Abstract
Background and Aim:
Renal artery stenosis (RAS) and acute renal failure may be due to the intimal hyperplasia and sympathetic fibers of the renal artery (RA), respectively. The purpose of this study was to characterize arterial wall and sympathetic innervation of the human RA.
Materials and Methods:
Fifty-two fresh human RA samples (proximal part) were collected from 26 cadavers (19 males and 7 females), between the ages of 19 and 83 years, during autopsy. Samples were divided into three age groups: Group 1, 19-40 years; Group 2, 41-60 years; Group 3, over 61 years. 5-μm thick sections of each sample were taken and stained with hematoxylin-eosin and Verhoeff-Van Gieson. Five out of 52 samples were processed for tyrosine hydroxylase (TH) immunostaining.
Results:
Our histological studies revealed that tunica media of RA showed smooth muscle cells and fine irregularly arranged elastic fibers. Intimal hyperplasia was the most common finding. The present study showed that thickness of tunica intima and media were found to increase with age. Sympathetic nerves were present in the tunica adventitia and outer media of the RA. The mean adventitial and sympathetic nerve fiber areas were found to be 0.595 and 0.071 mm2, respectively. Sympathetic index (SI) to RA was calculated by dividing the sympathetic fiber area by the adventitial area of the RA. SI of RA was found to be 0.140.
Conclusion:
We conclude that RA showed the structure of musculo-elastic artery. SI may be used for the analysis of sympathetic fiber related problems of the human RA or kidneys.
Keywords: Histology, intimal hyperplasia, stenosis, sympathetic nerve
INTRODUCTION
Renal artery stenosis (RAS) is the narrowing of the renal artery (RA), most often caused by atherosclerosis or intimal hyperplasia, and leads to reduced renal perfusion. RAS may cause hypertension, progressive renal failure and recurrent pulmonary edema.[1] Studies on RAs in human cadavers reported that pathological changes like intimal hyperplasia, atherosclerosis, calcification, ulceration, hyalinization and fibroelastic hyperplasia progress with age.[2–6] A few reports have been published on atherosclerosis in RA.[7–9] These studies are based on the clinico-radiological aspects of the disease. Histomorphometric studies have been conducted in two sexes of sheep at different ages and showed no significant differences in the thickness of tunica intima (Ti) and tunica media (Tm).[10] Morphometric analysis of renal arteries in the group of patients with renal cell carcinoma showed thicker tunica media when compared with the control group.[11] To the best of our knowledge and belief, there is lack of data in the available literature on histomorphometry about the thickness of Ti, Tm and Ti/Tm ratio in healthy human RAs and the changes in human RA related to age.
Renal sympathetic nervous system plays an important role in the development of the ischemic acute renal failure.[12,13] Renal sympathetic postganglionic nerves supply blood vessels, the juxtaglomerular apparatus, and tubules, and control renal blood flow, renin release, and sodium reabsorption.[14–17] The perivascular innervation of the RA has been studied by immunohistochemistry in golden hamster, rat, rabbit and guinea pig.[18–20] The intrinsic innervation of the human adult and fetal kidney has been documented.[21,22] In contrast, there is still a lack of detailed description of the sympathetic nerves of the main RA in humans. Hence, the purpose of this work was to study the characteristics of arterial wall and sympathetic innervations of the human RA.
MATERIALS AND METHODS
Sample collection
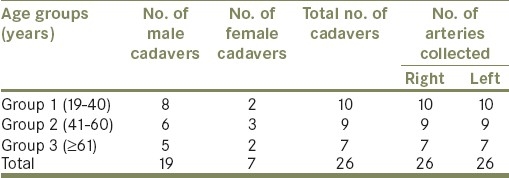
Fifty-two fresh human RA samples (proximal part) were collected from 26 cadavers (19 males and 7 females), between the ages of 19 and 83 years, during autopsy. Samples from the individuals who had died with vascular diseases, diabetes, hypertension and renal diseases were excluded from the present study. Distributions of RA samples are shown in Table 1.
Table 1.
