Abstract
Vitamin D has a potential role in preventing HIV-related complications, based on its extensive involvement in immune and metabolic function, including preventing osteoporosis and premature cardiovascular disease. However, this association has not been examined in large studies or in resource-limited settings. Vitamin D levels were assessed in 884 HIV-infected pregnant women at enrollment in a trial of multivitamin supplementation (excluding vitamin D) in Tanzania. Information on HIV related complications was recorded during follow-up (median, 70 months). Proportional hazards models and generalized estimating equations were used to assess the relationship of vitamin D status with these outcomes. Women with low vitamin D status (serum 25-hydroxyvitamin D<32 ng/mL) had 43% higher risk of reaching a body mass index (BMI) less than 18 kg/m2 during the first 2 years of follow-up, compared to women with adequate vitamin D levels (hazard ratio [HR]: 1.43; 95% confidence intervals: [1.03–1.99]). The relationship between continuous vitamin D levels and risk of BMI less than 18 kg/m2 during follow-up was inverse and linear (p=0.03). Women with low vitamin D levels had significantly higher incidence of acute upper respiratory infections (HR: 1.27 [1.04–1.54]) and thrush (HR: 2.74 [1.29-5.83]) diagnosed during the first 2 years of follow-up. Low vitamin D status was a significant risk factor for wasting and HIV-related complications such as thrush during follow-up in this prospective cohort in Tanzania. If these protective associations are confirmed in randomized trials, vitamin D supplementation could represent a simple and inexpensive method to improve health and quality of life of HIV-infected patients, particularly in resource-limited settings.
Introduction
Progressive deterioration in immune function is the major hallmark of HIV disease. Interventions such as vitamin D supplementation, by virtue of their extensive involvement in the human immune response,1 may help slow HIV disease progression and improve quality of life in the millions of HIV-infected individuals worldwide. In addition to the regulation of calcium movement in gastrointestinal mucosa, renal tubules, and the skeleton, vitamin D has several other important metabolic functions.2 An increase in the occurrence of infections in children with rickets, the classic vitamin D deficiency disease, has been reported although the mechanisms implicated are not fully understood.3 Vitamin D also has been shown to have an integral role in innate immunity and response to infections such as tuberculosis, one of the most common opportunistic illnesses among HIV-infected individuals.4,5 Additionally, vitamin D supplementation can potentially help prevent or ameliorate the deleterious effects of both the HIV infection and antiretroviral treatment on bone mineral density and cardiovascular disease.6–8 Vitamin D can improve insulin secretion and sensitivity, inhibit vascular smooth cell proliferation, and downregulate the renin-angiotensin system; these biologic actions may help explain the potential benefits of vitamin D on metabolic disease.9–11
In recent work, we have shown that low vitamin D levels (defined as serum 25-hydroxyvitamin D<32 ng/mL) among HIV-infected pregnant women at enrolment in a trial of vitamin supplementation12 are associated with increased risks of HIV disease progression, anemia, and mortality during follow-up.13 In this article, we examined the relationship between low vitamin D status and HIV-related complications, as it may provide an explanation for the increase in disease progression and mortality among women with low vitamin D status.
Methods
Study population
The study design has been previously described in detail.14 Briefly, participants were HIV-infected pregnant women enrolled in a randomized, double-blinded, placebo-controlled trial of vitamin supplementation (1995–1997). Pregnant women (12–27 weeks gestation) were randomized to receive vitamin A alone, multivitamins (vitamins B-complex, C, and E), both vitamin A and multivitamins, or placebo. All women received iron-folate daily and chloroquine weekly as malaria prophylaxis, according to national guidelines for antenatal care at the time the trial was conducted. At the time of the study, antiretroviral therapy (ART) was not available to most women in Tanzania, including participants in the trial.
Informed consent was obtained from all participants.14 The study protocol was approved by the Research and Publications Committee of the Muhimbili University College of Health Sciences, the Ethical Committee of the National AIDS Control Program of the Tanzanian Ministry of Health, and the Institutional Review Board of the Harvard School of Public Health.
Assessment of baseline covariates
Structured interviews were conducted during the baseline visit to collect information on demographic characteristics including age, educational level, and money spent on food per day. HIV disease stage was classified in accordance with the World Health Organization (WHO) guidelines.15 Trained research assistants obtained anthropometric measurements, including height, weight, and mid–upper arm circumference, using standardized procedures and calibrated instruments.
