Abstract
BACKGROUND AND OBJECTIVE:
Gram-positive bacteria are important nosocomial pathogens. The objective of this study was to estimate the frequencies and resistance rates of gram-positive pathogens isolated from hospitals in Makkah, Saudi Arabia.
DESIGN AND SETTING:
Prospective study at three Makkah hospitals from May 2008 to April 2009.
PATIENTS AND METHODS:
Clinical isolates were collected and demographic and laboratory data were recorded. Standard microbiological methods were used to identify the organisms and test for antimicrobial susceptibility. The results were interpreted according to the Clinical Laboratory Standards Institute (CLSI) guidelines.
RESULTS:
Clinical isolates were collected from 1087 patients. Gram-positive pathogens infected all age groups, but had no gender predominance. Staphylococcus aureus was the most common cause of wound infection and accounted for more than half of the clinical isolates (688 cases). Coagulase-negative staphylococcus (CONS) was a common isolate from blood cultures. Wounds were the most common site of infection (37.6%). Enterococcus spp. and Streptococcus agalactiae were the second most common bacteria (26%). The resistance rates of S aureus and CONS isolates were 39.4% and 82.4% for oxacillin, respectively. Among the streptococci, the resistance rates of Streptococcus pneumoniae were 21.1% and 16.7% for ampicillin and erythromycin, respectively.
CONCLUSIONS:
S aureus infections were found to be very common in the Makkah hospitals. Infection prevention, control measures and continuous monitoring for antibiotic susceptibility are necessary to reduce these and other nosocomial infections.
The introduction and increasing use of antibiotics for treatment have led to the rapid development and spread of antibiotic resistance in microorganisms, particularly in human pathogens. In addition, a tendency towards an increased number and severity of gram-positive infections has been observed in the last decade.1 Gram-positive bacteria are extremely important pathogens, both inside and outside the hospital environment. These pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), vancomycin-intermediate and -resistant S aureus (VISA and VRSA), coagulase-negative staphylococcus (CONS) and penicillin-resistant Streptococcus pneumoniae, have become a serious problem due to their increased resistance rates, resulting in increasing morbidity and mortality because of limited treatment options.2,3 Susceptibility to resistant strains is a treatment challenge.4 An important issue to control resistance among gram-positive pathogens is surveillance to determine the extent of the problem and identify epidemiological factors involved in the development and spread of resistance.
Each year, more than 2 million pilgrims travel to Makkah to perform Umrah and Hajj rituals. Mass gathering of individuals from different parts of the world in a confined area increases their susceptibility to infections, including those caused by gram-positive bacteria. The aim of this study was to estimate the frequencies and resistance rates of gram-positive pathogens isolated from selected hospitals in Makkah, Saudi Arabia.
PATIENTS AND METHODS
This prospective study was undertaken at three hospitals in Makkah: Al-Noor Specialist Hospital (ASH), Hera General Hospital (HGH), and King Abdul-Aziz Hospital (KAH). ASH is the largest hospital with a 560-bed capacity and is located east of Makkah near the pilgrimage area. KAH is a 272-bed hospital and is 7 km away from the Holy mosque (north of Makkah), while HGH is a 280-bed hospital 12 km away from the central city (north). The study protocol was approved by the local ethical committee and was in accordance with the Helsinki Declaration of 1975, as well as the 2000 revision. Data were collected from May 2008 to April 2009, corresponding to Islamic months Jumada’I 1429 to Rabi’II 1430H.
In addition to clinical isolates, demographic information collected included age, gender, nationality, site of infection and clinical wards. The data collected from the laboratory records included the type of organism and antimicrobial susceptibility. The bacteria were identified by using standard microbiological methods, including morphology on culture media, gram staining and biochemical tests. Antimicrobial susceptibility testing was performed with MicroScan WalkAway 40si (Siemens Healthcare Diagnostics, Deerfield, IL, USA) in HGH; and with Phoenix Automated Microbiology System (BD, Franklin Lakes, NJ, USA) in ASH and KAH. The results were analyzed by using IBM SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA), and the P values were calculated by using GraphPad InStat 3.0 software (GraphPad, Inc., San Diego, California, USA).