Distribution of the human RA samples
Method of collection
A midline incision was made on the anterior abdominal wall of the cadaver, abdominal contents were pushed aside, and RAs arising from abdominal aorta were identified. About 1.5 cm length of RAs was cut close to the abdominal aorta.[5]
Tissue processing for histological methods
Samples were fixed with 4% paraformaldehyde for 24 hours and immediately processed for histological procedures. 5-μm thick sections were taken with a rotary microtome and mounted on gelatin coated slides. All the sections were stained with hematoxylin-eosin (H and E) and Verhoeff-Van Gieson (VVG).
Tissue processing for immunohistochemistry
Five out 52 paraformaldehyde-fixed samples were processed for tyrosine hydroxylase (TH) immunostaining. 5-μm thick sections were taken using Leitz cryostat at -20°C and collected onto the aminopropyl triethoxysilane (APES) coated slides.
TH immunostaining
Sections were washed in phosphate-buffered saline (PBS; 2 × 5 min), treated with peroxidase block for 30 min, and then washed in PBS (2 × 5 min). Following this, the sections were blocked with normal goat serum for 1 hour, followed by incubation in rabbit polyclonal anti-TH primary antibody (AB152, Millipore, CA, U.S.A) diluted to 1:100 in PBS for 48 hours at 4°C. Sections were washed in PBS (2 × 5 min), incubated in biotinylated goat anti-rabbit secondary antibody (sc2051, Santa Cruz, CA, U.S.A) for 2 hours followed by incubation in the horseradish peroxidase (HRP)-streptavidin (sc2051, Santa Cruz, CA, U.S.A) complex for 2 hours. Finally, color was developed by treating these sections with DAB (diaminobenzidine) (sc2051, Santa Cruz, CA, U.S.A) for 5 min. The sections were then washed with distilled water, counterstained with hematoxylin and dehydrated with two changes of alcohol, cleared in xylene and coverslipped.
Human adrenal glands were used as positive controls and processed as above at the same time. For the negative control, sections were incubated in normal goat serum replacing primary antibody.
Analyzed morphometric parameters
Stained sections were observed under binocular light microscope and digital images were obtained. Digital images were analyzed for the following histomorphometric parameters. Thickness of Ti and Tm were measured by using Leica Qwin V3 program at a magnification of ×200. Thickness of Ti and Tm were measured at five random places and then means were obtained. Adventitial area (Ada) and sympathetic fiber content (Sympa) were obtained by using a software named “Tissue Quant” (TQ, Version 1.0), which is designed for color quantification in Manipal Centre for Information Science, Manipal.
Statistical analysis
Statistical analysis was performed using the SPSS 11.5 software. Data were expressed as mean ± standard deviation (SD) and 95% confidence interval (CI). Data were analyzed using one-way analysis of variance (ANOVA) followed by Tukey's Honestly Significant Difference (HSD) post-hoc test. Probability (P) values less than 0.05 were considered significant.
RESULTS
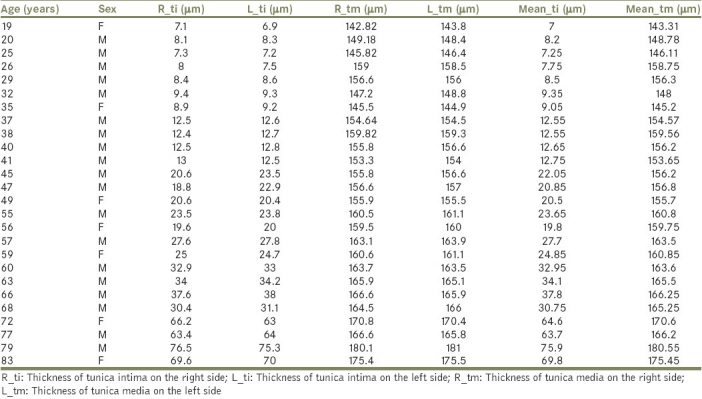
In the current study, the differences between the right and left RAs were not significant; thus, the mean values of right and left arteries had been taken together [Table 2].
