Abstract
Background and Objectives:
To evaluate the long-term prognostic value of the combination of the EORTC risk calculator and proapoptotic, antiapoptotic, proliferation, and invasiveness molecular markers in predicting the outcome of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) treated with intravesical Bacille Calmette-Guérin (BCG) therapy.
Materials and Methods:
This study included 42 patients accrued prospectively presenting with intermediate- to high-risk NMIBC (high-grade T1 tumors or multiple rapidly recurrent tumors refractory to intravesical chemotherapy) treated with transurethral resection (TUR) and BCG. TUR samples were analyzed for the molecular markers p53, p21 waf1/cip, Bcl-2, CyclinD1, and metallothionein 9 (MMP9) using immunohistochemistry. Frequency of positivity, measured as a percentage, was assessed alone or in combination with EORTC risk calculator, for interaction with outcome in terms of recurrence and progression using univariate analysis and Kaplan-Meier survival curves.
Results:
Median follow-up was 88 months (mean, 99; range, 14-212 months). The overall recurrence rate was 61.9% and progression rate was 21.4%. In univariate analysis, CyclinD1 and EORTC risk groups were significantly associated with recurrence (P value 0.03 and 0.02, respectively), although none of the markers showed a correlation to progression. In combining EORTC risk groups to markers expression status, high-risk group associated with positive MMP9, Bcl-2, CyclinD1, or p21 was significantly correlated to tumor recurrence (log rank P values <0.001, 0.03, 0.02, and 0.006, respectively) and when associated with positive MMP9 or p21, it was significantly correlated to progression (log rank P values 0.01 and 0.04, respectively).
Conclusion:
Molecular markers have a long-term prognostic value when combined with EORTC scoring system and they may be used to improve the predictive accuracy of currently existing scoring system. Larger series are needed to confirm these findings.
Keywords: BCG, Bcl-2, bladder cancer, cyclinD1, EORTC, high risk, long-term response, metallothionein 9, p53, p21, waf1/cip
INTRODUCTION
Intravesical immunotherapy with Bacille Calmette-Guérin (BCG) plays a major role in the treatment and prophylaxis of non-muscle-invasive bladder cancer (NMIBC). However, predicting which patients are destined to fail BCG is a difficult task. Some patients managed conservatively may not be salvaged with additional treatment when progression is identified, so that early identification of patients at risk of progression is crucial.[1–6] A growing body of evidence suggests that BCG works as an immunotherapy. A strong pattern of immune response has been suggested to be associated with an effective clinical response after BCG therapy.[7–10] In addition to BCG effects on the immune system, the inherent characteristics of the tumor are also likely to have an influence on the effectiveness of BCG therapy. The clinical usefulness of biomarkers in bladder cancer in addition to clinical parameters has been controversial given the discrepancy between various studies. Very few studies have analyzed the ability of biomarkers to predict long-term clinical outcome after BCG.[2] One of the reasons for the limited usefulness of biomarkers in bladder cancer prognostication is that failure to detect mutations or aberrant expression at one point of a biological pathway does not necessarily rule out defects further downstream as this was demonstrated for p53, for instance.[11] Multiple biomarkers determination rather than single markers are possibly more useful because they investigate various rather than one biological pathway.[12] Fundamentally, tumorigenicity develops from a combined loss of tumor suppressor genes and the disproportionate expression of oncogenes.[13] In urothelial cell carcinoma (UCC), deficiencies in the production or function of the tumor suppressor genes p53 and p21 are associated with a loss of apoptosis and are more frequently found in higher stage and grade lesions.[14,15] Alternatively, upregulation of the expression of various oncogene protein products such as Bcl-2, metallothionein 9 (MMP9), and cyclin D1 may also impair apoptotic pathways providing a cell survival advantage which may in turn relate to disease-specific survival.[16–20] The balance of interactions between pro- and antiapoptotic factors might be expected to determine the final biologic behavior of tumor cells. Indeed, the combination, among others, of Bcl-2 and p53 status has been used to predict disease progression.[21] Most of the studies using biomarkers in this setting have analyzed short or mid-term clinical response.