RESULTS
Clinical isolates and complete data were obtained from 1087 patients with gram-positive bacterial infection and they were included in the study. Patients with incomplete clinical data were excluded. Most isolates were collected from ASH (43%) and HGH (41%). S aureus was commonly isolated from ASH (54.4%), whereas Streptococcus agalactiae (76.2%) and Enterococcus spp. (63.5%) were frequently obtained from HGH. Moreover, most streptococcal strains were isolated from HGH. The highest number of infections occurred during the Umrah and Hajj seasons (i.e., Shabaan [10.1%], Ramadan [7.6%], Dhul Al-Hijjah [10.2%] and Muharram [12.3%]). The patients belonged to 22 different nationalities, the majority being Saudi (81%), followed by Pakistani (4.4%), Yemeni (2%), Nigerian (1.9%) and Egyptian (1.7%).
The most common pathogen was S aureus (63.3%) (Figure 1). Although the gram-positive pathogens infected all age groups, the number of cases increased in the 21-30 (19%) and “above 60” (21.1%) age groups, with staphylococci having a higher distribution in these age groups (17.9% and 21.5%, respectively) (Table 1). Streptococcus pyogenes was found less frequently (only 13 cases); however, it affected all age groups. Infection by Enterococcus spp. was more common in older patients than in young children. Overall, there was no major difference between genders with regard to susceptibility to infection. However, males were more susceptible to S aureus infection (59%), and females were more susceptible to Streptococcus agalactiae infection (79.2%) (Figure 2).
Figure 1.
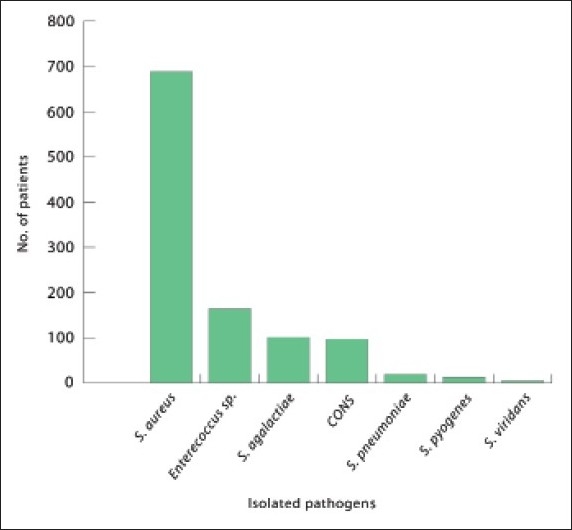
Isolated pathogens of gram-positive bacteria during the study period.
Table 1.
Frequencies of gram-positive bacterial isolates according to patient age.
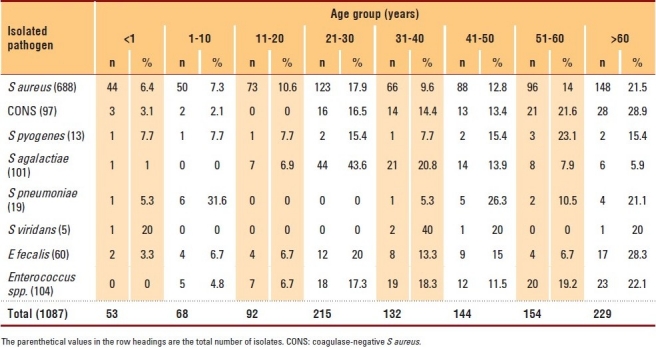
Figure 2.
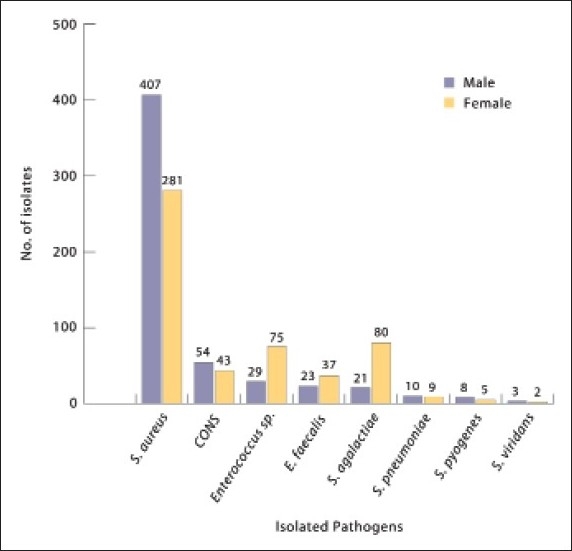
Frequency of pathogenic isolates according to patient gender.