Table 2.
Histomorphometric values of the RA
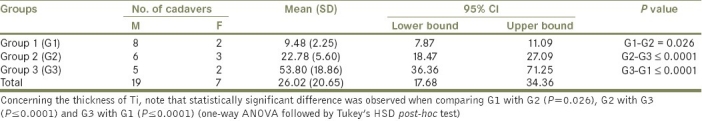
Intimal hyperplasia was the most common observation and incidences of thickening were found to increase with age. The mean, SD, 95% CI (lower bound and upper bound) and P values of Ti thickness of Group 1 (G1), Group 2 (G2) and Group 3 (G3) are shown in Table 3. Regarding the thickness of Ti, we found statistically significant differences in the thickness of Ti, when comparing G1 with G2 (P=0.026), G2 with G3 (P≤0.0001) and G3 with G1 (P≤0.0001).
Table 3.
Descriptive statistics of thickness of Ti of the RA
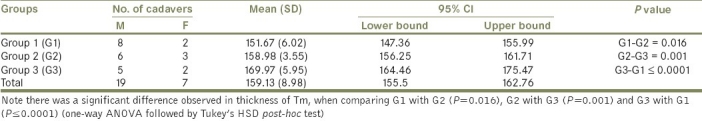
The present study showed that thickness of Tm was found to increase with age. The means thickness of the Tm in G1, G2, and G3 were 151.67, 158.98 and 169.97 μm, respectively [Table 4]. Concerning the thickness of Tm, there were statistically significant differences observed when G1 was compared with G2 (P=0.016), G2 with G3 (P=0.001) and G3 with G1 (P≤0.0001) [Table 4].
Table 4.
Descriptive statistics of thickness of Tm of RA
Ti/Tm ratio was calculated for the three age groups. Ti/Tm ratio was found to increase with age. The mean (SD) Ti/Tm ratios of the RA in G1, G2 and G3 were 0.06 (0.01), 0.14 (0.03) and 0.31 (0.10), respectively. Ti/Tm ratios were found to be significant when G1 was compared with G2 (P<0.01), G2 with G3 (P≤0.001) and G3 with G1 (P≤0.001) (one-way ANOVA, Tukey's post-hoc test).
Our histological studies revealed that proximal part of RA had a musculo-elastic artery structure. Tm showed smooth muscle cells and fine irregularly arranged elastic fibers [Figure 1a–c]. VVG staining showed fragmented internal elastic lamina (IEL) and elastic fibers in the thickened Ti. In few cases, IEL was double [Figure 1b]. The external elastic lamina (EEL) was prominent, well defined and appeared intact in the entire periphery of the vessel wall in all the samples studied [Figure 1d]. Discontinuations in IEL were found to be increased in elderly cases [Figure 1d]. There was also the deposition of calcium in the Tm observed in elderly cases. Clinical consequences of medial calcification would be that vascular surgery becomes much more difficult.
Figure 1.
