Abstract
Acute kidney injury (AKI) is one of the most challenging problems faced by clinicians in the tropics owing to its fast-changing burden. AKI in the tropics is strikingly different from that in the developed world in terms of etiology and presentation. In addition, there is a stark contrast between well-developed and poor areas in the tropics. The true epidemiological picture of AKI in the tropics is not well understood due to the late presentation of patients to tertiary centers. Infections remain the major culprit in most cases of AKI, with high mortality rates in the tropics. Human immunodeficiency virus–related AKI, related to nephrotoxicity due to antiretroviral therapy, is on the rise. Acute tubular necrosis and thrombotic microangiopathy are the most common mechanisms of AKI. A notable problem in the tropics is the scarcity of resources in health centers to support patients who require critical care due to AKI. This article reviews the unique and contrasting nature of AKI in the tropics and describes its management in each situation.
The tropical zone, geographically limited by the Tropics of Cancer and Capricorn, is an area with extreme heterogeneity in terms of ethnic composition, as well as socioeconomic and developmental status. Acute kidney injury (AKI), characterized by a reversible decline in the glomerular filtration rate, leading to retention of nitrogenous waste products and an inability to maintain fluid and electrolyte homeostasis, remains one of the most enigmatic problems, with reported incidence rates between 25% and 80%.1 Well-written clinical review articles, randomized controlled trials (RCTs), meta-analyses and editorials were searched using MEDLINE/Index Medicus and IndMed search engines for the preparation of this review.
What Makes Tropical AKI Unique?
There is a stark contrast between AKI in the tropics and that in temperate zones; however, the basic pathophysiological changes and principles of management remain the same. In contrast to trauma, industrial accidents, drugs, cardiogenic shock and renal transplantation rejection being the common causes of AKI in the developed world, acute tubular necrosis (ATN) due to community-acquired infections remains the commonest cause in the tropics.2 Natural medicines, used by traditional healers, add to the burden of AKI in some tropical areas.3 Two studies from India on patients with AKI over the past three decades have shown the percentage of medical, surgical and obstetrical conditions resulting in acute renal failure (ARF) as 60%, 25% and 15%; and 88%, 3.4% and 9%; respectively.4,5 The second difference between the two zones is the relatively younger age of patients in the tropics. In contrast to the mean age of around 72 years in patients with AKI in the temperate zone,6 in India it was 37.1 years.5 Yet another difference is the gross inadequacy of treatment facilities in the tropics, which accounts for the increasing mortality. Intermittent peritoneal dialysis remains the mainstay of treatment in several areas.7 Other unique features of tropical AKI include endemic malnutrition, a relative state of hypovolemia due to increased sweating and peripheral vasodilatation due to the hot climate and the triggering of hemolytic crisis in some glucose 6-phosphate dehydrogenase–deficient ethnic groups when exposed to certain drugs and toxins.8 This rich and diverse nature of the illness makes tropical AKI unparalleled and poses a major medical challenge.
Epidemiology: The Changing Trend
The lack of a uniform definition, the paucity of multicentric, large studies, and under-reporting in the tropics due to local conditions have created a void in the proper understanding of the epidemiology of AKI.9 The various incidence rates reported in the literature from areas in the tropics are: Kuwait (4.1 per year per million population),10 South Africa (20 per year per million population),11 Brazil (7.9 per 1000 admissions)12 and India (6.4 per 1000 admissions).13 The inability of a majority of the patients to make it to tertiary reporting centers remains one of the major reasons for the very low incidence rates when compared to those in temperate regions.14 Among the tropical countries, the developed areas, of late, have shown a paradigm shift in the epidemiology, with a predominance of medical conditions like human immunodeficiency virus (HIV) infection accounting for AKI, and obstetric causes almost becoming extinct. Common causes of AKI in the tropics are shown in Table 1. A variety of prospective studies, using the new definition and staging system of AKI are needed to understand epidemiological trends for this disease in the tropics.15
Table 1.
Causes of acute kidney injury in the tropics.