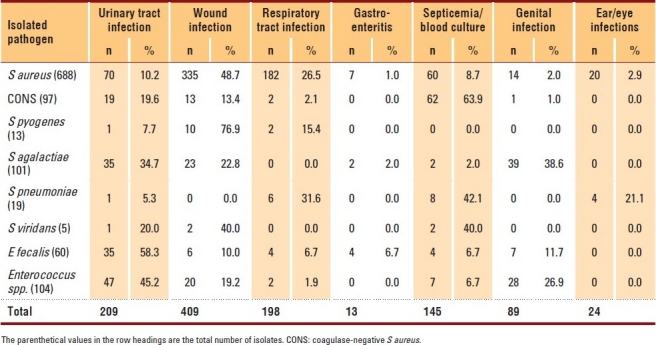
Wounds were the most common infection site (37.6%). S aureus was isolated from patients with wound infection at a frequency of 48.7%, followed by respiratory tract infection (RTI) (26.5%), urinary tract infection (UTI) (10.2%) and septicemia (8.7%) (Table 2). CONS was the common isolate from blood cultures (63.9%), followed by UTI (19.6%) and wound infection (13.4%). Streptococcus pyogenes was isolated commonly from the patients with wound infection (76.9%), followed by those with RTI (15.4%) and UTI (7.7%). Most clinical isolates of Streptococcus agalactiae originated from genital infections (38.6%), followed by UTI and wound infection (34.7% and 22.8%, respectively). Streptococcus pneumoniae was mainly isolated from the patients with septicemia (42.1%), followed by those with RTI (31.6%) and ear or eye infection (21.1%). Enterococcus spp. was very common in the patients with UTI (45.2%).
Table 2.
Distribution of the gram-positive pathogens according to site of infection.
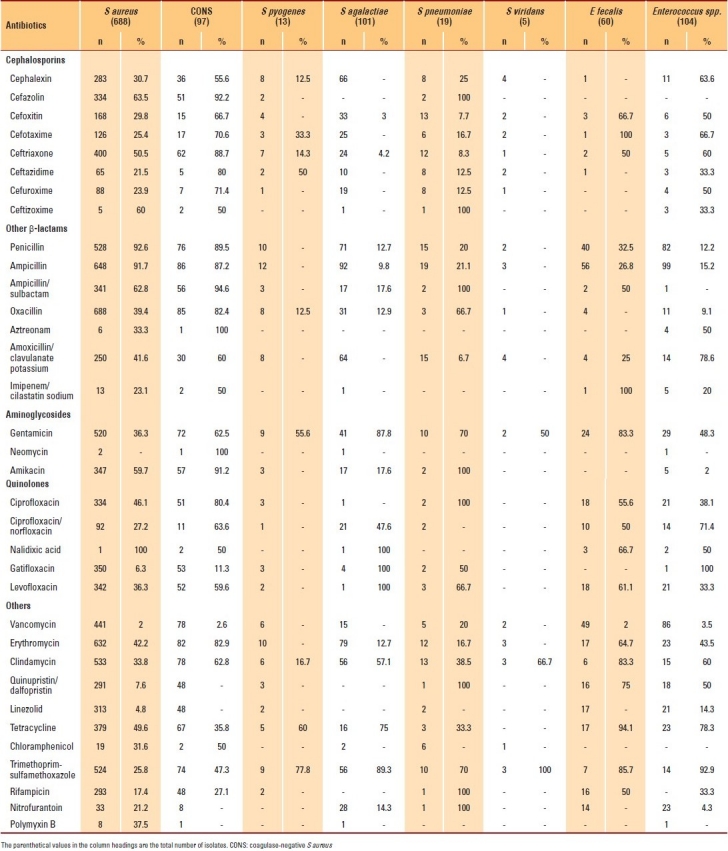
The susceptibility patterns of 8 gram-positive bacterial isolates were compared (Table 3). The resistance rates of S aureus to penicillin-G, oxacillin and vancomycin were 92.6%, 39.4% and 2%, respectively; for CONS, the resistant rates were 89.5%, 82.4% and 2.6%, respectively. There was a significant correlation between the resistance to oxacillin and the resistance to gentamicin, erythromycin and ciprofloxacin (P>.01). Among the streptococci, the resistance rates of Streptococcus pneumoniae were 21.1%, 16.6% and 70% to ampicillin, erythromycin and trimethoprim-sulfamethoxazole, respectively. The resistance rate of Streptococcus agalactiae to penicillin and erythromycin, respectively, was 12.7%. No Streptococcus pyogenes or Streptococcus viridans isolate showed resistance to penicillin and erythromycin. The resistance rates of E fecalis were 26.8%, 2% and 83% to ampicillin, vancomycin and gentamicin, respectively.
