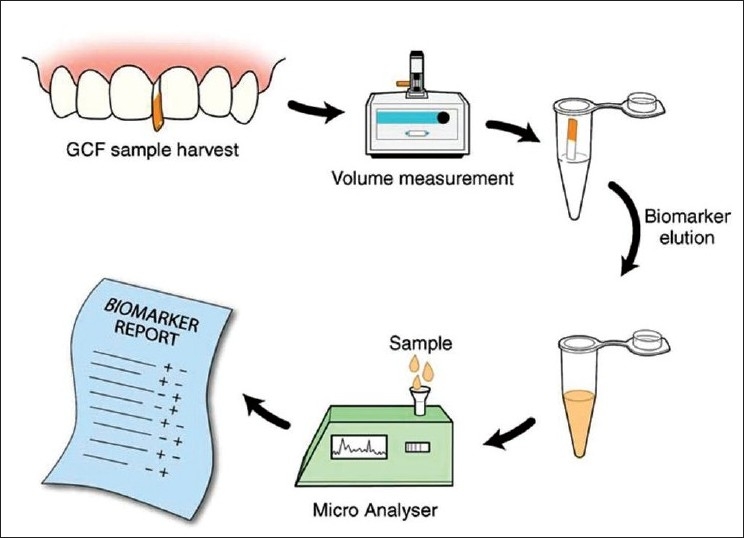
Futuristic chair-side diagnostic test based on GCF sampling
Critical analysis
Prostaglandin E2
Page in 1997 studied the relationship of the prostaglandin E2 concentration in GCF and found that the levels are significantly high during the active phase of periodontitis.[24]
β-glucuronidase
Lamster suggested that β-glucuronidase was involved in the degradation of the connective tissue ground substance. Studies confirmed that the levels increase sixfold in periodontal diseases.[25]
Neutrophil elastase
Paster in 2001 suggested that neutrophil elastase (NE) is a marker of the intracrevicular PMN activity and animal and human studies have shown that NE levels increase in GCF in periodontitis.[26]
Aspartate aminotransferase
Persson in 1999 conducted longitudinal and cross-sectional studies and multicenter trials. All have established a definite correlation between increased levels of AST and an increased activity of the periodontal disease process.[27]
Collagen in GCF
Garnero in 2003 found variations in collagen product levels resulting from disease states which are reflected in the GCF and showed the active status of the periodontal disease process.[28]
Proteoglycans
Page and Kornman in their study showed that increased levels of proteoglycans in GCF were in the active phase of periodontitis. Increased amounts chondroitin 4 and 6 sulfate isomers and hyaluronan in GCF indicate the increased periodontal disease activity.[24]
Glycoproteins
Paster in 2001 reported that the glycoprotein concentration increases in GCF along with the increased disease process and decreases in concentration when the healing process takes place in periodontium.[26]
USE OF GENOMICS IN PERIODONTAL DIAGNOSIS – A CRITICAL ANALYSIS
Single-nucleotide polymorphism
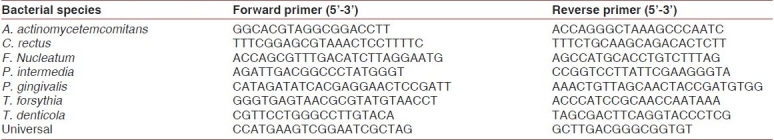
Several researchers have focused on genetic single-nucleotide polymorphisms in the study of periodontitis. There is a genetic susceptibility test currently available for severe chronic periodontitis (Interleukin Genetics, Waltham, MA, USA). This system works by the detection of two types of IL-1 genetic alleles, IL-1α +4845 and IL-1β +3954 [137]. Individuals identified as “genotype positive,” or found to have both of these alleles, are more likely to have the phenotype of overexpression of this gene [Table 6].
Table 6.
Primers of qPCR analysis of plaque biofilm bacteria
Li et al. investigated the potential use of genomics in the development of salivary diagnostics. They performed microarray testing of cell-free saliva for RNA profiling. RNA was isolated from unstimulated saliva that was collected from healthy subjects. After analysis by microarray and quantitative polymerase chain reaction, they found that it was possible to profile messenger RNAs, of which there were thousands present in the saliva. More recently, the group demonstrated the potential of salivary IL-8 levels to predict whether the patient was affected with squamous cell carcinoma.[29]
NUCLEIC ACID PROBES
In recent years, DNA probes have been developed to identify nucleotide sequences that are specific for individual bacterial species, believed to be of diagnostic significance. DNA and RNA probes are available [Figure 5].