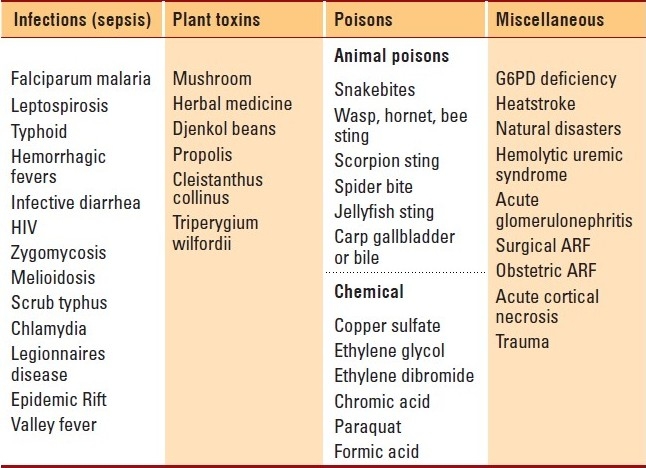
Causes of AKI in the Tropics
Sepsis due to bacterial invasion, parasitic and viral infections, toxins and acute diarrheal diseases still top the list in the etiology of AKI in the tropics. A drastic improvement in maternal and child health care in many of the tropical areas has resulted in a steep decline in the incidence of AKI due to obstetric causes and acute diarrheal diseases. The common conditions causing AKI in the tropics and their management in different situations are discussed in detail.
Falciparum malaria
Malaria remains one of the major public health hazards in the tropics. Malarial AKI (MAKI) occurs in less than 1% to 5% of all cases of AKI in endemic areas, whereas the prevalence in nonimmune individuals is around 25% to 30%.16 In a central Indian population of 419 confirmed patients with AKI, MAKI was detected in 22% of the patients.17 MAKI may present either as AKI alone or as part of a multi-organ dysfunction. Jaundice is the most common association of MAKI, occurring in more than 75% of cases.18 Electrolyte abnormalities like hyperkalemia, hypokalemia, hyponatremia, hypocalcemia and hypophosphatemia have been reported.19 Volume depletion, gastrointestinal bleeding, sepsis, nephrotoxic drugs and hyperbilirubinemia act as predisposing factors.20 The pathogenesis of MAKI is not clearly known. Different hypotheses proposed include hypovolemia, cytokine- and nitric oxide–mediated arterial vasodilatation, resistance to vasoactive hormones, cytopathic hypoxia leading to decreased ATP synthesis and mechanical obstruction by infected erythrocytes (cytoadherence).16 Renal histology consistently shows ATN, with a varied spectrum of cell necrosis, swelling and deposits of hemosiderin granules, predominantly affecting the distal tubules. Interstitial nephritis and glomerulonephritis may also be seen.16 Mortality from MAKI varies between 15% and 50% in different series.16 Expeditious assessment of hypovolemia, blood glucose level and acid-base status is essential. All patients with severe falciparum malaria should be presumed to be resistant to chloroquine, and intravenous quinine should be initiated as the first-line drug. The dose of quinine may be reduced owing to renal failure, but only after the first 48 hours. Artemisinin compounds like artesunate and artemether are valuable in areas with quinine resistance.21 Gametocidal therapy with doxycycline is recommended. Electrolyte disturbances should be watched for and promptly treated. Dopamine infusion and diuretics have not been shown to improve outcome.22,23 Dialysis when initiated early in the course of treatment has had a very good outcome in patients with MAKI. Different series have reported a requirement for dialysis in 50% to 80% of patients.24 Intermittent hemodialysis (IHD), continuous venovenous hemofiltration (CVVH) or continuous arteriovenous hemofiltration (CAVH) may be used, although there is no consensus on the best replacement therapy in MAKI. The efficacy of peritoneal dialysis (PD) is reduced by the clogging of peritoneal microcirculation with infected erythrocytes. However, in centers where facilities for hemodialysis are not available, initiating PD early in the course of treatment may be life-saving. A single study of 70 patients with infectious AKI, mostly malarial, compared outcomes between CVVH and PD, with a mortality rate of 15% in patients randomized to CVVH and 47% in patients treated with PD (P=.005).25 A randomized controlled trial to compare the effect of high-volume PD with that of daily HD on 120 patients with ATN showed a similar metabolic control, mortality rate (58% and 53%, respectively) and renal function recovery (28% and 26%, respectively) in both groups.26 Exchange blood transfusion, plasma exchange and plasmapheresis (erythrocytapheresis) may be tried in well-equipped centers, although there are no sufficiently powered RCTs.27 Albumin infusion for volume expansion has shown to reduce mortality rates.28
Leptospirosis