Table 3.
Antimicrobial resistance patterns of the gram-positive bacterial isolates.
DISCUSSION
The demographic data revealed that gram-positive bacterial infections can affect all age groups without predominant gender differences. The study showed that the highest number of cases of such infections occurred during the Umrah and Hajj seasons, possibly because of the high number of visitors and pilgrims from around the world and the effects of overcrowding, exhaustion and undernourishment on these visitors.
Wounds were the most common site of infection (409 cases), and S aureus was the causative agent in about half of these cases. Previous studies have shown a high prevalence of skin infection compared with other sites of infection both inside and outside the hospital environment.5,6 In a recent study, the incidence of skin infection was estimated to be 24.6 per 1000 person-years, and skin infection was estimated to be the third most common diagnosis after chest pain and asthma in the emergency-care setting.5,6 In other studies, S aureus was considered to be the main cause of skin infection acquired nosocomially, with a high resistance rate to oxacillin (40%).7,8 CONS was responsible for about two thirds of the positive blood cultures in the present study. As the organism is normally found on the skin, many positive blood cultures are frequently a result of contaminants from the skin. In fact, the interpretation of positive blood cultures of CONS is a major challenge facing clinicians worldwide, and it has not been well studied in Saudi hospitals.9
Enterococcus spp. and Streptococcus agalactiae were the second most common pathogens, and accounted for about 26% of the total cases. Enterococcus spp. were most commonly isolated from female UTIs. A study in India provided similar results: enterococci were found in 22.2% of the clinical specimens, with Foley catheters and burn wounds being the major sites of isolation.10 Data from the CDC indicate that enterococci are the second leading cause of nosocomial infection, joining Escherichia coli, Pseudomonas aeruginosa, and S aureus in the list of the most prevalent pathogens.11 The frequency of enterococci in clinical specimens is possibly related to their ability to grow and survive because of selective pressure of antimicrobial agents.10 The lack of significant differences between genders with regard to susceptibility to infection with gram-positive pathogens in this study is probably because both genders were subjected to the same environment and physical activities.
The resistance rate of S aureus isolates to oxacillin was 39.4%, and that of CONS isolates was 82.4% in this study. The oxacillin-resistance rate varies among countries and depends on several factors, including infection control activities and antimicrobial selection pressure.12 For example, the prevalence of MRSA was found to vary from approximately 64.5% in the United States and Taiwan to negligible levels in Sweden and Denmark.12,13 The present data is in agreement with the findings of previous studies performed in different regions of Saudi Arabia, where MRSA was detected in 30% to 40% of the clinical isolates, although some of these studies were performed several years ago.14–17 As demonstrated by many investigators from other geographical regions, we found a strong correlation between oxacillin resistance and co-resistance to non–β-lactam antimicrobials such as gentamicin, erythromycin and ciprofloxacin (P>.01).18–20 Such high rates of co-resistance suggest the presence of multidrug-resistant MRSA strains.
Among the streptococci in this study, the resistance rates of Streptococcus pneumoniae were 21.1% and 16.6% to ampicillin and erythromycin, respectively. The resistance rate of these isolates against penicillin was much lower than that estimated in many local and international studies. For example, in a study performed at King Saud University in Riyadh, Fouda et al found that 44.6% of the Streptococcus pneumoniae isolates were susceptible to penicillin.21 In a study conducted in Japan, Qin et al found that 27.4% of the Streptococcus pneumoniae isolates were susceptible to penicillin.22 Similarly, in another study conducted in Taiwan, the resistance rate of Streptococcus pneumoniae to penicillin was 69%.13
The resistance rates of E fecalis were 26.8%, 2% and 83%, for ampicillin, vancomycin and gentamicin,respectively. The ampicillin-resistance rate in this study was much lower than the estimate in many international studies, although there are no recent local studies available for comparison. The results show that the rate of vancomycin resistance remains low in Saudi hospitals. Data from the SENTRY program confirms that the rate of vancomycin resistance in the Asia-Pacific region is similar to that in the current study (1% to 2%).23 Such a low rate may reflect the fact that vancomycin is used less often in Saudi hospitals than in other countries such as the United States, where resistance is more common.24 High-level resistance to gentamicin was observed in this study. A similar finding was reported by McDonald et al, who calculated a resistance rate of 66%.13 In Taiwan, although human outpatient use of gentamicin may be very common, aminoglycosides are also used in agriculture, and high levels of gentamicin-resistant E fecalis and E fecium can be detected in the human food supply. Thus, one possibility, in addition to the frequent use of parenteral aminoglycosides among outpatients, is food-borne transmission of high-level resistance to gentamicin, contributing to elevated rates of resistance among outpatient isolates.13 Johnson et al stated that, among the enterococci, high-level resistance to gentamicin, which abolishes synergy between cell-wall–active agents and all aminoglycosides except streptomycin, is a major problem; in their study, 40% of the E fecalis isolates and 25% of the E fecium isolates were highly resistant to gentamicin.25
In conclusion, S aureus infections were found to be very common in Makkah. The expansion of resistance to antimicrobial agents is currently the main concern of the medical community worldwide. Infections caused by gram-positive antibiotic-resistant strains seem to be associated with increased morbidity and mortality in hospitals. Infection prevention and control measures should be established to reduce such and other health-care-associated infections. Antibiotic susceptibility of gram-positive bacteria should be continuously monitored to avoid treatment difficulties.