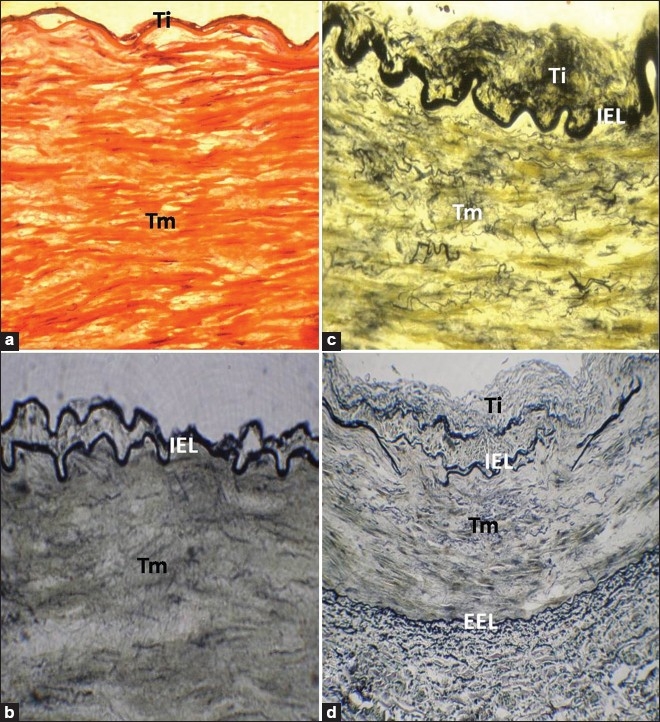
(a) RA of a 25-year-old individual, stained with H and E, showing no intimal changes (×400). (b) The cross section of RA of a 20-year-old individual, stained with VVG stain, showing duplicated IEL (×400). (c) RA of a 45-year-old individual, stained with VVG stain, showing intimal thickening and numerous fine elastic fibers in Tm (×400). (d) Disorganized IEL with elastic fibers in Tm in RA of a 59-year-old individual, stained with VVG stain. EEL was well defined and appears intact in the entire periphery of the vessel wall (×200). Ti: Tunica intima; Tm: Tunica media; IEL: Internal elastic lamina; EEL: External elastic lamina
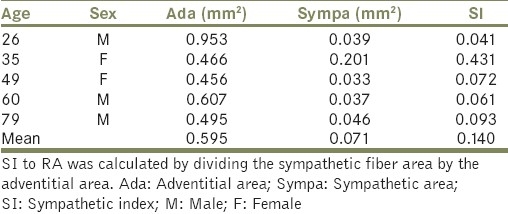
TH immunostaining revealed that sympathetic nerve fibers were present in the RA. TH positive sympathetic nerve fibers were situated mainly in the tunica adventitia and outer media [Figure 2a and b]. Mean Ada and Sympa areas are found to be 0.595 and 0.071 mm2, respectively. Sympathetic index (SI) to RA was calculated by dividing the sympathetic fiber area by the adventitial area. SI was found to be 0.140 [Table 5].
Figure 2.
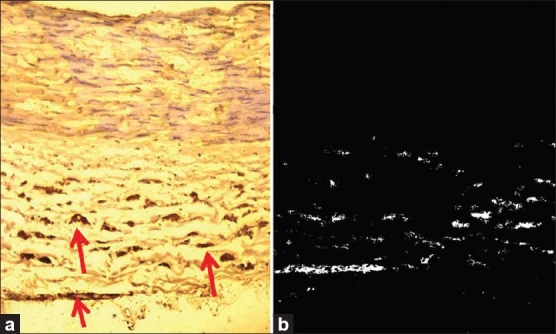
(a) Arrows pointing to the sympathetic fibers in a RA of a 26-year-old individual, stained with TH immunostaining (×250). (b) Results of the automated measurement of sympathetic fiber area (white dots) of the same RA that was calculated by Tissue Quant image analysis software (×250)
Table 5.
Adventitial and sympathetic nerve areas of the human RA
DISCUSSION
In the present study, RA showed the structure of a musculo-elastic artery. Thickness of Tm increased in relation to age. This may be due to the incorporation of fibrous tissue. There was also deposition of calcium in the Tm observed in two samples of Group 3. Clinical consequences of medial calcification would be that vascular surgery becomes much more difficult. Discontinuations/fragmentations in IEL were found in all the samples above the fourth decade and its incidence of fragmentation increased in elderly cases. In a few cases, IEL was double. The IEL represents a flexible barrier between the Ti and Tm and may have a role in atherogenesis via its modulation of diffusion across the artery wall.[23,24] According to Sims (1985), discontinuity of the IEL causes migration of myocytes from media to intima and activates atherosclerosis.[25] In this study, thickness of intima and media hyperplasia were observed to cause the increased thickness of the arterial wall. This may be attributed to the breaks/discontinuations in the IEL. Smooth muscles from the media may migrate into the media through the broken IEL, resulting in atherogenesis. The EEL was prominent, well defined and appeared intact in the entire periphery of the vessel wall in all the samples studied. The mean Ti thickness of RA in G1, G2, and G3 were found to be 9.48, 22.78, and 53.80 μm, respectively. In the present study, incidence of intimal thickness or atherosclerosis progressed with age and was found in all cases after the fourth decade. Incidence and severity of RA atherosclerosis was found to increase with age. Thickened intima was composed of lipid, fibrous tissue and foam cells. The incidence of intimal thickening was similar on both right and left sides. Schwartz and White (1964) noticed a slight but inconsistent difference in right and left RAs.[26] Wollenweber (1968), on the other hand, claimed that in cases with unilateral atherosclerosis, the right RAs were involved more frequently.[27] However, Aggarwal et al. (2008) have reported that incidence of atherosclerotic changes in most of the cases showed bilateral involvement; in unilaterally affected cases, left artery was more affected.[5] In this study, Ti/Tm ratio of RA in G1, G2, and G3 were found to be 0.06 (0.01), 0.14 (0.03) and 0.31 (0.10), respectively. Ti/Tm ratio increased with age. Increased Ti/Tm ratio may be attributed to the intimal changes in reaction to pressure and blood flow dynamics of the RA.