Assessment of outcomes
Women were followed through monthly study clinic visits, and women who missed a clinic visit or traveled out of Dar es Salaam were followed via a home visit. At each visit, physicians performed a clinical examination, and a nurse assessed self-reported symptoms and HIV-related complications in the preceding period. HIV disease stage was assessed at each visit, in accordance with WHO criteria.15 Height, weight, and mid–upper arm circumference were also measured at each visit.
Laboratory methods
Blood samples were obtained from study participants at enrollment (12–27 weeks of gestation), and plasma was stored at or below −70°C. Vitamin D status was assessed using serum levels of 25-hydroxyvitamin D [25(OH)D]. Serum 25(OH)D levels were measured by the fully automated chemiluminescence ADVANTAGE 25(OH)D assay system obtained from Nicholas Institute Diagnostics, San Juan Capistrano, California. Absolute CD4, CD8, and CD3 T-cell counts were evaluated with the FACSCount system (Becton-Dickinson, San Jose, CA). Hemoglobin levels were assessed using either a CBC5 Coulter Counter (Coulter Corporation, Miami, FL) or by the cyanmethemoglobin method with use of a colorimeter (Corning Inc., Corning, NY). HIV-1 serostatus was determined by the Enzygnost anti-HIV-1/2 Plus assay (Dade Behring, Germany) followed by the Wellcozyme HIV-1 recombinant test (Murex Biotech Ltd., Dartford, UK). Discordant results were resolved via Western blot test (Bio-Rad Laboratories Ltd., Hertfordshire, UK).16
Statistical analysis
Vitamin D status was defined as insufficient (<32 ng/mL or<80 nmol/L) versus adequate. The cutoff of 32 ng/mL for vitamin D insufficiency was based on requirements for optimal calcium homeostasis17 and previous studies.18
Wasting was defined as a body mass index (BMI) of less than 18 kg/m2; this is in accordance with our previous studies19 and was selected on the basis of findings from a study of HIV-infected African adults demonstrating that BMI less than 18 kg/m2 had comparable validity as a CD4 T-cell count of less than 200 cells per microliter in predicting mortality.20 We also considered mid–upper arm circumference (MUAC) less than 22 cm as an end point in this analysis in accordance with the value recommended for screening for malnutrition in women.21 Weight loss of more than 10% was also used as an end point based on the conventional definition of HIV-related wasting.22,23
We examined the relationship of vitamin D status at baseline with wasting outcomes using proportional hazards models.24 We examined the times to first episode of wasting as defined by a BMI of less than 18 kg/m2, MUAC of less than 22 cm, and a weight loss of greater than 10% during follow-up. For those without the outcomes, follow-up ended on the date on which they were last assessed.
We investigated potential nonlinear relationship of continuous vitamin D levels with the risk of wasting outcomes nonparametrically with stepwise restricted cubic splines.25,26 Tests for nonlinearity used the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline term.
The relationship between vitamin D levels and clinical signs and symptoms including ulcers in the mouth and throat, painful tongue or mouth, difficult or painful swallowing, nausea and vomiting, diarrhea, dysentery, fatigue, and rash was analyzed using generalized estimating equations and SAS Proc Genmod software.27
We adjusted for all known and suspected confounders of the relationship of baseline vitamin D levels with the outcomes.28 These included age, CD4 cell count at baseline, height, and education levels for the wasting outcomes and age, HIV disease stage, CD4 cell count, BMI, primiparity, anemia (Hb<7.0 g/dL), and erythrocyte sedimentation rate (>81 mm/h) at baseline, and multivitamin regime received for the comorbidities. Observations with missing data for covariates were retained in the analysis using the missing indicator method for variables missing greater than 1% of the observations.29 Statistical analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC).
Results
The baseline characteristics of the 884 women participating in the trial of vitamin supplementation, who had information on vitamin D status at enrolment, are presented in Table 1. There were no major differences in sociodemographic characteristics such as age, WHO HIV disease stage, CD4 T-cell counts, BMI, or hemoglobin concentrations between women with insufficient levels of vitamin D and women with adequate vitamin D status. The mean (standard deviation) serum 25-hydroxyvitamin D concentration in women classified as having insufficient vitamin D was 24.2 (6.2) ng/mL and in women with adequate vitamin D status was 43.1 (9.4) ng/mL.