Leptospirosis, a zoonotic disease caused by spirochetes of the genus Leptospira, has increasingly been recognized to affect travelers and residents in the tropics. The incidence of AKI in severe leptospirosis varies from 40% to 60%.29 Clinical manifestations are classically biphasic, with an initial septicemic phase lasting for 3 to 7 days followed by an immune phase, during which nephropathy occurs. The pathogenetic mechanisms suggested include bacterial invasion, inflammatory processes, hemodynamic alterations, and the direct toxicity of bacterial products.30 AKI in leptospirosis is primarily non-oliguric, and hypokalemia occurs frequently due to kaliuresis. Interstitial nephritis is the basic lesion of leptospirosis. Renal biopsy reveals interstitial edema and infiltration with mononuclear cells and few eosinophils. ATN, primarily affecting the proximal tubules, may also be present. Glomeruli are usually spared. AKI leads to a rapid elevation of blood urea nitrogen and serum creatinine levels. Diagnosis is usually by serological tests in the second phase. Detection of antigens by polymerase chain reaction offers early diagnosis. The macroscopic agglutination test may be used as a screening test. Leptospirae may be detected in the urine between 1 and 4 weeks of infection, using dark-field illumination.30 Crystalline penicillin or doxycycline shorten the duration of fever and hospital stay and may hasten amelioration of leptospiruria. Intravenous ceftriaxone (1 g once daily) or cefotaxime (1 g every 6 hours) has shown efficacy equal to that of penicillin.31 There are controversial reports on the use of dopamine and diuretics. However, a combination of furosemide and dopamine has been reported to be useful in mild AKI in leptospirosis.32 Poor prognostic factors include oliguric renal failure, increasing age, jaundice, pulmonary complications and hypotension, accompanied by hyperkalemia and serum creatinine more than 3 mg/dL, requiring dialysis.33 Other modalities, including CVVH, IHD, plasmapheresis and exchange transfusions, are beneficial in decreasing blood levels of cytokines and mediators. Andrade et al compared the efficacy of ‘delayed alternate-day’ HD (15 patients) with that of ‘prompt and daily’ HD (18 patients) and reported the futility of alternate-day HD.34 Complete recovery from renal failure is typical. AKI in the presence of failure of two or more organ-systems carries an unfavorable prognosis.35
Human immunodeficiency virus infection
AKI is common in HIV-infected patients, with an odds ratio of 2.8 for acquiring the disease in those treated with highly active antiretroviral therapy (HAART) and a 6-fold higher risk of in-hospital mortality in HAART-naive hospitalized HIV patients.36 The prevalence rates reported from sub-Saharan Africa are 6% in South Africa, 38% in Nigeria, 28% in Tanzania and 20% to 48.5% in Uganda.37 The known causes of AKI are ATN secondary to sepsis, hypotension, dehydration and nephrotoxins. The post-HAART era has seen a rise in HIV-related AKI secondary to the nephrotoxicity of tenofovir and other antibiotics. Other rare causes include intra-renal (medication-induced crystalluria) obstruction, extra-renal (retroperitoneal fibrosis, bladder dysfunction, nephrolithiasis) obstruction and acute interstitial nephritis secondary to immune restoration inflammatory syndrome.38 Potential risk factors for developing AKI include increased HIV viral loads, reduced CD4 cell counts, hepatitis C virus co-infection, a history of diabetes, black race and male gender.39 Successful prevention depends on optimizing immune status and aggressively treating other comorbidities.
Viral hemorrhagic fevers
These are diseases caused by RNA viruses belonging to Flaviridiae, Arenaviridiae, Bunyaviridiae and Filoviridiae. Renal failure is nonoliguric in about 60% of cases. Dengue fever rarely causes AKI, which is usually associated with hypotension, rhabdomyolysis or hemolysis. Lee et al have reported a 4.9% incidence rate of AKI in Chinese patients suffering from severe dengue.40 Yellow fever is endemic in tropical Africa. Anuria and ATN are seen in severe forms. Viral antigens can be demonstrated in the renal epithelium.41
Other infections
Renal mucormycosis, with vascular invasion and thrombosis resulting in infarction and necrosis, is a rare cause of AKI. Hematuria and lumbar pain in a patient with high fever are usual pointers for this condition. Ultrasonography and computed tomography (CT) reveal enlarged kidneys with evidence of peri-renal collection or intra-renal abscesses. A contrast CT scan shows an absence of renal perfusion.42 Treatment is extensive debridement and systemic amphotericin B.