Figure 5.
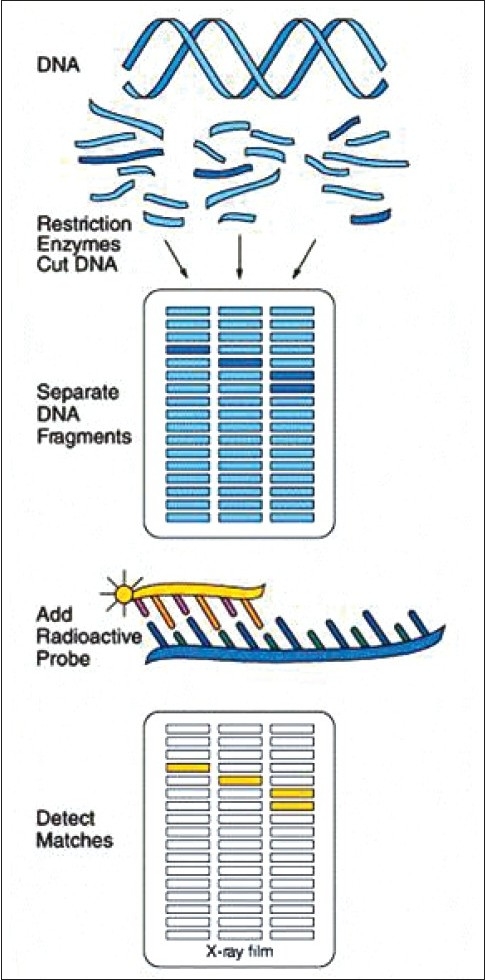
DNA probe technique
Critical analysis
In the future, mini molecular laboratories will be available for chair-side DNA probe testing in an hour or less (Periodontal Microbial Identification Test, Saigene Corp, Bothell, WA, USA). However, the advice of a microbiologist might still sometimes be necessary to avoid problems that may occur in the diagnostic process.
INFRARED SPECTROSCOPY
Compared to analyzing one or more particular biomarkers in the tissue or body fluid, infrared (IR) spectroscopy analyzes complex biologic systems by capturing the entire IR spectrum that represents the sum of the contributions of the biomolecules present, such as proteins, lipids, sugars, and nucleic acids. The IR spectrum of GCF is a rich source of information regarding the oral cavity and associated inflammation.[30]
Critical analysis
IR spectroscopy can distinguish between the characteristics of diverse molecules by probing vibrations of chemical bonds and can use these molecular and submolecular profiles to define and differentiate between diseased and healthy tissues.
IR analysis of GCF, unlike traditional biochemical analyses, measures the total contents of GCF and may prove to be a more powerful diagnostic and prognostic tool for periodontal diseases. Subtle differences in the spectral band intensity and positions arising from the three major components, i.e., lipid, protein and DNA, were observed in GCF from healthy, gingivitis, and periodontitis groups.
NEAR -IR SPECTROSCOPY
Near-IR (NIR) spectroscopy can be used to monitor hemodynamic and edema-based markers of soft tissues of the oral cavity. Several light-absorption bands in the visible and NIR spectral region reflect key inflammatory events. The 500- to 600-nm wavelength region is dominated by the absorption from oxygenated hemoglobin (HbO2) and deoxygenated hemoglobin (Hb) in the capillary bed gingival tissue, whereas the absorption from water results in an increased attenuation at longer wavelengths centered at 970 nm. In addition, tissue edema, an index that is commonly used as a marker of gingival inflammation, can be measured using NIR spectroscopy.[30]
Critical analysis
Monitoring the intensity of water bands in gingival tissues provides an index of tissue hydration representing a simple indicator of inflammation at specific periodontal sites. Optical spectroscopy provides a measure of the hemoglobin-oxygen saturation of tissues and the degree of tissue perfusion as well as a measure of tissue edema.