Table 1.
Baseline Characteristics of Women Enrolled in the Trial with Available Vitamin D Levels (n=884)
| |
|
Low vitamin D (n = 347) |
Adequate vitamin D (n = 537) |
|---|---|---|---|
| Variable | Mean ± SD or frequency (%) | Mean ± SD or frequency (%) | |
| Age | |||
| Less than 20 years | 45 (12.97) | 71 (13.22) | |
| 20–24 years | 144 (41.50) | 213 (39.66) | |
| 25–29 years | 105 (30.26) | 166 (30.91) | |
| Greater than or equal to 30 years | 53 (15.27) | 87 (16.20) | |
| WHO HIV stage | |||
| I | 278 (80.12) | 457 (85.10) | |
| II | 60 (17.29) | 75 (13.97) | |
| III | 9 (2.59) | 5 (0.93) | |
| CD4 category, cells/μL | 413.45±202.32 | 428.65±210.14 | |
| Body Mass Index (BMI), kg/m2 | 23.01±3.20 | 23.39±3.20 | |
| Height, cm | 156.48±5.86 | 156.86±5.94 | |
| Mid–upper arm circumference (MUAC), cm | 25.37±2.85 | 25.67±2.85 | |
| Hemoglobin at baseline, g/L | 93.50±17.29 | 95.25±16.70 | |
| Vitamin D [Serum 25(OH)D] at baseline, ng/mL | 24.22±6.16 | 43.07±9.40 | |
SD, standard deviation.
In the results from the analysis examining the relationship between vitamin D status and wasting outcomes during a median of 70 months of follow-up, a total of 222 women experienced an episode of wasting as defined by BMI less than 18 kg/m2 during follow-up. More than 500 women experienced a weight loss of more than 10% during follow-up. There was no significant relationship between low vitamin D status and any of the wasting outcomes, when the complete duration of follow-up was included in the analysis (data not shown).
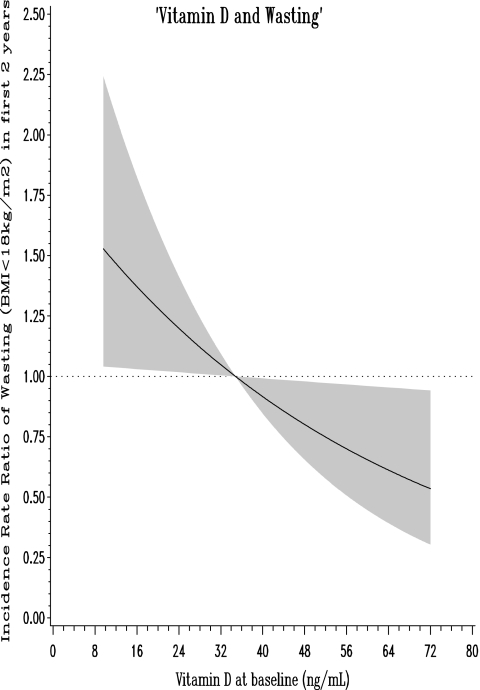
In Table 2, we restricted the analysis to the first 2 years of follow-up and observed that low vitamin D status was associated with a significantly increased risk of wasting defined as BMI less than 18 kg/m2 (hazard ratio [HR]: 1.43; 95% confidence intervals [CI]: 1.03, 1.99; p value: 0.03). Notably, 145 women experienced an episode of wasting in the first 2 years of follow-up, of the total 222 women who experienced such an event in the complete duration of follow-up. In analysis with continuous vitamin D levels using restricted cubic splines, we found that risk of wasting during the first 2 years of follow-up was inversely and linearly related to vitamin D concentrations; the higher the vitamin D levels, lower the risk of wasting defined as BMI less than 18 kg/m2 (Fig. 1; p: 0.03).
Table 2.
