Abstract
BACKGROUND AND OBJECTIVE:
Home intravenous (IV) antibiotic programs are becoming increasingly popular worldwide because of their efficacy and safety. However, in Saudi Arabia these programs have not yet become an integrated part of the health care system. We present our experience with a home IV antibiotic program, as one of the major health care providers in Saudi Arabia.
DESIGN AND SETTING:
Retrospective chart review of patients enrolled in the King Abdulaziz Medical City Home Health Care IV Antibiotic Program from 1 May 2005 (the start of the program) until 30 December 2007.
METHODS:
In addition to demographic characteristics, we collected data on the site of infection, the clinical diagnosis, the isolated microorganisms, and the type of antibiotics given. Outcome measures evaluated included the relapse rate, failure rate, the safety of the program, and readmission rates.
RESULTS:
Of the 155 patients enrolled, 152 patients completed the program. Those who completed the program had a mean (SD) age of 52.8 (23.9) years. The mean (SD) duration of the IV antibiotic treatment was 20.6 (17) days. Three patients refused to complete the intended duration of therapy. Peripherally inserted central catheter (PICC) lines were utilized in 130 patients (86%). One-hundred and thirty-one patients completed the intended duration of therapy, although the therapy was changed from the initial plan for 21 (13.8%) patients. Readmission to the hospital during therapy was required for 13 patients (8.5%). Osteomyelitis was the most frequently encountered diagnosis (65 patients, 42.8%), followed by urinary tract infection (36 patients, 23.7%).
CONCLUSIONS:
The home health care-based IV antibiotic program was an effective and safe alternative for in-patient management of patients with non-life-threatening infections, and was associated with a very low complication rate. Home IV antibiotic programs should be used more frequently as part of the health care system in Saudi Arabia.
Out-patient parenteral antibiotic therapy (OPAT) programs, also known as outpatient intravenous antibiotic therapy (OPIVAT), community-based parenteral antimicrobial therapy (CoPAT), and outpatient and home parenteral antibiotic therapy (OHPAT), for non-life-threatening infections have been gaining acceptance as a standard of care, since it was defined in 1974, by Rucker and Harrison.1,2 These programs have become well-accepted and are growing around the world because of their advantages to institutions and patients.3
Hospitalization has a great financial impact on the budget of any organization.2 Hospital stays are often prolonged because of the need for IV antibiotic therapy.3,4 Most antimicrobial agents are given intravenously in hospitalized patients, and some conditions require prolonged courses of treatment.4 For example, infective endocarditis, osteomyelitis, and cellulitis typically require three to six weeks of IV antibiotics.4,5 Home IV antibiotics are also given to AIDS patients to treat cytomegalovirus retinitis, with considerable cost-saving compared to in-hospital stays.6 Other conditions treated on an outpatient basis with success include Lyme borreliosis, urinary tract infections, community-acquired pneumonia, septic arthritis, sinusitis, abdominal abscesses, and pyelonephritis.7,8 It has been proved that these programs are safe and effective and have clinical outcomes equivalent to in-hospital care.1,7,9,10 Furthermore, many institutions worldwide have adopted these programs as a cost-effective alternative to in-hospital therapy with a better quality of care.1,3,4,7,9,10,11
The King Abdulaziz Medical City (KAMC) Home Health Care Department (HHC) started a Home Intravenous Antibiotics Program as part of the HHC program in 2005 for National Guard-eligible patients to minimize the duration of hospital stay and the cost associated with IV antibiotic therapy. To our knowledge, there is no national (local) data assessing the efficacy of any similar programs in terms of cost reduction and safety, even though this program has been implemented in other private and governmental hospitals in Saudi Arabia. Therefore, this retrospective study was aimed at evaluating patient acceptance, the dropout rate, efficiency, safety, types and rates of complications, most frequently used antibiotics, and the most frequently encountered diagnoses in a tertiary hospital in Saudi Arabia.
METHODS
This study was based on a retrospective chart review of all patients enrolled in the KAMC HHC IV antibiotic Program from 1 May 2005, (the start of the program) until 30 December 2007. Before patients were enrolled in the program, they were evaluated by an infectious disease specialist for clinical stability and to establish the proper antibiotic options, treatment plan, and duration of therapy. They were then evaluated by a clinical pharmacist for dosing adjustment, drug interactions, and drug level monitoring, if necessary. Other preconditions that needed to be met included clinical stability, the absence of acute psychiatric disorders, good family support, home telephone facilities, and residence within 50 km of the hospital.