Another promising area is the use of molecular markers for improving the predictive accuracy of currently existing scoring systems such as that developed by the EORTC.[22]
In the present study, we sought to analyze the prognostic value of combining pro- and antiapoptotic factors as well as markers of proliferation and invasiveness in the prediction of the long-term response (mean follow-uP >8 years) to BCG for the treatment of NMIBC and whether they added prognostic significance to the EORTC scoring system.[23]
MATERIALS AND METHODS
Subjects
Patients with pathologically confirmed intermediate- or high-risk NMIBC were considered eligible for this trial. Patients were prospectively accrued, part of a study looking at the influence of immunological parameters on the clinical response to BCG therapy.[7,8] The study was performed after approval by the local Research Ethics Board Committee.
Intermediate- or high-risk NMIBC patients were defined according to the EORTC[23] as: Ta with two or more recurrences within the last year or T1 tumors; tumors larger than 3 cm with at least one recurrence within the last year; multiple synchronous Ta or T1, grade 3 tumors or presence of carcinoma in situ (CIS). A total of 42 consecutively accrued patients met these inclusion criteria.
Patients follow-up
Transurethral resection of the identified bladder tumors (TURBT) was followed by six weekly intravesical instillations of BCG (120 mg of Pasteur strain BCG in 50 ml sterile saline). Standard follow-up included cystoscopy and urinary cytology at 3 monthly intervals for 2 years, then 6 monthly intervals for 2 years, and yearly intervals thereafter and upper tract imaging was performed on an annual basis. Upper tract lesions were excluded with urography and/or washing cytology or ureteroscopy prior to TURBT at entry of the study. Evidence of recurrence in the form of noninvasive tumors, CIS, or positive cytology led to re-resection as indicated and a further 6-week course of BCG. Progression to muscle invasive disease or refractory CIS led to the recommendation of radical cystectomy for definitive treatment.
Immunohistochemical evaluation
TURBT specimens were embedded in paraffin and sectioned at 4 μm intervals. The tissue was then deparaffinized, hydrated, and washed for five minutes in phosphate-buffered saline. The sections were placed in 0.01% citrate buffer and heated for 5 minutes on three occasions in a 500W microwave oven. Specimens were then rinsed in Tris-buffered saline and cooled for 20 minutes. Incubation in 3% hydrogen peroxide in methanol was undertaken to prevent the actions of endogenous peroxidase. There was a further overnight incubation at 4°C with primary multiclonal antibodies. These included p53-DO7 diluted 1:100 in phosphate-buffered solution (Dakopatts, High Wycombe, U.K), p21: WAF1-(Ab-1)/EA10 diluted 1: 25 in phosphate-buffered solution (Oncogene Science, Inc, Cambridge, Massachusetts), Bcl-2:124 (Dako, Glosstrup, Denmark), cyclin D1: P2D11F11, and MMP9: E9 (Abcam, Cambridge Science Park).
After washing with phosphate-buffered saline, the specimens were incubated for 20 minutes with biotinylated anti-mouse antibodies diluted 1: 100 (Van Ramm, MO, USA), then horseradish peroxidase-labeled streptavidin diluted 1: 100 for 20 minutes (Supersensitive concentrated multilink - HRP, Biogenex, San Ramon, CA, USA). Diaminobenzidine substrate solution was added as a chromogen. Sections were stained with Mayer's hematoxylin, dehydrated, and mounted.
Positive controls for the biomarkers analyzed were taken from known colonic and sebaceous skin gland adenocarcinomata respectively. Negative controls were generated by adding distilled water as a substitute for the primary antibodies. One pathologist reviewed all cases for grade and stage (SR).