Wasting Outcomes and Low Vitamin D Status: First 2 Years of Follow-Up
| |
Low vitamin D |
Univariate |
Multivariatea |
|||
|---|---|---|---|---|---|---|
| Outcome | Yes n (N) | No n (N) | RR (95% CI) | p Valueb | RR (95% CI) | p Valueb |
| BMI<18 kg/m2 | 65 (330) | 80 (521) | 1.42 (1.02, 1.96) | 0.04 | 1.43 (1.03, 1.99) | 0.03 |
| MUAC<22 cm | 68 (313) | 108 (501) | 1.05 (0.78, 1.43) | 0.73 | 1.04 (0.77, 1.41) | 0.80 |
| Weight loss>10% | 165 (336) | 275 (527) | 1.02 (0.84, 1.24) | 0.84 | 1.02 (0.84, 1.24) | 0.82 |
All multivariate models adjusted for age, CD4 cell counts at baseline, height, and education levels.
p Values from Cox proportional hazards regression models.
Low vitamin D status was defined as serum 25-hydroxyvitamin D concentrations<32 ng/mL.
BMI, body mass index; MUAC, mid–upper arm circumference; RR, Incidence rate ratio.
FIG. 1.
Association of vitamin D status with wasting (body mass index [BMI]<18 kg/m2) in the first 2 years of follow-up. Adjusted for baseline age, CD4 T-cell count, height, and education levels.
There was no relationship between low vitamin D status and clinical signs and symptoms during follow-up (Table 3) except for acute upper respiratory tract infection (relative risk [RR]: 1.18; 95% CI: 1.03, 1.37; p value: 0.02). When we restricted the analysis to the first 2 years of follow-up (Table 4), we found that low vitamin D status was significantly associated with increased risks of thrush (RR: 2.74; 95% CI: 1.29, 5.83; p value: 0.01), in addition to acute upper respiratory tract infection.
Table 3.
Vitamin D and HIV-Related Complications Among Women During Follow-Up (n=884)
| Complication | Episodes mean (SD)a | Relative risk for low vitamin D (95% CI) | p Valueb | Adjusted relative risk for low vitamin D (95% CI)c | p Valueb |
|---|---|---|---|---|---|
| Thrush | 0.15 (0.50) | 0.99 (0.70, 1.41) | 0.96 | 0.94 (0.66, 1.34) | 0.74 |
| Acute upper respiratory tract infection | 0.85 (1.19) | 1.18 (1.02, 1.36) | 0.02 | 1.18 (1.03, 1.37) | 0.02 |
| Reported mouth and throat ulcers | 0.29 (0.98) | 1.15 (0.86, 1.56) | 0.34 | 1.05 (0.77, 1.44) | 0.74 |
| Painful tongue or mouth | 0.32 (1.03) | 1.07 (0.80, 1.43) | 0.66 | 0.97 (0.71, 1.32) | 0.83 |
| Difficult or painful swallowing | 0.16 (0.56) | 1.06 (0.77, 1.46) | 0.74 | 1.04 (0.74, 1.47) | 0.83 |
| Nausea and vomiting | 0.36 (1.03) | 1.06 (0.82, 1.36) | 0.66 | 1.03 (0.79, 1.34) | 0.83 |
| Diarrhea | 0.54 (1.20) | 0.92 (0.73, 1.16) | 0.50 | 0.91 (0.72, 1.14) | 0.40 |
| Dysentery | 0.18 (0.63) | 0.77 (0.56, 1.05) | 0.10 | 0.77 (0.57, 1.03) | 0.08 |
| Fatigue | 0.60 (1.42) | 1.26 (1.00, 1.58) | 0.05 | 1.15 (0.92, 1.45) | 0.21 |
| Rash | 0.98 (1.77) | 1.09 (0.88, 1.34) | 0.43 | 1.04 (0.84, 1.28) | 0.73 |
Mean number of episodes per year per woman (the average number of episodes per visit*12). Low vitamin D status was defined as serum 25-hydroxyvitamin D concentrations<32 ng/mL.
p values from Generalized Estimating Equations using Proc Genmod in SAS.
All multivariate models adjusted for age, HIV disease stage, CD4 cell count, BMI, primiparity, anemia (Hb<7.0 g/dL), and erythrocyte sedimentation rate (>81 mm/h) at baseline, and multivitamin regime received.
SD, standard deviation; CI, confidence interval; BMI, body mass index.
Table 4.