All the patients who enrolled in the program stayed in the hospital for at least 48 hours and received the recommended antibiotic for at least 24 hours to check for anaphylactic manifestations and to ensure suitable vascular access. Peripherally inserted central catheters (PICC) were mandatory if IV antibiotics were to be used for more than 7 to 10 days. For shorter antibiotic courses, peripheral vascular access that was changed every third day was considered adequate. All PICC lines were inserted by an interventional radiologist. During the stay in the hospital, a home health care nurse interviewed the patient, explained the goals of the program and instructed the patient and/or his caregiver on how to care for the vascular access site. After discharge from the hospital, a nurse from HHC visited the patient daily until discharge from the program. The nurse determined whether a visit by a HHC primary care physician was needed. Vascular access was managed as per CDC recommendations for maintenance of vascular access guidelines. All peripheral vascular access devices were routinely changed every third day. The nurse reported any concerns or problems encountered during the therapy to either the program-assigned physician or to the infectious disease consultant responsible for the overall management and treatment plan of the patient. The enrolled patients were observed for adverse drug reactions, such as nephrotoxicity, allergic reactions, and venous access complications, including phlebitis, leakage, line fracture, blockage, line slippage exit site redness, pain, and discharge.
In addition to the general demographic characteristics of the participants, such as age and gender, we collected data about the site of infection, the clinical diagnosis, the isolated microorganisms, the type of antibiotics given, the duration, complications encountered, hospital readmissions during the treatment period and after discharge, and the type of venous catheterization. Only the microbiological results for the samples obtained before the initiation of the antibiotic regimens that the patients were given at the time of discharge were included in the analysis. The outcome measures evaluated included the relapse rate, failure rate, and safety of the program (determined by the number of line infections (using the CDC definition of central line infection and the central line infection rate per 1000 catheter days) and the number of readmissions due to complications related to IV home antibiotics). Ethical approval was obtained for the study from the research center. All data were analyzed using SPSS for Windows, version 13.0 (SPSS Inc., Chicago, IL).
RESULTS
There were 155 patients enrolled in our program between 1 May 2005, and 30 December 2007. Of these patients, three dropped out of the program, one because of travel outside of Riyadh and the other two for no documented reasons. Data on these patients were presented, but were not included in the calculation of the complication rate, including the infection rate. The intended course of treatment in two of these three patients was 21 days, but they completed seven and 12 days. Both had PICC lines that were removed without apparent complications. The third patient had a peripheral vascular access site and completed nine days out of an intended 10 days. Ninety-four (61.8%) patients were male, and the mean age of the patients was 52.8 (23.9) years. Fourteen patients (9%) were less than 14 years old, which was the age limit for pediatrics in our institution, and 38 (25%) patients were older than 70 years of age. The mean (SD) duration of IV antibiotic treatment was 20.6 (17) (range, 1-150). A PICC line was used in 130 (86%) patients, while peripheral vascular access was adequate in 22 (14%) patients.
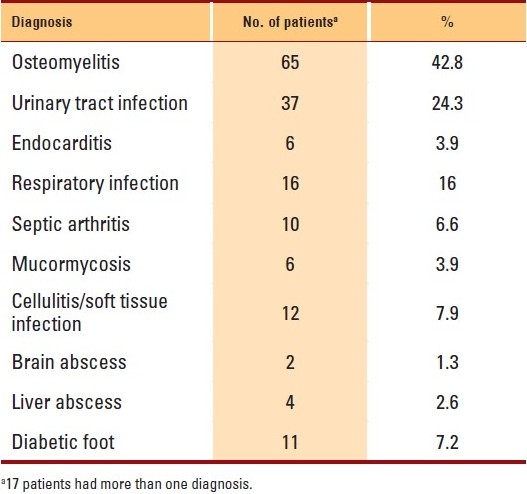
Osteomyelitis was the most frequently encountered diagnosis (65 patients, 42.8%), followed by urinary tract infection (UTI; 36 patients, 23.7%), and respiratory infection, including pneumonia and bronchiectasis, (16 patients, 10.5%). Soft tissue infections, including cellulitis and diabetic foot, were the diagnoses in 22 (14%) patients, and more than one diagnosis was present in 17 patients (Table 1).
Table 1.
Clinical diagnoses of patients enrolled in the home IV program (indications for home intravenous antimicrobials).