Immunohistochemistry slides were reviewed by two blinded, independent pathologists. At least 1 000 nuclei were viewed in 10 representative fields viewed at 400× magnification to give an indication of the degree of staining. The exact percentage of tumor cells with positive staining defined as nuclear immunoreactivity was determined. Membranous staining in a manner similar to urothelial cells was considered normal. Cutoffs for positivity were set at 10% for both p53 and p21 and for the other markers.
Data analysis
Correlation between the tumors’ pathological features (stage, grade, multifocality, and size) and mean percentage of markers’ expression was performed using analysis of variance (ANOVA).
Patients were stratified according to the EORTC scoring system[7] into either intermediate or high risk.
The outcome in terms of progression and recurrence was analyzed in correlation with the molecular marker expression and the EORTC risk group using univariate analysis and Kaplan-Meier survival curves. Log Rank test was used to compare factors; all P values of <0.05 were considered to be statistically significant.
Furthermore, patients were stratified into two groups according to their EORTC risk status and molecular markers (i.e., one group with high risk and positive marker vs another group with intermediate risk and any marker status). The outcome in terms of progression-free survival (PFS) and recurrence-free survival (RFS) was then compared between these two groups using Kaplan-Meier survival curves.
Due to the small sample size, we did not perform multivariate analysis. Statistical analyses were performed using SPSS software, version 16 (Chicago, IL, USA).
RESULTS
Patient and tumor characteristics
There were 42 patients (38 men and 4 women). Mean age was 68.3 years (median, 70.5; range, 50-78). Patients were followed for a mean of 99 months (median, 88; range, 14-212). The patient's clinical and pathological features are summarized in Table 1. In this intermediate- and high-risk group of patients, 16 (38%) patients achieved complete response following BCG. The overall recurrence rate was 61.9% with a median time to recurrence of 26 months (mean, 61 months; range, 3 to 181) and the overall progression rate was 21.4% (9 patients) of which eight patients (19%) had disease that progressed to muscle-invasion and required cystectomy.
Table 1.
General demographics

Relationship of markers to tumor characteristics
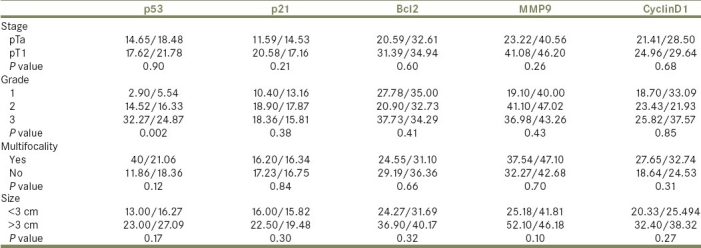
In univariate analysis of the relationship between tumor stage, grade, multifocality, and size with the mean percentage of expression of each molecular marker [Table 2], the only statistically significant relationship was found between tumor grade and p53 expression (P =0.002).
Table 2.
Markers’ mean percentage/standard deviation in relation to tumor pathological features: stage, grade, multifocality, and size
Relationship to recurrence
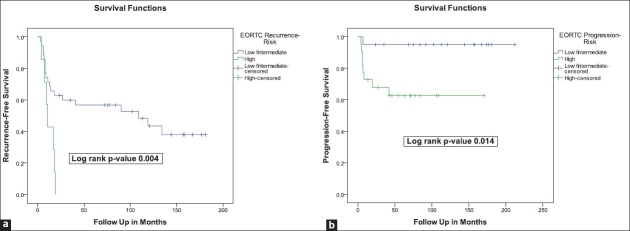
In univariate analysis [Table 3], CyclinD1 was the only molecular marker that showed a statistically significant correlation to recurrence rates (45.0% vs 77.3% for negative expression vs positive expression, respectively, P value 0.03). The EORTC risk stratification was strongly associated with recurrence rates (54.3% for intermediate-risk vs 100% for high-risk, P value 0.02) [Table 3] and RFS (log rank P value 0.004) [Figure 1a].
Table 3.