Vitamin D and HIV-Related Complications Among Women During First 2 Years of Follow-Up (n=884)
| Complication | Episodes mean (SD)a | Relative risk for low vitamin D (95% CI) | p Valueb | Adjusted relative risk for low vitamin D (95% CI)c | p Valueb |
|---|---|---|---|---|---|
| Thrush | 0.06 (0.40) | 2.67 (1.31, 5.44) | 0.01 | 2.74 (1.29, 5.83) | 0.01 |
| Acute upper respiratory tract infection | 0.72 (1.30) | 1.34 (1.10, 1.62) | <0.01 | 1.27 (1.04, 1.54) | 0.02 |
| Reported mouth and throat ulcers | 0.30 (1.15) | 1.54 (1.06, 2.23) | 0.02 | 1.44 (0.98, 2.14) | 0.07 |
| Painful tongue or mouth | 0.36 (1.20) | 1.35 (0.96, 1.90) | 0.08 | 1.22 (0.85, 1.73) | 0.28 |
| Difficult or painful swallowing | 0.16 (0.62) | 1.15 (0.75, 1.77) | 0.51 | 1.11 (0.71, 1.75) | 0.64 |
| Nausea and vomiting | 0.44 (1.19) | 1.05 (0.79, 1.39) | 0.75 | 1.01 (0.75, 1.36) | 0.95 |
| Diarrhea | 0.67 (1.37) | 0.98 (0.77, 1.26) | 0.88 | 0.95 (0.74, 1.21) | 0.66 |
| Dysentery | 0.21 (0.74) | 0.88 (0.60, 1.28) | 0.50 | 0.84 (0.59, 1.18) | 0.31 |
| Fatigue | 0.72 (1.66) | 1.35 (1.05, 1.75) | 0.02 | 1.20 (0.92, 1.56) | 0.17 |
| Rash | 1.14 (2.05) | 1.17 (0.93, 1.48) | 0.18 | 1.08 (0.85, 1.37) | 0.53 |
Mean number of episodes per year per woman (the average number of episodes per visit*12). Low vitamin D status was defined as serum 25-hydroxyvitamin D concentrations<32 ng/mL.
p-values from Generalized Estimating Equations using Proc Genmod in SAS.
All multivariate models adjusted for age, HIV disease stage, CD4 cell count, BMI, primiparity, anemia (Hb<7.0 g/dL), and erythrocyte sedimentation rate (>81 mm/h) at baseline, and multivitamin regime received.
SD, standard deviation; CI, confidence interval; BMI, body mass index.
Discussion
While there are an increasing number of studies being published about HIV-infected patients having inadequate concentrations of vitamin D,30–36 there is limited literature on the association of low vitamin D status with long-term clinical outcomes. In previous work in the same cohort, we have shown that low vitamin D levels are associated with increased risk of HIV disease progression, anemia, and mortality.13 In this study, we observed that low vitamin D status is associated with increased risk of HIV-related complications including wasting (BMI<18 kg/m2), thrush, and acute upper respiratory tract infections through the first 2 years of follow-up.
Wasting is a hallmark of HIV disease in adults22,23 and is associated with adverse HIV-related health outcomes and survival.37 For example, in a study in the Gambia, a BMI of less than 18 kg/m2 was associated with a more than twofold increase in risk of mortality.20 BMI and changes in weight also determine the clinical stage of HIV disease and affect timing and initiation of antiretroviral therapy.38–41 The etiology of wasting is complex and includes the increased secretion of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interferon-gamma (INF-γ), and interleukins (IL) 1 and 6.42,43 Both TNF-α and INF-γ are known to inhibit myosin expression in muscle cells,44 and TNF-α also induces anorexia.45 A potential explanation of the observed association between vitamin D and wasting may be the known anti-inflammatory role of Vitamin D that includes decreasing the levels of TNF-α.46–52
Vitamin D is also a known immunomodulator and is extensively involved in both innate and adaptive immunity.1 Vitamin D is needed for induction of cathelicidin, an antimicrobial peptide, which is responsible for intracellular killing of pathogens such as Mycobacterium tuberculosis.5 Vitamin D also induces autophagy in infected macrophages.53 These effects on the immune system may explain the relationship of low vitamin D levels with increased risk of thrush and acute upper respiratory tract infections observed in this study.