Acknowledgments
The author is grateful to The Custodian of The Two Holy Mosques Institute of Hajj Research for supporting this study. The author thanks Wail Abdul-Salam, Abdul-Elah Al-Jeaid, Abdul-Kareem Abid, Abdul-Aziz Al-Qarni and Abdulla Allehyani, the medical science students, for their help in specimen collection and practical work. I would also like to thank the staff of ASH, HGH and KAH, in Makkah, involved in this study; without their help, this work could not have been accomplished.
REFERENCES
- 1.Berger-Bächi B. Resistance mechanisms of gram-positive bacteria. Int J Med Microbiol. 2002;292:27–35. doi: 10.1078/1438-4221-00185. [DOI] [PubMed] [Google Scholar]
- 2.Menichetti F. Current and emerging serious Gram-positive infections. Clin Microbiol Infect. 2005;11:22–8. doi: 10.1111/j.1469-0691.2005.01138.x. [DOI] [PubMed] [Google Scholar]
- 3.Nordmann P, Naas T, Fortineau N, Poirel L. Superbugs in the coming new decade; multidrug resistance and prospects for treatment of Staphylococcus aureus Enterococcus spp. and Pseudomonas aeruginosa in 2010. Curr Opin Microbiol. 2007;10:436–40. doi: 10.1016/j.mib.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Alzeer AH. Respiratory tract infection during Hajj. Ann Thorac Med. 2009;4:50–3. doi: 10.4103/1817-1737.49412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellis Simonsen SM, van Orman ER, Hatch BE, Jones SS, Gren LH, Hegmann KT, et al. Cellulitis incidence in a defined population. Epidemiol Infect. 2006;134:293–9. doi: 10.1017/S095026880500484X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eron LJ, Lipsky BA, Low DE, Nathwani D, Tice AD, Volturo GA. Expert panel on managing skin and soft tissue infections. Managing skin and soft tissue infections: Expert panel recommendations on key decision points. J Antimicrob Chemother. 2003;52:i3–17. doi: 10.1093/jac/dkg466. [DOI] [PubMed] [Google Scholar]
- 7.Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: A review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol. 2008;19:173–84. doi: 10.1155/2008/846453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vinh DC, Embil JM. Rapidly progressive soft tissue infections. Lancet Infect Dis. 2005;5:501–13. doi: 10.1016/S1473-3099(05)70191-2. [DOI] [PubMed] [Google Scholar]
- 9.Chiu L, Huang Y, Chen Y. Infection with coagulase-negative staphylococci in a neonatal intensive care unit. J Med Sci. 2000;20:481–7. [Google Scholar]
- 10.Desai PJ, Pandit D, Mathur M, Gogate A. Prevalence, identification and distribution of various species of enterococci isolated from clinical specimens with special reference to urinary tract infection in catheterized patients. Indian J Med Microbiol. 2001;19:132–7. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Nosocomial enterococci resistant to vanomycin-United States, 1989-1993. MMWR Morb Mortal Wkly Rep. 1993;42:597–9. [PubMed] [Google Scholar]
- 12.Wise R. Introduction: Treatment of Gram-positive infections. J Antimicrob Chemother. 2003;51:ii5–7. doi: 10.1093/jac/dkg252. [DOI] [PubMed] [Google Scholar]
- 13.McDonald LC, Lauderdale TL, Shiau YR, Chen PC, Lai JF, Wang HY, et al. TSAR Participating Hospitals.The status of antimicrobial resistance in Taiwan among Gram-positive pathogens: The Taiwan Surveillance of Antimicrobial Resistance (TSAR) programme, 2000. Int J Antimicrob Agents. 2004;23:362–70. doi: 10.1016/j.ijantimicag.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Asghar AH, Momenah AM. Methicillin resistance among Staphylococcus aureus isolates from Saudi hospitals. Med Princ Pract. 2006;15:52–5. doi: 10.1159/000089386. [DOI] [PubMed] [Google Scholar]