AKI associated with scrub typhus is not rare in the tropics and should be suspected in patients with environmental exposure who develop high-grade fever and varying degrees of ARF along with the presence of an eschar.43 Diagnosis can be made by immunofluorescence techniques, along with renal biopsy. The mechanisms include ATN caused by direct invasion of Orientia tsutsugamushi and intravascular hemolysis due to rhabdomyolysis.44,45 Patients respond well to doxycycline.
AKI was observed in up to 61% of patients with acute septic melioidosis.46 Renal histology has shown ATN, interstitial nephritis and rarely micro-abscesses. Treatment is by long-term parenteral ceftazidime. Typhoid can rarely cause AKI due to intravascular hemolysis or myoglobinuria.
Snakebite
Poisonous snakebites pose a crucial health problem in the tropics, constituting 1.2% of the total cases of AKI in Thailand, 3% in India, and 70% in Myanmar.47 Snakes belonging to the families Elapidae, Viperidae, Colubridae and Hydrophidae cause AKI. The common clinical presentations include proteinuria, hematuria, pigmenturia, rhabdomyolysis and myoglobinuria.48 Kidney injury occurs within a few hours to as late as 96 hours after the bite and is oligo-anuric in up to 94% of patients.49 ATN, with varying degrees of interstitial edema and inflammatory cell infiltration, is the commonest histology feature. AIN, necrotizing arteritis of interlobular arteries and occasionally crescentic glomerulonephritis are also described.50 Direct nephrotoxicity, hypovolemia, hemolysis, myoglobinuria and disseminated intravascular coagulation are the usual pathogenic mechanisms. Early administration of specific monovalent antivenom, before AKI sets in, is pivotal. In most of the tropical countries, only polyvalent antivenom is available. Plasmapheresis and blood exchange can be used if antivenom is unavailable. Dialysis should be performed early and frequently.51
Plant Toxins
Tropical nephrotoxins are derived from local fauna and flora or plant and chemical sources. A good proportion of the population in the tropics depends on traditional medicine, which constitutes a mix of herbs and unknown chemicals. Direct injury to tubular cells, renal ischemia, pigmenturia and allergic AIN are the different mechanisms of AKI.52 Common hisotological lesions include ATN, acute cortical necrosis and AIN. Poisoning by traditional medicines—Callilepis laureola (Impila) and Cape aloe—is one of the commonest causes of AKI in South Africa. A mixture of henna and paraphenylenediamine induces severe rhabdomyolysis. AKI also occurs due to interactions of herbal medicines with conventional drugs. Djenkol bean, considered to be a delicacy in South East Asia, and ingestion of certain genera of mushrooms are common causes of AKI. Direct toxicity, along with volume depletion and hepatic failure, is an important mechanism.
Chemical Poisons
Oliguric AKI after ingestion of copper sulfate, used in leather industry; and formic acid used in rubber plantations has been reported in 25% and 38.7% of total cases of AKI, respectively.53,54 Renal histology usually shows ATN. Hyperkalemia is severe due to ongoing hemolysis. Early and frequent dialysis can change the outcomes.
Glucose-6-Phosphate Dehydrogenase (G6PD) Deficiency
This is exclusively found in some ethnic tropical populations. Acute intravascular hemolysis due to precipitation of hemoglobin when red blood cells are subjected to oxidative stress is the cause of AKI in these individuals. Its incidence varies from 2.2% to 15% in various regions of India, 13% in Saudi Arabia, 15% in East Africa, 20% in Nigeria, 20% in oriental Jews, and 2% in the Chinese.50 Hemolytic crisis develops within hours of exposure to stress, most commonly by drugs, toxins or infections. Common infections include viral hepatitis, rickettsial infections, typhoid and urinary tract infection. Passage of dark-colored urine followed by oliguria is common. Renal histology shows ATN. Treatment includes high fluid intake to increase urine output, rendering the urine alkaline early in the course of the illness. Dialysis may be required in cases of severe, life-threatening hyperkalemia.
Miscellaneous Medical Causes
Post-infectious glomerulonephritis, different forms of rapidly progressive glomerulonephritis; rhabdomyolysis, compounded by dehydration and metabolic acidosis; natural disasters like earthquakes, tsunamis and floods are the various other rare causes of AKI in the tropics. Early evaluation and intervention can reduce the incidence of renal complications.