In summary, in addition to decreasing the risk of HIV disease progression, anemia, and mortality, vitamin D levels are associated with decreased incidence of HIV-related complications such as wasting and opportunistic illnesses. The findings of our study need to be confirmed in the setting of a randomized controlled trial; if found to be effective, vitamin D supplementation may be a potential adjunct treatment to antiretroviral therapy to ameliorate HIV-related complications and improve the quality of life of HIV-infected patients.
Acknowledgments
We thank the mothers and children, and field teams, including physicians, nurses, midwives, supervisors, laboratory staff, and the administrative staff, who made the study possible; and Muhimbili Medical Centre, Muhimbili University College of Health Sciences, and the National AIDS Control Program in Dar es Salaam for their institutional support.
S.M. analyzed the data and wrote the initial draft of the manuscript; D.S., E.L.G., E.V., J.L.F., and W.W.F. helped develop the data analysis plan and interpret the data; W.W.F., G.I.M., F.M.M., and D.S. were investigators of the trial on which this study is based and contributed to study design and data collection; E.H. and J.L.F. helped with the data analysis; all authors contributed to final manuscript preparation.
Supported by the National Institute of Child Health and Human Development (grant R01 32257), the Fogarty International Center (National Institutes of Health grant D43 TW00004), and the Harvard School of Public Health.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adams JS. Liu PT. Chun R. Modlin RL. Hewison M. Vitamin D in defense of the human immune response. Ann NY Acad Sci. 2007;1117:94–105. doi: 10.1196/annals.1402.036. [DOI] [PubMed] [Google Scholar]
- 2.deLuca HF. Metabolism and molecular mechanism of action of vitamin D: 1981. Biochem Soc Trans. 1982;10:147–158. doi: 10.1042/bst0100147. [DOI] [PubMed] [Google Scholar]
- 3.Stroder J. Immunity In Vitamin D Deficient Rickets. Vitamin D And Problems Of Uremic Bone Disease. Berlin: de Gruyter; 1975. pp. 675–687. [Google Scholar]
- 4.Liu PT. Stenger S. Tang DH. Modlin RL. Cutting edge: Vvitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J Immunol. 2007;179:2060–2063. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- 5.Liu PT. Stenger S. Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 6.Bischoff-Ferrari HA. Vitamin D and fracture prevention. Endocrinol Metab Clin North Am. 2010;39:347–353. doi: 10.1016/j.ecl.2010.02.009. table of contents. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff-Ferrari HA. Shao A. Dawson-Hughes B. Hathcock J. Giovannucci E. Willett WC. Benefit-risk assessment of vitamin D supplementation. Osteoporos Int. 2010;21:1121–1132. doi: 10.1007/s00198-009-1119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souberbielle JC. Body JJ. Lappe JM, et al. Vitamin D and musculoskeletal health, cardiovascular disease, autoimmunity and cancer: Recommendations for clinical practice. Autoimmun Rev. 2010;9:709–715. doi: 10.1016/j.autrev.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Wang L. Manson JE. Song Y. Sesso HD. Systematic review: Vitamin D and calcium supplementation in prevention of cardiovascular events. Ann Intern Med. 2010;152:315–323. doi: 10.7326/0003-4819-152-5-201003020-00010. [DOI] [PubMed] [Google Scholar]
- 10.Li YC. Qiao G. Uskokovic M. Xiang W. Zheng W. Kong J. Vitamin D: A negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Parker J. Hashmi O. Dutton D, et al. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas. 2010;65:225–236. doi: 10.1016/j.maturitas.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Fawzi WW. Msamanga GI. Spiegelman D, et al. A randomized trial of multivitamin supplements and HIV disease progression and mortality. N Engl J Med. 2004;351:23–32. doi: 10.1056/NEJMoa040541. [DOI] [PubMed] [Google Scholar]