Overall recurrence and progression rates in relation to EORTC risk-stratification and different molecular markers
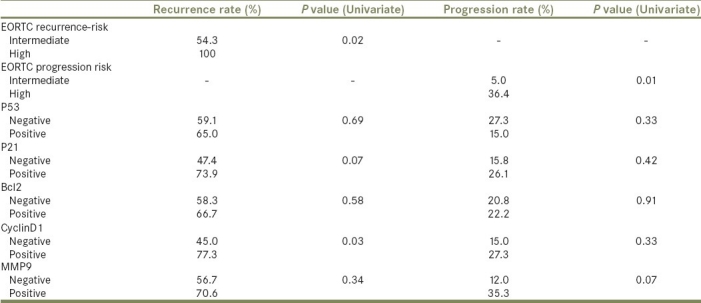
Figure 1.
Kaplan-Meier survival curves for (a) recurrence- and (b) progression-free survivals in relation to EORTC risk-stratification
When RFS rates were compared between patients with EORTC high risk and positive marker vs those with intermediate risk, there was a statistically significant differences between the two groups with a worse RFS for those with high risk and positive expression of MMP9, Bcl2, CyclinD1, and p21 (log rank P values <0.001, 0.03, 0.02, and 0.006, respectively).
Relationship to progression
In univariate analysis, none of the markers showed a statistically significant correlation with progression rates [Table 3]. On the contrary, the EORTC risk stratification was strongly associated with progression rates (5.0% for intermediate-risk vs 36.4% for high-risk, Pvalue 0.01) [Table 3] and PFS (log rank P value 0.014) [Figure 1b].
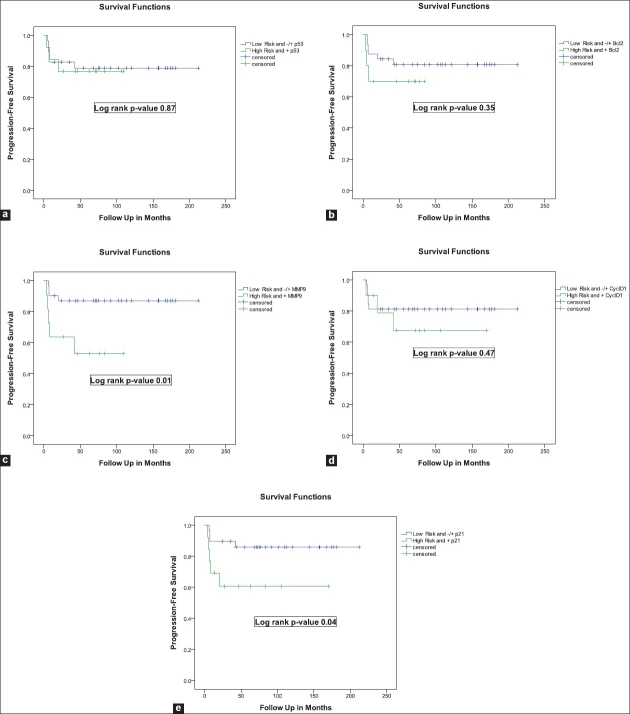
When PFS rates were compared between patients with EORTC high-risk and positive marker vs those with intermediate-risk, there was a statistically significant differences between the two groups with a worse PFS for those with high-risk and positive expression of MMP9 and p21 (log rank P values 0.01 and 0.04, respectively) [Figure 2].
Figure 2.
Kaplan-Meier curves for progression-free survivals in relation to a combination of EORTC risk-stratification and molecular marker status (Low-Risk and -/+ Marker vs High-Risk and + Marker)
Combination of different markers and outcome
Combining the different markers did not add any prognostic significance to RFS or to PFS, with the exception that when the expression of all five markers were positive, RFS was significantly worse than if with positive expression of four or less markers in combination (Log Rank P value <0.0001, data not shown).