Surgical AKI
The incidence of surgical AKI has considerably increased in the tropics over the past decade. Obstructive uropathy causing AKI continues to top the list of causes. Trauma and operative complications are contributory in 2% to 5% and postoperative causes are contributory in 24% of cases of AKI in the tropics.50
Obstetric AKI
Better education, higher standards of living and improvements in obstetric care have drastically brought down the incidence of pregnancy-related AKI in the tropics caused by unsafe home deliveries and illegal abortions using sticks, chemicals and toxins. Renal ischemia caused by hemorrhagic shock or hypotension due to sepsis is the dominant factor leading to AKI, which is usually oligo-anuric. ATN is the usual pathology.
Acute Cortical Necrosis
Described occurring commonly as an obstetric complication or due to snakebite, acute cortical necrosis (ACN) is the most calamitous form of AKI. A large series from India reported obstetric causes to be responsible for 56% of all cases of ACN.55 Prolonged oliguria and anuria are pathognomonic. The main hypotheses in the pathogenesis are vasospasm of small vessels, toxic capillary endothelial damage, the role of endothelium-derived vasoactive substances and a hypercoagulable state.50 The gold standard of diagnosis is renal biopsy. A CT scan can be used as a noninvasive diagnostic tool for early diagnosis. Renal recovery is usually slow and may be incomplete.
Tropical AKI In Children
The spectrum and outcomes of AKI in children in thetropics different from those in non-tropical countries. Commonly, AKI occurs secondary to diarrheal diseases. The most striking feature is dehydration. Renal failure is usually oliguric. Patients have hypotension and metabolic acidosis out of proportion to the degree of failure. ATN is the commonest histological lesion. Early and adequate rehydration with oral rehydration solution or intravenous Ringer lactate in severe cases is the cornerstone of treatment. Children with established AKI are treated by peritoneal dialysis. Hemolytic uremic syndrome, seen mainly in pre-school children, is responsible for 25% to 55% of all cases of AKI in Asia.56 Urinalysis shows proteinuria with microscopic hematuria. Treatment is mainly supportive and includes volume replacement, control of hypertension and dialysis.
Conclusion
AKI is a challenging problem in the tropics in view of the changing burden of the disease (inreasing HIV-related AKI), the late presentation of patients to health care facilities and the lack of resources to support patients with established AKI in many regions. The mortality of patients with AKI in the tropics is unacceptably high. A balanced clinical approach to the problem is very crucial in the tropics. The treating physician in rural centers should be competent to treat trivial causes of AKI and refer those individuals who require critical care at the right moment. Moving the patient to an advanced center for renal replacement therapy is fundamental to treatment of AKI in rural areas. Figure 1 shows an algorithm on the treatment approach for a patient with AKI in the tropics. Patient education, basic research facilities to get the true picture of the epidemiology, utilization of new clinical biomarkers and more resources are required to catalyze the development of health care in these regions.
Figure 1.
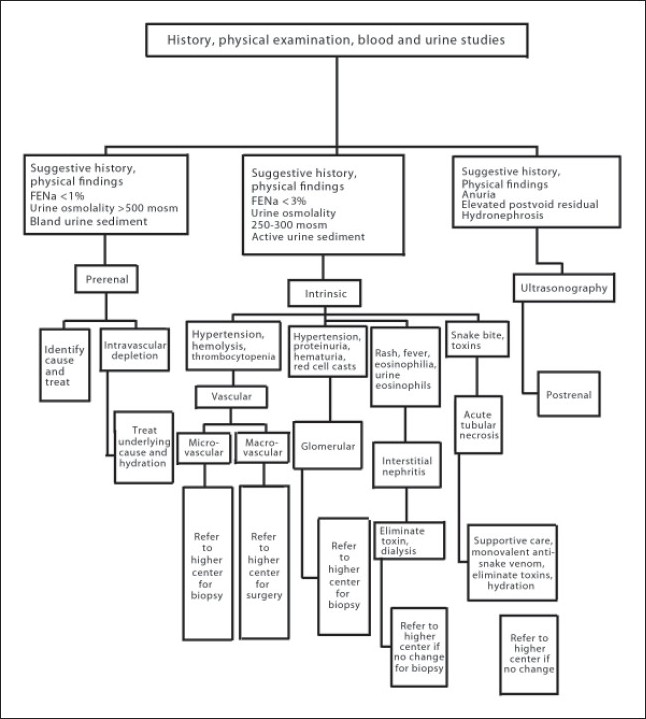
Treatment approach for a patient with acute kidney injury in the tropics.