- 13.Mehta S. Giovannucci E. Mugusi FM, et al. Vitamin D status of HIV-infected women and its association with HIV disease progression, anemia, and mortality. PLoS ONE. 2010;5:e8770. doi: 10.1371/journal.pone.0008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fawzi WW. Msamanga GI. Spiegelman D. Urassa EJ. Hunter DJ. Rationale and design of the Tanzania Vitamin and HIV Infection Trial. Control Clin Trials. 1999;20:75–90. doi: 10.1016/s0197-2456(98)00045-2. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Proposed ‘World Health Organization staging system for HIV infection and disease’: Preliminary testing by an international collaborative cross-sectional study. The WHO International Collaborating Group for the Study of the WHO Staging System. AIDS. 1993;7:711–718. [PubMed] [Google Scholar]
- 16.Urassa W. Matunda S. Bredberg-Raden U. Mhalu F. Biberfeld G. Evaluation of the WHO human immunodeficiency virus (HIV) antibody testing strategy for the diagnosis of HIV infection. Clin Diagn Virol. 1994;2:1–6. doi: 10.1016/0928-0197(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 17.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 18.Bodnar LM. Simhan HN. Powers RW. Frank MP. Cooperstein E. Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villamor E. Saathoff E. Manji K. Msamanga G. Hunter DJ. Fawzi WW. Vitamin supplements, socioeconomic status, and morbidity events as predictors of wasting in HIV-infected women from Tanzania. Am J Clin Nutr. 2005;82:857–865. doi: 10.1093/ajcn/82.4.857. [DOI] [PubMed] [Google Scholar]
- 20.van der Sande MA. Schim van der Loeff MF. Aveika AA, et al. Body mass index at time of HIV diagnosis: A strong and independent predictor of survival. J Acquir Immune Defic Syndr. 2004;37:1288–1294. doi: 10.1097/01.qai.0000122708.59121.03. [DOI] [PubMed] [Google Scholar]
- 21.James WP. Mascie-Taylor GC. Norgan NG. Bistrian BR. Shetty PS. Ferro-Luzzi A. The value of arm circumference measurements in assessing chronic energy deficiency in Third World adults. Eur J Clin Nutr. 1994;48:883–894. [PubMed] [Google Scholar]
- 22.Wanke C. Kotler D. Collaborative recommendations: The approach to diagnosis and treatment of HIV wasting. J Acquir Immune Defic Syndr. 2004;37:S284–S288. doi: 10.1097/01.qai.0000144384.55091.0f. [DOI] [PubMed] [Google Scholar]
- 23.Polsky B. Kotler D. Steinhart C. HIV-associated wasting in the HAART era: Guidelines for assessment, diagnosis, and treatment. AIDS Patient Care STDs. 2001;15:411–423. doi: 10.1089/108729101316914412. [DOI] [PubMed] [Google Scholar]
- 24.Cox D. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 25.Govindarajulu US. Spiegelman D. Thurston SW. Ganguli B. Eisen EA. Comparing smoothing techniques in Cox models for exposure-response relationships. Stat Med. 2007;26:3735–3752. doi: 10.1002/sim.2848. [DOI] [PubMed] [Google Scholar]
- 26.Durrleman S. Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 27.Diggle P, editor; Liang KY, editor; Zeger SL, editor. Analysis of Longitudinal Data. Oxford, England: Oxford University Press; 1995. [Google Scholar]
- 28.Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miettinen O. Theoretical Epidemiology. Vol. 107. New York: John Wiley & Sons; 1985. [Google Scholar]
- 30.Mueller NJ. Fux CA. Ledergerber B, et al. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- 31.Bang UC. Shakar SA. Hitz MF, et al. Deficiency of 25-hydroxyvitamin D in male HIV-positive patients: A descriptive cross-sectional study. Scand J Infect Dis. 2010;42:306–310. doi: 10.3109/00365540903463981. [DOI] [PubMed] [Google Scholar]
- 32.Overton ET. Yin MT. The rapidly evolving research on vitamin D among HIV-infected populations. Curr Infect Dis Rep. 2011;13:83–93. doi: 10.1007/s11908-010-0144-x. [DOI] [PubMed] [Google Scholar]
- 33.Wasserman P. Rubin DS. Highly prevalent vitamin D deficiency and insufficiency in an urban cohort of HIV-infected men under care. AIDS Patient Care STDs. 2010;24:223–227. doi: 10.1089/apc.2009.0241. [DOI] [PubMed] [Google Scholar]