We assessed the sympathetic nerve content of the human RAs by using adrenergic marker TH. The intrinsic innervation within mammalian kidneys has been extensively described in the literature.[14–19] Tiniakos et al. (2004) reported that the human fetal kidney appears richly innervated during the 2nd and 3rd trimesters. There is a progressive increase in the density of parenchymal nerve fibers toward term from the corticomedullary region to the cortex. Most intrarenal nerves are adrenergic and have a predominant perivascular distribution, implying that renal innervation plays an important functional role during intrauterine life.[22] Renal sympathetic nerves and circulating cathecholamines are considered to be involved in the development of acute renal failure.[28,29] Renal sympathetic postganglionic fibers innervate the RA, afferent and efferent arterioles, the juxtaglomerular apparatus, and tubules. Renal sympathetic nerves control renal blood flow, renin release, and sodium reabsorption.[14–17] The present study revealed that dense plexus of sympathetic nerves was present in the tunica adventitia and outer media of the RA. SI may provide the morphological basis for further studies involving the structural basis of altered renal responses in conditions such as hypertension, aging, diabetes and peripheral neuropathies.
ACKNOWLEDGMENT
The authors sincerely thank Manipal University, Manipal, for providing experimental facilities to carry out this work.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.McLaughlin K, Jardine AG, Moss JG. ABC of arterial and venous disease. Renal artery stenosis. BMJ. 2000;320:1124–7. doi: 10.1136/bmj.320.7242.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wright I. The microscopical appearances of human peripheral arteries during the growth and aging. J Clin Pathol. 1963;16:499–522. doi: 10.1136/jcp.16.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamaguchi T, Omae T, Katsuki S. Quantitative determination of renal vascular changes related to age and hypertension. Jpn Heart J. 1969;10:248–58. doi: 10.1536/ihj.10.248. [DOI] [PubMed] [Google Scholar]
- 4.Vink A, Schoneveld AH, Poppen M, de Kleijn DP, Borst C, Pasterkamp G. Morphometric and immunohistochemical characterization of the intimal layer throughout the arterial system of elderly humans. J Anat. 2002;200:97–103. doi: 10.1046/j.0021-8782.2001.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal A, Kapoor K, Singh B. Prevalence and severity of atherosclerosis in renal artery in Northwest Indian population: An autopsy study. Surg Radiol Anat. 2009;31:349–56. doi: 10.1007/s00276-008-0452-0. [DOI] [PubMed] [Google Scholar]
- 6.Mathur KS, Patney NL, Kumar V. Atherosclerosis in India: An autopsy study of the aorta and coronary cerebral, renal and pulmonary arteries. Circulation. 1961;24:68–75. doi: 10.1161/01.cir.24.1.68. [DOI] [PubMed] [Google Scholar]
- 7.Hansen KJ. Prevalence of ischemic nephropathy in the atherosclerotic population. Am J Kidney Dis. 1994;24:615–21. doi: 10.1016/s0272-6386(12)80222-8. [DOI] [PubMed] [Google Scholar]
- 8.Strandness DE., Jr Duplex imaging for the detection of renal artery stenosis. Am J Kidney Dis. 1994;24:674–8. doi: 10.1016/s0272-6386(12)80230-7. [DOI] [PubMed] [Google Scholar]