FUTURE DIRECTONS AND SUMMARY
There is a plethora of possibilities for the future use of oral fluids in biotechnology and health care applications, especially in the field of diagnostics. From physical measurements by periodontal probing to sophisticated genetic susceptibility analysis and molecular arrays for the detection of biomarkers on the different stages of the disease, substantial improvements have been made in the understanding of the mediators implicated on the initiation, pathogenesis, and progression of periodontitis. Through the biomarker discovery process, new therapeutics have been designed linking therapeutic and diagnostic approaches together, especially in the area of host modulatory drugs for periodontal disease treatment.
Moreover, new diagnostic technologies, such as microarray and microfluidics, are now currently available for risk assessment and comprehensive screening of biomarkers. The future is bright for the use of rapid, easy-to-use diagnostics that will provide an enhanced patient assessment that can guide and transform customized therapies for dental patients, leading to more individualized, targeted treatments for oral health.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Frodge BD, Ebersole JL, Kryscio RJ, Thomas MV, Miller CS. Bone Remodeling Biomarkers of Periodontal Disease in Saliva. J Periodontol. 2008;79:1913–19. doi: 10.1902/jop.2008.080070. [DOI] [PubMed] [Google Scholar]
- 2.Teles R, Sakellari D, Teles F, Konstantinidis A, Kent R, Socransky S, et al. Relationships among Gingival Crevicular Fluid Biomarkers, Clinical Parameters of Periodontal Disease, and the Subgingival Microbiota. J Periodontol. 2010;81:89–98. doi: 10.1902/jop.2009.090397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seibel MJ. Clinical Application of Biochemical Markers of Bone Turnover. Arq Bras Endocrinol Metabol. 2006;50:603–20. doi: 10.1590/s0004-27302006000400006. [DOI] [PubMed] [Google Scholar]
- 4.Miller CS, King CP, Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: A cross-sectional study. J Am Dent Assoc. 2006;137:322–9. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 5.Taba M, Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic Biomarkers for Oral and Periodontal Diseases. Dent Clin North A. 2005;49:551–72. doi: 10.1016/j.cden.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Subrahmanyam MV, Sangeetha M. Gingival Crevicular Fluid A Marker of the Periodontal Disease Activity. Indian J Clin Biochem. 2003;18:5–7. doi: 10.1007/BF02867658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinney JS, Ramseier CA, Giannobile WV. Oral fluid-based biomarkers of alveolar bone loss in periodontitis. Ann N Y Acad Sci. 2007;1098:230–51. doi: 10.1196/annals.1384.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malamud D. Salivary diagnostics- The future is now. J Am Dent Assoc. 2006;137:284–286. doi: 10.14219/jada.archive.2006.0158. [DOI] [PubMed] [Google Scholar]
- 9.Huynh QN, Wang S, Tafolla E, Gansky SA, Kapila S, Armitage GC, et al. Specific fibronectin fragments as markers of periodontal disease status. J Periodontol. 2002;73:1101–10. doi: 10.1902/jop.2002.73.10.1101. [DOI] [PubMed] [Google Scholar]
- 10.Holmlund A, Hanstrom L, Lerner UH. Bone resorbing activity and cytokine levels in gingival crevicular fluid before and after treatment of periodontal disease. J Clin Periodontol. 2004;31:475–82. doi: 10.1111/j.1600-051X.2004.00504.x. [DOI] [PubMed] [Google Scholar]
- 11.Oringer RJ, Howell TH, Nevins ML, Reasner DS, Davis GH, Sekler J, et al. Relationship between crevicular aspartate aminotransferase levels and periodontal disease progression. J Periodontol. 2001;72:17–24. doi: 10.1902/jop.2001.72.1.17. [DOI] [PubMed] [Google Scholar]