- 34.Dao CN. Patel P. Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 35.Stein EM. Yin MT. McMahon DJ, et al. Vitamin D deficiency in HIV-infected postmenopausal Hispanic and African-American women. Osteoporos Int. 2011;22:477–487. doi: 10.1007/s00198-010-1299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adeyemi OM. Agniel D. French AL, et al. Vitamin D deficiency in HIV-infected and un-infected women in the US. J Acquir Immune Defic Syndr. doi: 10.1097/QAI.0b013e31821ae418. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkelstein JL. Mugusi FM. Mehta S. Fawzi WW. HIV/AIDS and nutrition in the era of antiretroviral therapy: Programmatic implications for care and treatment. In: Marlink RG, editor; Teitelman SJ, editor. From the Ground Up: Building Comprehensive HIV/AIDS Care Programs in Resource-Limited Settings. Vol. 2. Washington, D.C.: Elizabeth Glaser Pediatric AIDS Foundation; 2009. pp. 193–229. [Google Scholar]
- 38.Palenicek JP. Graham NM. He YD, et al. Weight loss prior to clinical AIDS as a predictor of survival. Multicenter AIDS Cohort Study Investigators. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:366–373. [PubMed] [Google Scholar]
- 39.Hsu JW. Pencharz PB. Macallan DC. Tomkins A. Macronutrients and HIV/AIDS: a review of current evidence. Durban, South Africa: World Health Organization; Apr 10–13, 2005. [Google Scholar]
- 40.Guenter P. Muurahainen N. Simons G, et al. Relationships among nutritional status, disease progression, and survival in HIV infection. J Acquir Immune Defic Syndr. 1993;6:1130–1138. [PubMed] [Google Scholar]
- 41.Chlebowski RT. Grosvenor MB. Bernhard NH. Morales LS. Bulcavage LM. Nutritional status, gastrointestinal dysfunction, and survival in patients with AIDS. Am J Gastroenterol. 1989;84:1288–1293. [PubMed] [Google Scholar]
- 42.Rimaniol AC. Zylberberg H. Zavala F. Viard JP. Inflammatory cytokines and inhibitors in HIV infection: Correlation between interleukin-1 receptor antagonist and weight loss. AIDS. 1996;10:1349–1356. doi: 10.1097/00002030-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Godfried MH. van der Poll T. Jansen J, et al. Soluble receptors for tumour necrosis factor: A putative marker of disease progression in HIV infection. AIDS. 1993;7:33–36. [PubMed] [Google Scholar]
- 44.Acharyya S. Ladner KJ. Nelsen LL, et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest. 2004;114:370–378. doi: 10.1172/JCI20174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tracey KJ. Beutler B. Lowry SF, et al. Shock and tissue injury induced by recombinant human cachectin. Science. 1986;234:470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z. Yuan W. Sun L, et al. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- 47.Teegarden D. Donkin SS. Vitamin D: Emerging new roles in insulin sensitivity. Nutr Res Rev. 2009;22:82–92. doi: 10.1017/S0954422409389301. [DOI] [PubMed] [Google Scholar]
- 48.Sun J. Kong J. Duan Y, et al. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–322. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 49.Laverny G. Penna G. Vetrano S, et al. Efficacy of a potent and safe vitamin D receptor agonist for the treatment of inflammatory bowel disease. Immunol Lett. 2010;131:49–58. doi: 10.1016/j.imlet.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Cohen-Lahav M. Shany S. Tobvin D. Chaimovitz C. Douvdevani A. Vitamin D decreases NFkappaB activity by increasing IkappaBalpha levels. Nephrol Dial Transplant. 2006;21:889–897. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 51.Cohen-Lahav M. Douvdevani A. Chaimovitz C. Shany S. The anti-inflammatory activity of 1,25-dihydroxyvitamin D3 in macrophages. J Steroid Biochem Mol Biol. 2007;103:558–562. doi: 10.1016/j.jsbmb.2006.12.093. [DOI] [PubMed] [Google Scholar]
- 52.Adams LS. Teegarden D. 1,25-dihydroxycholecalciferol inhibits apoptosis in C3H10T1/2 murine fibroblast cells through activation of nuclear factor kappaB. J Nutr. 2004;134:2948–2952. doi: 10.1093/jn/134.11.2948. [DOI] [PubMed] [Google Scholar]
- 53.Spector SA. Vitamin D earns more than a passing grade. J Infect Dis. 2009;200:1015–1017. doi: 10.1086/605723. [DOI] [PubMed] [Google Scholar]