- 15.Bukharie HA, Abdelhadi MS. The epidemiology of methicillin-resistant Staphylococcus aureus at a Saudi university hospital. Microb Drug Resist. 2001;7:413–6. doi: 10.1089/10766290152773428. [DOI] [PubMed] [Google Scholar]
- 16.Madani TA. Epidemiology and clinical features of methicillin-resistant Staphylococcus aureus in the University Hospital, Jeddah, Saudi Arabia. Can J Infect Dis. 2002;13:245–50. doi: 10.1155/2002/235213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madani TA, Al-Abdullah NA, Al-Sanousi AA, Ghabrah TM, Afandi SZ, Bajunid HA. Methicillin-resistant Staphylococcus aureus in two tertiary-care centers in Jeddah, Saudi Arabia. Infect Control Hosp Epidemiol. 2001;22:211–6. doi: 10.1086/501891. [DOI] [PubMed] [Google Scholar]
- 18.Lee NY, Song JH, Kim S, Peck KR, Ahn KM, Lee SI, et al. Carriage of antibiotic-resistant pneumococci among Asian children: A multinational surveillance by the Asian Network for Surveillance of Resistant Pathogens (ANSORP) Clin Infect Dis. 2001;32:1463–9. doi: 10.1086/320165. [DOI] [PubMed] [Google Scholar]
- 19.Tripodi MF, Attanasio V, Adinolfi LE, Florio A, Cione P, Cuccurullo S, et al. Prevalence of antibiotic resistance among clinical isolates of methicillin-resistant staphylococci. Eur J Clin Microbiol Infect Dis. 1994;13:148–52. doi: 10.1007/BF01982189. [DOI] [PubMed] [Google Scholar]
- 20.von Eiff C, Reinert RR, Kresken M, Brauers J, Hafner D, Peters G. Multicenter Study on Antibiotic Resistance in Staphylococci and Other Gram-Positive Cocci Study (Mars) Group. Nationwide German multicenter study on prevalence of antibiotic resistance in staphylococcal bloodstream isolates and comparative in vitro activities of quinupristin-dalfopristin. J Clin Microbiol. 2000;38:2819–23. doi: 10.1128/jcm.38.8.2819-2823.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fouda SI, Kadry AA, Shibl AM. Beta-lactam and macrolide resistance and serotype distribution among Streptococcus pneumoniae isolates from Saudi Arabia. J Chemother. 2004;16:517–23. doi: 10.1179/joc.2004.16.6.517. [DOI] [PubMed] [Google Scholar]
- 22.Qin L, Masaki H, Watanabe K, Furumoto A, Watanabe H. Antimicrobial susceptibility and genetic characteristics of Streptococcus pneumoniae isolates indicating possible nosocomial transmission routes in a community hospital in Japan. J Clin Microbiol. 2007;45:3701–6. doi: 10.1128/JCM.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low DE, Keller N, Barth A, Jones RN. Clinical prevalence, antimicrobial susceptibility, and geographic resistance patterns of enterococci: Results from the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001;32:S133–45. doi: 10.1086/320185. [DOI] [PubMed] [Google Scholar]
- 24.McDonald LC, Yu HT, Yin HC, Hsiung CA, Hung CC, Ho M. Antibiotic Use Working Group. Correlates of antibiotic use in Taiwan hospitals. Infect Control Hosp Epidemiol. 2001;22:565–71. doi: 10.1086/501953. [DOI] [PubMed] [Google Scholar]
- 25.Johnson AP, Henwood C, Mushtaq S, James D, Warner M, Livermore DM. ICU Study Group. Susceptibility of Gram-positive bacteria from ICU patients in UK hospitals to antimicrobial agents. J Hosp Infect. 2003;54:179–87. doi: 10.1016/s0195-6701(03)00145-2. [DOI] [PubMed] [Google Scholar]