- 12.Oringer RJ, Palys MD, Iranmanesh A, Fiorellini JP, Haffajee AD, Socransky SS, et al. C-telopeptide pyridinoline cross-links (ICTP) and periodontal pathogens associated with endosseous oral implants. Clin Oral Implants Res. 1998;9:365–73. doi: 10.1034/j.1600-0501.1996.090602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibert P, Tramini P, Sieso V, Piva MT. Alkaline phosphatase isozyme activity in serum from patients with chronic periodontitis. J Periodontal Res. 2003;38:362–5. doi: 10.1034/j.1600-0765.2003.00388.x. [DOI] [PubMed] [Google Scholar]
- 14.Wong DT. Salivary diagnostics powered by nanotechnologies, proteomics and genomics. J Am Dent Assoc. 2006;137:313–21. doi: 10.14219/jada.archive.2006.0180. [DOI] [PubMed] [Google Scholar]
- 15.Rai B, Kharb S, Jain R, Anand SC. Biomarkers of periodontal disease in oral fluids. J Oral Sci. 2008;50:53–6. doi: 10.2334/josnusd.50.53. [DOI] [PubMed] [Google Scholar]
- 16.Eley BM, Cox SW. The relationship between gingival crevicular fluid cathepsin B activity and periodontal attachment loss in chronic periodontitis patients: a 2-year longitudinal study. J Periodontal Res. 1996;31:381–92. doi: 10.1111/j.1600-0765.1996.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 17.Teng YT, Sodek J, McCulloch CA. Gingival crevicular fluid gelatinase and its relationship to periodontal disease in human subjects. J Periodontal Res. 1992;27:544–52. doi: 10.1111/j.1600-0765.1992.tb01830.x. [DOI] [PubMed] [Google Scholar]
- 18.de Queiroz AC, Taba M, Jr, O’Connell PA, da Nóbrega PB, Costa PP, Kawata VK, et al. Inflammation Markers in Healthy and Periodontitis Patients. A Preliminary Data Screening. Braz Dent J. 2008;19:3–8. doi: 10.1590/s0103-64402008000100001. [DOI] [PubMed] [Google Scholar]
- 19.Nakashima K, Giannopoulou C, Andersen E, Roehrich N, Brochut P, Dubrez B, et al. A longitudinal study of various crevicular fluid components as markers of periodontal disease activity. J Clin Periodontol. 1996;23:832–8. doi: 10.1111/j.1600-051x.1996.tb00620.x. [DOI] [PubMed] [Google Scholar]
- 20.Kido J, Nakamura T, Asahara Y, Sawa T, Kohri K, Nagata T. Osteopontin in gingival crevicular fluid. J Periodontal Res. 2001;36:328–33. doi: 10.1034/j.1600-0765.2001.360509.x. [DOI] [PubMed] [Google Scholar]
- 21.Bowers MR, Fisher LW, Termine JD, Somerman MJ. Connective tissue-associated proteins in crevicular fluid: Potential markers for periodontal diseases. J Periodontol. 1989;60:448–51. doi: 10.1902/jop.1989.60.8.448. [DOI] [PubMed] [Google Scholar]
- 22.Sharma CG, Pradeep AR. Gingival crevicular fluid osteopontin levels in periodontal health and disease. J Periodontol. 2006;77:1674–80. doi: 10.1902/jop.2006.060016. [DOI] [PubMed] [Google Scholar]
- 23.Giannobile WV. C-telopeptide pyridinoline cross-links. Sensitive indicators of periodontal tissue destruction. Ann N Y Acad Sci. 1999;878:404–12. doi: 10.1111/j.1749-6632.1999.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page RC, Kornman KS. The pathogenesis of human periodontitis: An introduction. Periodontol. 2000;1997(14):9–11. doi: 10.1111/j.1600-0757.1997.tb00189.x. [DOI] [PubMed] [Google Scholar]
- 25.Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol. 1997;2:123–37. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- 26.Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–83. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persson L, Bergström J, Gustafsson A, Åsman B. Tobacco smoking and gingival neutrophil activity in young adults. J Clin Periodontol. 1999;25:9–13. doi: 10.1034/j.1600-051x.1999.260102.x. [DOI] [PubMed] [Google Scholar]
- 28.Garnero P, Delmas PD. An immunoassay for type I collagen alpha 1 helicoidal peptide 620-633, a new marker of bone resorption in osteoporosis. Bone. 2003;32:20–6. doi: 10.1016/s8756-3282(02)00922-5. [DOI] [PubMed] [Google Scholar]
- 29.Yoshie H, Kobayashi T, Tai H, Galicia JC. The role of genetic polymorphisms in periodontitis. Periodontol. 2000;2007(43):102–32. doi: 10.1111/j.1600-0757.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- 30.Xiang X, Sowa MG, Iacopino AM, Maev RG, Hewko MD, Man A, et al. An Update on Novel Non-Invasive Approaches for Periodontal Diagnosis. J Periodontol. 2010;81:186–98. doi: 10.1902/jop.2009.090419. [DOI] [PubMed] [Google Scholar]