DISCUSSION
Extensive scientific endeavor notwithstanding, the accumulation of knowledge regarding genetic makeup of bladder cancer has largely failed to impinge on the consciousness of the clinician.[24] Given the redundancy in biological pathways, there is an obvious need to investigate multiple steps of a pathway before determining if the pathway may or may not be involved in cancer progression. In order to prophesy tumor behavior effectively, the most plausible approach might involve relating the combination of multiple genetic markers to eventual outcome. Although the current “holy grail” of management of NMIBC is the ability to accurately forecast when a tumor acquires the ability to invade muscle and metastasize beyond the bladder, this is difficult to define.
In this study, we attempted to concentrate on the G1/S checkpoint and specifically on the role of known pro-, antiapoptotic, and proliferative gene products to see whether they predicted disease response to BCG. We also investigated markers of cancer invasiveness to address the issue of progression to muscle-invasive disease. Our study of a cohort of patients with intermediate- to high-risk NMIBC treated with BCG revealed a 61.9% recurrence and a 19% progression to cystectomy rate over a long-term mean follow-up of 99 months. The well-established EORTC risk stratification was a strong predictor for both recurrence and progression in our series. Moreover, an interesting finding of the current study is that the positive expression of MMP9, Bcl2, CyclinD1, and p21 showed a strong prediction of RFS in the EORTC high-risk group. And, positive expression of MMP9 and p21 strongly correlated with PFS in the EORTC high-risk group as well. The use of molecular markers in improving the predictive accuracy of a scoring system such as EORTC risk calculator is a promising area.[22] Our findings support this concept, although they require further confirmation in larger cohorts.
p53 was the sole biomarker related to grade. This is not an unexpected finding since high proliferation index and p53 accumulation occurs frequently in high-grade NMIBC.[13,25] p53 expression did not predict outcome in our cohort. This only adds to the conflicting discrepancy in the literature regarding its prognostic value in bladder cancer.[25]
We did not find a correlation between the expression of p21 and outcome; p21 is a cyclin kinase inhibitor that promotes cell cycle arrest in response to many stimuli. Control of p21 expression is complex and can be mediated by p53-independent pathways.[11,26] There is controversy in the literature regarding the predictive ability of p21 in bladder cancer.[14–16,18] Reports have outlined the central role for p21 in mediating the response of the tumor to BCG therapy as p21 was found to be necessary for BCG cytotoxic effects.[27] There is a growing body of evidence suggesting that functional loss of p21 can mediate a drug-resistance phenotype.[28]
Our study also tested the expression of Cyclin D1 as a marker of outcome in NMIBC. Cyclin D family form complexes with cyclin-dependent kinase 4 and 6, which promote phosphorylation and inactivation of the retinoblastoma protein, thus mediating the progression of cells from G1- to S-phase transition.[29–31] We found that Cyclin D1 was a predictor of recurrence, which supports the role of Cyclin D family members in bladder cancer.[30,31]
The metallothionein family has been associated with poor prognosis and shown to predict disease-free survival for patients undergoing radical cystectomy.[19] However, no significant association between MMP9 and outcome was found in the current study, possibly because of the small sample size.
Bcl-2 is an antiapoptotic G1/S modulator, the action of which is restrained by p53 and BAX proteins; overexpression provides protection from apoptosis.[18] This may occur, at least in part, by excluding the p53 protein from the cell nucleus.[18] The role of Bcl-2 in UCC outcome is controversial.[32] In our study, Bcl 2 expression did not predict recurrence or progression.
The combination of multiple markers failed to show predictive value, except in the presence of positive expression of five markers together which was associated with a worse outcome in terms of recurrence. This is in contrast with the findings of other groups who found that combining more than two markers together improved their predictive ability in terms of recurrence and progression.[17,21] P53, p27, and Ki-67 were found to improve the prediction of recurrence-free and bladder-specific survival in patients with pT1 disease at radical cystectomy.[33] A possible explanation is that, especially in a small sample population, one strong biomarker may decrease the usefulness of the others. We do not rule out that by analyzing larger patients populations, combination of markers might prove more useful than isolated parameters.