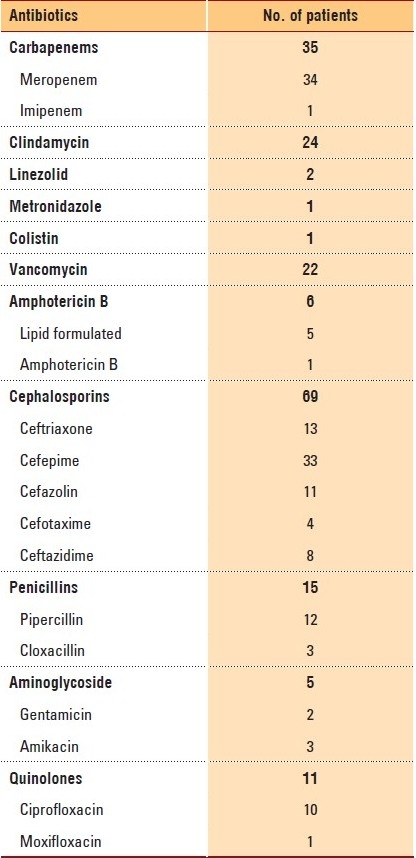
A total of 167 microbiological specimens were sent to the laboratory for culturing. Of these, 137 (82%) were positive and 30 (19.7%) were negative. Gram-negative organisms were the most commonly isolated bacteria (86 samples isolates), while gram-positive organisms were present in 44 isolates. Mixed gram-positive and gram-negative bacteria were present in 18 samples, while fungi were isolated in eight specimens (Table 2). Cephalosporins were the most widely used antibiotics, with cefepime and ceftriaxone being the most commonly prescribed. Meropenem was the second most widely used antibiotic. Combination antibiotics were the third most commonly used regimens, with clindamycin and ceftriaxone being the most widely used. Amphotericin B was the main antifungal antimicrobial drug used (Table 3).
Table 2.
Microorganisms isolated.
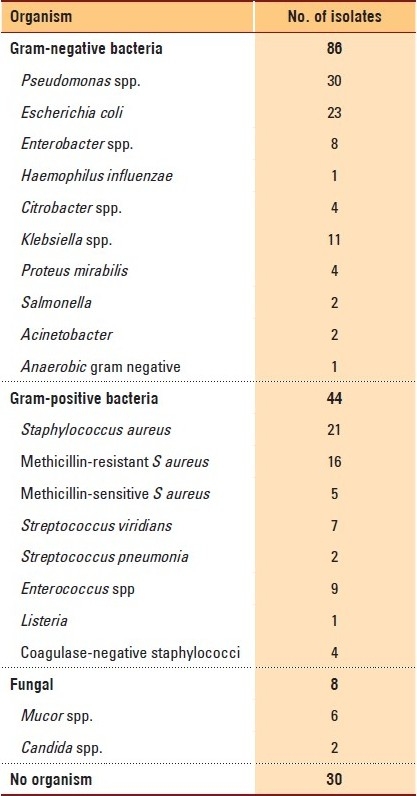
Table 3.
Antibiotic selection preference among physicians.
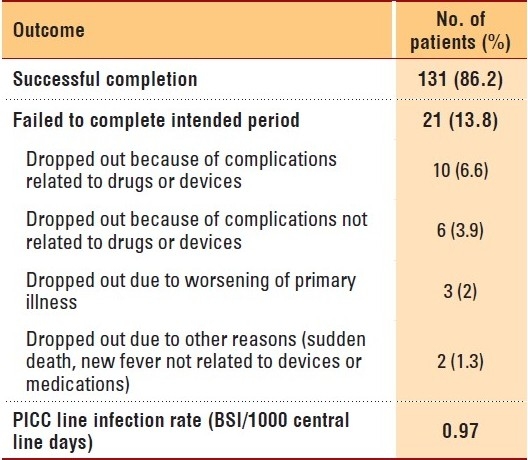
The total number of home visits to all patients was 348 during the study period; 68 patients received three visits per day (antibiotic administrated thrice daily), while 60 received two visits per day, and 24 patients received one visit per day. One hundred and thirty-one patients (86%) completed the intended duration of therapy, while the therapy was changed from the initial plan for 21 (13.8%) patients. Ten patients dropped out due to drug or device complications, while six dropped out due to complications not related to drugs or devices. Other reasons for failure to complete therapy are outlined in Table 4. Three patients refused to continue in the program after starting (one because of travel outside of Riyadh, while the reasons were not documented for the other two), and one was taken out of the program because of security concerns. These patients were not included in the final calculation of the infection rate. No access complications were recorded for any of them. The primary physician changed the route from IV to oral or discontinued therapy before completion of the targeted duration in two patients (Table 4). Complications related to PICC lines occurred in five patients. There were two blocked lines, one exit site infection, one case of bleeding from a site, and one case in which the access was accidentally removed by the patient. There were four patients with new onset fever during therapy. All four patients were eventually admitted and had the vascular access device removed. There were five antimicrobial-related complications. A worsening renal profile was seen in two patients, and skin rash was seen in an additional three patients, all of whom were being treated with piperacillin-tazobactam. There was one sudden death at home. No clear diagnosis was given. Readmission to the hospital during therapy was required for 13 patients (8.5%). The reasons were worsening clinical condition in three patients, PICC line-related complications in four patients, new onset fever in four patients (three had PICC line complications and one had a new fever due to a UTI), adverse drug reactions in five patients, and suspected pulmonary embolism in one patient. The PICC line infection rate was calculated to be 0.97 per 1000 central line days.