REFERENCES
- 1.Lameire N, Van BW, Vanholder R. Acute renal failure. Lancet. 2005;365:417–30. doi: 10.1016/S0140-6736(05)17831-3. [DOI] [PubMed] [Google Scholar]
- 2.Chugh KS, Sitprija V, Jha V. Acute renal failure in tropical countries. In: Davidson AM, Cameron JS, Grunfeld JP, Ronco C, et al., editors. Oxford textbook of clinical nephrology. Oxford: Oxford University Press; 2005. pp. 639–58. [Google Scholar]
- 3.Burdmann EA. Acute kidney injury in the tropics: Introduction. Semin Nephrol. 2008;28:319. doi: 10.1016/j.semnephrol.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Chugh KS, Sakhuja V, Malhotra HS, Pereira BJ. Changing trends in acute renal failure in third-world countries - Chandigarh study. Q J Med. 1989;73:1117–23. [PubMed] [Google Scholar]
- 5.Jayakumar M, Prabahar MR, Fernando EM, Manorajan R, Venkatraman R, Balaraman V. Epidemiologic trend changes in acute renal failure - A tertiary centre experience from south India. Ren Fail. 2006;28:405–10. doi: 10.1080/08860220600689034. [DOI] [PubMed] [Google Scholar]
- 6.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–50. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 7.Hayat A, Kamili MA, Samia R, Yaseen M, Shakeel R, Qureshi W, et al. Peritoneal dialysis for adults with acute renal failure: an underutilized modality. Saudi J Kidney Dis Transpl. 2007;18:195–9. [PubMed] [Google Scholar]
- 8.Jain S, Suri V. Acute renal failure in the tropics.In Medicine Update 2009 Part II. Greater Noida: Proceedings of APICON; 2009. [Google Scholar]
- 9.Lameire N, Ven Biesen W, Vanholder R. The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol. 2006;2:364–77. doi: 10.1038/ncpneph0218. [DOI] [PubMed] [Google Scholar]
- 10.Abraham G, Gupta RK, Senthilselvan A, van der Meurlen J, Johny KV. Cause and prognosis of acute renal failure in Kuwait: A 2-year prospective study. J Trop Med Hyg. 1989;92:325–9. [PubMed] [Google Scholar]
- 11.Seedat YK. Acute renal failure in the black population of South Africa. Int J Artif Organs. 1993;16:801–2. [PubMed] [Google Scholar]
- 12.Noronha IL, Schor N, Coelho SN, Jorgetti V, Romao JE, Junior, Zatz R, et al. Nephrology, dialysis and transplantation in Brazil. Nephrol Dial Transplant. 1997;12:2234–43. doi: 10.1093/ndt/12.11.2234. [DOI] [PubMed] [Google Scholar]
- 13.Jha V, Chugh KS. Acute renal failure in the tropics. J Assoc Physicians India. 1997;45:18–23. [PubMed] [Google Scholar]
- 14.Cerda J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, et al. Epidemiology of acute kidney injury. Clin J Am Soc Nephrol. 2008;3:881–6. doi: 10.2215/CJN.04961107. [DOI] [PubMed] [Google Scholar]
- 15.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P the ADQI workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the acute dialysis quality initiative (ADQI) group. Crital Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra SK, Das BS. Malaria and acute kidney injury. Semin Nephrol. 2008;28:395–408. doi: 10.1016/j.semnephrol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Jain V, Nagpal AC, Joel PK, Shukla M, Singh MP, Gupta RB, et al. Burden of cerebral malaria in central India (2004-2007) Am J Trop Med Hyg. 2008;79:636–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Wilairatana P, Looareesuwan S, Charoenlarp P. Liver profile changes and complications in jaundiced patients with falciparum malaria. Trop Med Parasitol. 1994;45:298–302. [PubMed] [Google Scholar]
- 19.Sitprija V. Nephropathy in falciparum malaria. Kidney Int. 1988;34:867–77. doi: 10.1038/ki.1988.262. [DOI] [PubMed] [Google Scholar]
- 20.Das BS. Renal failure in malaria. J Vector Borne Dis. 2008;45:83–97. [PubMed] [Google Scholar]