It is evident from the controversies in this area that there is now an urgent need for much larger, multicenter studies to clarify the role of promising molecular markers in bladder cancer generally and in biomarkers for predicting response to treatments specifically.[34,35]
Our study has several limitations. First, the reliability of biomarker ascertainment from transurethral resection and immunochemical analysis is imperfect. Dependence on variables in specimen acquisition, handling, fixation, antibody choice, and concentration as well as the subjectivity of interpretative criteria all contribute to this inaccuracy.[36] Gene chip technology offers the prospect of improving on some of these factors and is likely to assume increasingly importance; however, immunochemistry retains some appeal due to its low cost, relative ease of administration, and the fact that it is readily available.
Second, the study is limited by its sample size and heterogeneity of patients. Given the previous discussion of the polygenetic nature of bladder cancer, it follows that large sample sizes are required to discriminate the predictive power of different biomarkers. This in combination with the need for lengthy follow-up to address disease outcomes has limited progress to date. Multivariate analysis was not preformed since it may lead to inconsistent results in low number of patients, as it is the case in our study.
Despite the sample size of the study, we believe that current study have two major strengths. First is the long follow-up. Second is the demonstration that molecular markers are able to predict response to BCG therapy when combined to an established prognostic score on the long run even in a limited patient population. And, that having established the value of combinations of molecular markers with EORTC risk groups as a proof of principle, further more effectively resourced study is now warranted.
CONCLUSION
Molecular markers provide additional long-term prognostic value when combined with EORTC scoring system in NMIBC. They may be used to improve the predictive accuracy of currently existing scoring system. Larger series are needed to confirm these findings including other pathways that represent interesting targets for biomarker development.
These markers could then be used to predict outcomes of conservative treatment and to select the most appropriate candidates for conservative or radical intervention.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodriguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000;163:73–8. doi: 10.1016/s0022-5347(05)67975-x. [DOI] [PubMed] [Google Scholar]
- 2.Soloway MS, Sofer M, Vaidya A. Contemporary management of stage T1 transitional cell carcinoma of the bladder. J Urol. 2002;167:1573–83. [PubMed] [Google Scholar]
- 3.Sylvester RJ, van der MA, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: A meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964–70. doi: 10.1016/S0022-5347(05)64273-5. [DOI] [PubMed] [Google Scholar]
- 4.Bohle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90–5. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 5.Shahin O, Thalmann GN, Rentsch C, Mazzucchelli L, Studer UE. A retrospective analysis of 153 patients treated with or without intravesical bacillus Calmette-Guerin for primary stage T1 grade 3 bladder cancer: Recurrence, progression and survival. J Urol. 2003;169:96–100. doi: 10.1016/S0022-5347(05)64044-X. [DOI] [PubMed] [Google Scholar]
- 6.Brake M, Loertzer H, Horsch R, Keller H. Recurrence and progression of stage T1, grade 3 transitional cell carcinoma of the bladder following intravesical immunotherapy with bacillus Calmette-Guerin. J Urol. 2000;163:1697–701. [PubMed] [Google Scholar]