Table 4.
Outcome measures.
The total cost of the HHC program was 644 627.48 SAR, which included nurse visits, physician visits, disposables, and antibiotics. If the cost of the PICC of 1 500 SAR was included, the total cost increased to 839 627.48 SAR. The calculated in-patient cost was 1 368 750.68 SAR, which included bed cost, nursing cost, physicians visit cost, administration of antibiotics, and disposables. Most of the patients who required more than 14 days of in-hospital administration of antibiotics would have had either a PICC line or other central access. The cost of these lines and insertion fees was not included.
DISCUSSION
Home-based antibiotic treatment programs are increasingly used around the world. The increasing knowledge of their safety for the treatment of a large variety of infectious diseases has prompted the creation of reference therapeutic guidelines like those compiled by the American Society of Infectious Diseases.12,13 These programs have advantages that span all age groups: elderly patients benefit because these programs reduce the incidence of many adverse events resulting from conventional hospitalization, including delirium and functional worsening, and young patients benefit by having more freedom through resuming a normal lifestyle.14–17
Despite worldwide implementation of programs of home IV antibiotic treatment, there are currently a limited number of non-private centers in Saudi Arabia that run these programs. There is an increasing demand for acute beds in the Saudi health care system, and this program is a safe and convenient alternative for admission to acute beds. This model of therapy provides a comfortable and acceptable alternative to in-hospital admission for Saudi patients. Only two patients refused to complete therapy (1.9%), one of whom was traveling outside of Riyadh. A high acceptance fot these programs has been reported in the literature.7–10
Overall, complications related to PICC lines occurred only in 1.6/1000 catheter days, which is lower than some previous complication rates reported for similar programs in North America.18 The rate of PICC line infection was 0.97 per 1000 central line days. There is no current National Nosocomial Infections Surveillance System (NNIS) benchmark for the PICC line infection rate. The NNIS rate for central line infection in general is 3.9 blood stream infections (BSIs)/1000 catheter days.19,20 Older studies have reported the use of a 5% vascular access site infection rate,4 while in one more recent study, the PICC line infection rate ranged between 0.4 and 1.15 BSI/1000 catheter days.18
Cephalosporins are the most frequently used antibiotics in our program. Other studies have also indicated a preference for using cephalosporins in similar programs.19 However, the overall high rate of using broad-spectrum medications such as meropenem and piperacillin-tazobactam is of concern. The higher prevalence of gram-negative organisms, including multi-resistant organisms, and the increasing isolation of ESBL in our organization, may be factors leading to a more frequent selection of broader spectrum antibiotics. The high rate of isolation of MRSA explains the high use of vancomycin in this studied population.
We consider the launching of this program to have been successful, with a low dropout rate, a low overall complication rate, and provision of a safe and cost-effective alternative to in-hospital management of similar cases. We believe that a wider use of similar programs should be considered among the health care sectors in Saudi Arabia. We hope that our positive experience will encourage health care planners to develop the infrastructure necessary for such programs across the country.