- 21.Mohanty S, Mishra SK, Pati SS, Pattnaik J, Das BS. Complications and mortality patterns due to Plasmodium falciparum malaria in hospitalized adults and children, Rourkela, India. Trans R Soc Trop Med Hyg. 2003;97:69–70. doi: 10.1016/s0035-9203(03)90027-7. [DOI] [PubMed] [Google Scholar]
- 22.Kellum JA, Decker M, Janine RN. Use of dopamine in acute renal failure: A meta-analysis. Crit Care Med. 2001;29:1526–31. doi: 10.1097/00003246-200108000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Karajala V, Mansour W, Kellum JA. Diuretics in acute kidney injury. Minerva Anestesiol. 2009;75:251–7. [PubMed] [Google Scholar]
- 24.Barsoum RS. Malarial acute renal failure. J Am Soc Nephrol. 2000;11:2147–54. doi: 10.1681/ASN.V11112147. [DOI] [PubMed] [Google Scholar]
- 25.Phu NH, Hien TT, Mai NT, Chau TT, Chuong LV, Loc PP, et al. Hemofiltration and peritoneal dialysis in infection-associated acute renal failure in Vietnam. N Eng J Med. 2002;19:895–902. doi: 10.1056/NEJMoa020074. [DOI] [PubMed] [Google Scholar]
- 26.Gabriel DP, Caramori JT, Martim LC, Barretti P, Balbi AL. High volume peritoneal dialysis vs daily hemodialysis: a randomized, controlled trial in patients with acute kidney injury. Kidney Int. 2008;108:S87–93. doi: 10.1038/sj.ki.5002608. [DOI] [PubMed] [Google Scholar]
- 27.White NJ. What is the future of exchange transfusion in severe malaria? J Infect. 1999;39:185–6. doi: 10.1016/s0163-4453(99)90046-4. [DOI] [PubMed] [Google Scholar]
- 28.Maitland K, Pamba A, English M, Peshu N, Marsh K, Newton C, et al. Randomized trial of volume expansion with albumin or saline in children with severe malaria: preliminary evidence of albumin benefit. Clin Infect Dis. 2005;40:538–45. doi: 10.1086/427505. [DOI] [PubMed] [Google Scholar]
- 29.Andrade L, Daher EF, Seguro AC. Leptospiral nephropathy. Semin Nephrol. 2008;28:383–94. doi: 10.1016/j.semnephrol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Sitprija V, Losuwanrak K, Kanjanabuch T. Leptospiral nephropathy. Semin Nephrol. 2003;23:42–8. doi: 10.1053/snep.2003.50004. [DOI] [PubMed] [Google Scholar]
- 31.Panaphut T, Domrongkitchaiporn S, Vibhagool A, Thinkamrop B, Susaengrat W. Ceftriaxone compared with sodium penicillin G for treatment of severe leptospirosis. Clin Infect Dis. 2003;36:1507–13. doi: 10.1086/375226. [DOI] [PubMed] [Google Scholar]
- 32.Niwattayakul K, Sitprija V. Leptospiral acute renal failure: effects of dopamine and furosemide. Ren Fail. 2007;29:159–62. doi: 10.1080/08860220601095850. [DOI] [PubMed] [Google Scholar]
- 33.Ricaldi JN, Vinetz JM. Leptospirosis in the tropics and in travellers. Curr Infect Dis Rep. 2006;8:51–8. doi: 10.1007/s11908-006-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: Impact on mortality. Clin J Am Soc Nephrol. 2007;2:739–44. doi: 10.2215/CJN.00680207. [DOI] [PubMed] [Google Scholar]
- 35.Covic A, Goldsmith DJ, Gusbeth-Tatomir P, Seica A, Covic M. A retrospective 5 year study in Moldova of acute renal failure due to leptospirosis: 58 cases and a review of the literature. Nephrol Dial Transplant. 2003;18:1128–34. doi: 10.1093/ndt/gfg095. [DOI] [PubMed] [Google Scholar]
- 36.Wyatt CM, Arons RR, Klotman PE, Klotman ME. Acute renal failure in hospitalized patients with HIV: risk factors and impact on in-hospital mortality. AIDS. 2006;20:561–5. doi: 10.1097/01.aids.0000210610.52836.07. [DOI] [PubMed] [Google Scholar]
- 37.Fabian J, Naicker S. HIV and kidney disease in sub-Saharan Africa. Nat Rev Nephrol. 2009;5:591–98. doi: 10.1038/nrneph.2009.141. [DOI] [PubMed] [Google Scholar]
- 38.Daugas E, Plaisier E, Boffa JJ, Guiard-Schmid JB, Pacanowski J, Mougenot B, et al. Acute renal failure associated with immune restoration inflammatory syndrome. Nat Clin Pract Nephrol. 2006;2:594–8. doi: 10.1038/ncpneph0282. [DOI] [PubMed] [Google Scholar]