- 7.Zlotta AR, Drowart A, Huygen K, De Bruyn J, Shekarsarai H, Decock M, et al. Humoral response against heat shock proteins and other mycobacterial antigens after intravesical treatment with bacille Calmette-Guerin (BCG) in patients with superficial bladder cancer. Clin Exp Immunol. 1997;109:157–65. doi: 10.1046/j.1365-2249.1997.4141313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zlotta AR, Drowart A, Van Vooren JP, de Cock M, Pirson M, Palfliet K, et al. Evolution and clinical significance of the T cell proliferative and cytokine response directed against the fibronectin binding antigen 85 complex of bacillus Calmette-Guerin during intravesical treatment of superficial bladder cancer. J Urol. 1997;157:492–8. [PubMed] [Google Scholar]
- 9.Thalmann GN, Sermier A, Rentsch C, Möhrle K, Cecchini MG, Studer UE. Urinary Interleukin-8 and 18 predict the response of superficial bladder cancer to intravesical therapy with bacillus Calmette-Guerin. J Urol. 2000;164:2129–33. [PubMed] [Google Scholar]
- 10.de Reijke TM, de Boer EC, Kurth KH, Schamhart DH. Urinary cytokines during intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer: Processing, stability and prognostic value. J Urol. 1996;155:477–82. [PubMed] [Google Scholar]
- 11.Zlotta AR, Noel JC, Fayt I, Drowart A, Van Vooren JP, Huygen K, et al. Correlation and prognostic significance of p53, p21WAF1/CIP1 and Ki-67 expression in patients with superficial bladder tumors treated with bacillus Calmette-Guerin intravesical therapy. J Urol. 1999;161:792–8. [PubMed] [Google Scholar]
- 12.Shariat SF, Zlotta AR, Ashfaq R, Sagalowsky AI, Lotan Y. Cooperative effect of cell-cycle regulators expression on bladder cancer development and biologic aggressiveness. Mod Pathol. 2007;20:445–59. doi: 10.1038/modpathol.3800757. [DOI] [PubMed] [Google Scholar]
- 13.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–23. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 14.Stein JP, Ginsberg DA, Grossfeld GD, Chatterjee SJ, Esrig D, Dickinson MG, et al. Effect of p21WAF1/CIP1 expression on tumor progression in bladder cancer. J Natl Cancer Inst. 1998;90:1072–9. doi: 10.1093/jnci/90.14.1072. [DOI] [PubMed] [Google Scholar]
- 15.Shariat SF, Tokunaga H, Zhou J, Kim J, Ayala GE, Benedict WF, et al. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol. 2004;22:1014–24. doi: 10.1200/JCO.2004.03.118. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee SJ, Datar R, Youssefzadeh D, George B, Goebell PJ, Stein JP, et al. Combined effects of p53, p21, and pRb expression in the progression of bladder transitional cell carcinoma. J Clin Oncol. 2004;22:1007–13. doi: 10.1200/JCO.2004.05.174. [DOI] [PubMed] [Google Scholar]
- 17.Karam JA, Lotan Y, Karakiewicz PI, Ashfaq R, Sagalowsky AI, Roehrborn CG, et al. Use of combined apoptosis biomarkers for prediction of bladder cancer recurrence and mortality after radical cystectomy. Lancet Oncol. 2007;8:128–36. doi: 10.1016/S1470-2045(07)70002-5. [DOI] [PubMed] [Google Scholar]
- 18.Pfister C, Flaman JM, Dunet F, Grise P, Frebourg T. p53 mutations in bladder tumors inactivate the transactivation of the p21 and Bax genes, and have a predictive value for the clinical outcome after bacillus Calmette-Guerin therapy. J Urol. 1999;162:69–73. doi: 10.1097/00005392-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Yamasaki Y, Smith C, Weisz D, van Huizen I, Xuan J, Moussa M, et al. Metallothionein expression as prognostic factor for transitional cell carcinoma of bladder. Urology. 2006;67:530–5. doi: 10.1016/j.urology.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Correlation of cyclin D1 and E1 expression with bladder cancer presence, invasion, progression, and metastasis. Hum Pathol. 2006;37:1568–76. doi: 10.1016/j.humpath.