REFERENCES
- 1.Steinmetz D, Berkovits E, Edelstein H, Flatau E, Almany A, Raz R. Home intravenous antibiotic therapy programme, 1999. J Infect. 2001;42:176–80. doi: 10.1053/jinf.2001.0824. [DOI] [PubMed] [Google Scholar]
- 2.Tice AD, Rehm SJ, Dalovisio JR, Bradley JS, Martinelli LP, Graham DR, et al. IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38:1651–72. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 3.Seaton RA, Nathwani D. Outpatient and home parenteral antibiotic therapy (OHPAT) in the UK: survey of infection specialists’ experience and views. Clin Microbiol Infect. 2000;6:385–8. [PubMed] [Google Scholar]
- 4.Kayley J, Berendt AR, Snelling MJ, Moore H, Hamilton HC, Peto TE, et al. Safe intravenous antibiotic therapy at home: Experience of a UK based programme. J Antimicrob Chemother. 1996;37:1023–9. doi: 10.1093/jac/37.5.1023. [DOI] [PubMed] [Google Scholar]
- 5.Bernard L, El-Hajj, Pron B, Lotthé A, Gleizes V, Signoret F, et al. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: Evaluation of efficacy, tolerance and cost. J Clin Pharm Ther. 2001;26:445–51. doi: 10.1046/j.1365-2710.2001.00380.x. [DOI] [PubMed] [Google Scholar]
- 6.Bucher G, Wiley D. International Conference on AIDS; 1993. Vol. 9. New Orleans, USA: International Conference on Aids; 1993. Performance of peripherally inserted central catheters (PICC lines) in HIV-positive patients for longterm IV therapy; p. 521. [Google Scholar]
- 7.DeMaio J. Outpatient parenteral antibiotic therapy. Infect Med. 2004;21:496–504. [Google Scholar]
- 8.Corwin P, Toop L, McGeoch G, Than M, Wynn-Thomas S, Wells JE, et al. Randomised controlled trial of intravenous antibiotic treatment for cellulitis at home compared with hospital. BMJ. 2005;330:129. doi: 10.1136/bmj.38309.447975.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalovisio JR, Juneau J, Baumgarten K, Kateiva J. Financial impact of a home intravenous antibiotic program on a Medicare managed care program. Clin Infect Dis. 2000;30:639–42. doi: 10.1086/313755. [DOI] [PubMed] [Google Scholar]
- 10.Dubois A, Santos-Eggimann B. Evaluation of patients’ satisfaction with hospital-at-home care. Eval Health Prof. 2001;24:84–98. doi: 10.1177/01632780122034812. [DOI] [PubMed] [Google Scholar]
- 11.Wolter JM, Cagney RA, McCormack JG. A randomised trial of home vs hospital intravenous antibiotic therapy in adults with infectious diseases. J Infect. 2004;48:263–8. doi: 10.1016/S0163-4453(03)00135-X. [DOI] [PubMed] [Google Scholar]
- 12.Esposito S, Noviello S, Leone S, Tice A, Seibold G, Nathwani D, et al. Outpatient parenteral antibiotic therapy (OPAT) in different countries: A comparison. Int J Antimicrob Agents. 2004;24:473–8. doi: 10.1016/j.ijantimicag.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Tice AD, Rehm SJ, Dalovisio JR, Bradley JS, Martinelli LP, Graham DR, et al. IDSA. Practice guidelines for outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2004;38:1651–72. doi: 10.1086/420939. [DOI] [PubMed] [Google Scholar]
- 14.Creditor MC. Hazards of hospitalization of the elderly. Ann Intern Med. 1993;118:219–23. doi: 10.7326/0003-4819-118-3-199302010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 16.Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–8. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Lopez J, San Jose Laporte A, Pardos-Gea J, Tapia Melencho E, Lozano Ortın E, Barrio Guirado A, et al. Safety and efficacy of home intravenous antimicrobial infusion therapy in older patients: A comparative study with younger patients. Int J Clin Pract. 2008;62:1188–92. doi: 10.1111/j.1742-1241.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 18.Funk D, Gray J, Plourde PJ. Two-year trends of peripherally inserted central catheter-line complications at a tertiary-care hospital: Role of nursing expertise. Infect Control Hosp Epidemiol. 2001;22:377–9. doi: 10.1086/501917. [DOI] [PubMed] [Google Scholar]
- 19.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. Centers for Disease Control and Prevention. Appendix A. MMWR Recomm Rep. 2002;51:27–8. [PubMed] [Google Scholar]
- 20.Royer T, Lang E, Neuzil K, Beneda H. Peripherally inserted central catheter (PICC) bloodstream infection surveillance rates in medical intensive care, medical-surgical wards, extended care, and outpatients. Am J Infect Control. 2005;33:e15–6. [Google Scholar]