- 39.Kalim S, Szczech LA, Wyatt CM. Acute kidney injury in HIV-infected patients. Semin Nephrol. 2008;28:556–62. doi: 10.1016/j.semnephrol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee IK, Liu JW, Yang KD. Clinical characteristics and risk factors for concurrent bacteremia in adults with dengue hemorrhagic fever. Am J Trop Med Hyg. 2005;72:221–6. [PubMed] [Google Scholar]
- 41.Lima EQ, Nogueira ML. Viral hemorrhagic fever - induced acute kidney injury. Semin Nephrol. 2008;28:409–15. doi: 10.1016/j.semnephrol.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Chugh KS, Sakhuja V, Gupta KL, Jha V, Chakravarty A, Malik N, et al. Renal mucormycosis: computerized tomographic findings and their diagnostic significance. Am J Kidney Dis. 1993;22:393–7. doi: 10.1016/s0272-6386(12)70141-5. [DOI] [PubMed] [Google Scholar]
- 43.Yen TH, Chang CT, Lin JL, Jiang JR, Lee KF. Scrub typhus: a frequently overlooked cause of acute renal failure. Ren Fail. 2003;25:397–410. doi: 10.1081/jdi-120021152. [DOI] [PubMed] [Google Scholar]
- 44.Kim DM, Kang DW, Kim JO, Chung JH, Kim HL, Park CY, et al. Acute renal failure due to acute tubular necrosis caused by direct invasion of Orientia tsutsugamushi. J Clin Microbiol. 2008;46:1548–50. doi: 10.1128/JCM.01040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young PC, Hae CC, Lee KH, Hoon CJ. Tsutsugamushi infection - associated acute rhabdomyolysis and acute renal failure. Korean J Intern Med. 2003;18:248–50. doi: 10.3904/kjim.2003.18.4.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Susaengrat W, Dhiensiri T, Sinavatana P, Sitprija V. Renal failure in melioidosis. Nephron. 1987;46:167–9. doi: 10.1159/000184334. [DOI] [PubMed] [Google Scholar]
- 47.Sitprija V. Snakebite nephropathy. Nephrol. 2006;11:442–8. doi: 10.1111/j.1440-1797.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 48.Kanjanabuch T, Sitprija V. Snakebite nephropathy in Asia. Semin Nephrol. 2008;28:363–72. doi: 10.1016/j.semnephrol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Chugh KS. Snake bite-induced acute renal failure in India. Kidney Int. 1989;35:891–907. doi: 10.1038/ki.1989.70. [DOI] [PubMed] [Google Scholar]
- 50.Sakhuja V, Sud K. Acute renal failure in the tropics. Saudi J Kidney Dis Transplant. 1999;9:247–60. [PubMed] [Google Scholar]
- 51.Kanjanabuch T, Sitprija V. Snakebite nephrotoxicity in Asia. Semin Nephrol. 2008;28:363–72. doi: 10.1016/j.semnephrol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Jha V, Chugh KS. Nephropathy associated with animal, plant and chemical toxins in the tropics. Semin Nephrol. 2003;23:49–65. doi: 10.1053/snep.2003.50003. [DOI] [PubMed] [Google Scholar]
- 53.Chugh KS, Sharma BK, Singhal PC, Das KC, Datta BN. Acute renal failure following copper sulphate intoxication. Postgrad Med J. 1977;53:18–23. doi: 10.1136/pgmj.53.615.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mathew AJ, Dalus D. Acute formic acid poisoning in south India. Proceedings of the 8th Asia Pacific Association of Medical Toxicology Congress, Beijing. 2009:52. OP04. [Google Scholar]
- 55.Prakash J, Vohra R, Wani IA, Murthy AS, Srivastava PK, Tripathi K, et al. Decreasing incidence of renal cortical necrosis in patients with acute renal failure in developing countries: a single centre experience of 22 years from Eastern India. Nephrol Dial Transplant. 2007;22:1213–7. doi: 10.1093/ndt/gfl761. [DOI] [PubMed] [Google Scholar]
- 56.Srivastava RN, Bagga A, Moudgil A. Acute renal failure in north Indian children. Indian J Med Res. 1990;92:404–8. [PubMed] [Google Scholar]