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Shariat SF, Ashfaq R, Sagalowsky AI, Lotan Y. Predictive value of cell cycle biomarkers in nonmuscle invasive bladder transitional cell carcinoma. J Urol. 2007;177:481–7. doi: 10.1016/j.juro.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 22.Sylvester RJ. Combining a molecular profile with a clinical and pathologicalprofile: biostatistical considerations. Scand J Urol Nephrol Suppl. 2008;218:185–90. doi: 10.1080/03008880802283847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466–5. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Alonso A, Pita-Fernandez S, Gonzalez-Carrero J, Nogueira-March JL. p53 and ki67 expression as prognostic factors for cancer-related survival in stage T1 transitional cell bladder carcinoma. Eur Urol. 2002;41:182–8. doi: 10.1016/s0302-2838(01)00038-0. [DOI] [PubMed] [Google Scholar]
- 25.Malats N, Bustos A, Nascimento CM, Fernandez F, Rivas M, Puente D, et al. P53 as a prognostic marker for bladder cancer: a meta-analysis and review. Lancet Oncol. 2005;6:678–86. doi: 10.1016/S1470-2045(05)70315-6. [DOI] [PubMed] [Google Scholar]
- 26.Michieli P, Chedid M, Lin D, Pierce JH, Mercer WE, Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994;54:3391–5. [PubMed] [Google Scholar]
- 27.Zhang G, Chen F, Cao Y, See WA, Milwaukee W. P21 expression by human urothelial carcinoma cells modulates the phenotypic response to BCG. J Urol. 2009;181:1155. doi: 10.1016/j.urolonc.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Abukhdeir AM, Park BH. P21 and p27: Roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19. doi: 10.1017/S1462399408000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sgambato A, Migaldi M, Faraglia B, De Aloysio G, Ferrari P, Ardito R, et al. Cyclin D1 expression in papillary superficial bladder cancer: its association with other cell cycle-associated proteins, cell proliferation and clinical outcome. Int J Cancer. 2002;97:671–8. doi: 10.1002/ijc.10055. [DOI] [PubMed] [Google Scholar]
- 30.Wagner U, Suess K, Luginbuhl T, Schmid U, Ackermann D, Zellweger T, et al. Cyclin D1 overexpression lacks prognostic significance in superficial urinary bladder cancer. J Pathol. 1999;188:44–50. doi: 10.1002/(SICI)1096-9896(199905)188:1<44::AID-PATH320>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Beltran A, Luque RJ, Alvarez-Kindelan J, Quintero A, Merlo F, Carrasco JC, et al. Prognostic factors in stage T1 grade 3 bladder cancer survival: The role of G1-S modulators (p53, p21Waf1, p27 kip1, Cyclin D1, and Cyclin D3) and proliferation index (ki67-MIB1) Eur Urol. 2004;45:606–12. doi: 10.1016/j.eururo.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 32.Kong G, Shin KY, Oh YH, Lee JJ, Park HY, Woo YN, et al. Bcl-2 and p53 expressions in invasive bladder cancers. Acta Oncol. 1998;37:715–20. doi: 10.1080/028418698430098. [DOI] [PubMed] [Google Scholar]
- 33.Shariat SF, Bolenz C, Godoy G, Fradet Y, Ashfaq R, Karakiewicz PI, et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol. 2009;182:78–84. doi: 10.1016/j.juro.2009.02.125. [DOI] [PubMed] [Google Scholar]
- 34.Bryan RT, Zeegers MP, James ND, Wallace DM, Cheng KK. Biomarkers in bladder cancer. BJU Int. 2009;105:608–13. doi: 10.1111/j.1464-410X.2009.08880.x. [DOI] [PubMed] [Google Scholar]
- 35.Riley RD, Sauerbrei W, Altman DG. Prognostic markers in cancer: the evolution of evidence from single studies to meta-analysis, and beyond. Br J Cancer. 2009;100:1219–29. doi: 10.1038/sj.bjc.6604999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McShane LM, Aamodt R, Cordon-Cardo C, Cote R, Faraggi D, Fradet Y, et al. Reproducibility of p53 immunohistochemistry in bladder tumors. National Cancer Institute, Bladder Tumor Marker Network. Clin Cancer Res. 2000;6:1854–64. [PubMed] [Google Scholar]