- 9.Grist TM. Magnetic resonance angiography of renal artery stenosis. Am J Kid Dis. 1994;24:700–12. doi: 10.1016/s0272-6386(12)80234-4. [DOI] [PubMed] [Google Scholar]
- 10.Norouzani FR, Haghighat M, Gholami S. Morphometry of renal artery in two sexes of sheep at different ages. J Anim Vet Adv. 2008;7:889–91. [Google Scholar]
- 11.Tomic K, Mladinov D, Batelja-Vuletic L, Spajic B, Mijic A, Tomas D, et al. Morphometric analysis of renal arteries in patients with renal cell carcinoma. Pathol Res Pract. 2007;203:647–52. doi: 10.1016/j.prp.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Fujii T, Kurata H, Takaoka M, Muraoka T, Fujisawa Y, Shokoji T, et al. The role of renal sympathetic nervous system in the pathogenesis of ischemic acute renal failure. Eur J Pharmacol. 2003;481:241–8. doi: 10.1016/j.ejphar.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Neumann J, Ligtenberg G, Klein II, Koomans HA, Blankestijn PJ. Sympathetic hyperactivity in chronic kidney disease: Pathogenesis, clinical relevance, and treatment. Kidney Int. 2004;65:1568–76. doi: 10.1111/j.1523-1755.2004.00552.x. [DOI] [PubMed] [Google Scholar]
- 14.Barajas L, Müller J. The innervation of the juxtaglomerular apparatus and surrounding tubules: A quantitative analysis by serial section electron microscopy. J Ultrastruct Res. 1973;43:107–32. doi: 10.1016/s0022-5320(73)90073-7. [DOI] [PubMed] [Google Scholar]
- 15.Moss NG. Renal function and renal afferent and efferent nerve activity. Am J Physiol. 1982;243:425–33. doi: 10.1152/ajprenal.1982.243.5.F425. [DOI] [PubMed] [Google Scholar]
- 16.Di Bona GF. The functions of the renal nerves. Rev Physiol Biochem Pharmacol. 1982;94:176–81. [Google Scholar]
- 17.Barajas L, Powers K, Wang P. Innervation of the renal cortical tubules: A quantitative study. Am J Physiol. 1984;247:F50–60. doi: 10.1152/ajprenal.1984.247.1.F50. [DOI] [PubMed] [Google Scholar]
- 18.McLachlan EM, Luff SE. Sympathetic innervation of renal and extra-renal arterial vessels. Kidney Int Suppl. 1992;37:S56–60. [PubMed] [Google Scholar]
- 19.Saitongdee P, Milner P, Loesch A, Knight G, Burnstock G. Electron-immunocytochemical studies of perivascular nerves of mesenteric and renal arteries of golden hamsters during and after arousal from hibernation. J Anat. 1999;195:121–30. doi: 10.1046/j.1469-7580.1999.19510121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato KL, do Carmo JM, Fazan VP. Ultrastructural anatomy of the renal nerves in rats. Brain Res. 2006;1119:94–100. doi: 10.1016/j.brainres.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Shannon JL, Headland R, MacIver AG, Ferryman SR, Barber PC, Howie AJ. Studies on the innervation of human renal allografts. J Pathol. 1998;186:109–15. doi: 10.1002/(SICI)1096-9896(199809)186:1<109::AID-PATH134>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 22.Tiniakos D, Anagnostou V, Stavrakis S, Karandrea D, Agapitos E, Kittas C. Ontogeny of intrinsic innervation in the human kidney. Anat Embryol (Berl) 2004;209:41–7. doi: 10.1007/s00429-004-0420-3. [DOI] [PubMed] [Google Scholar]
- 23.Osborne-Pellegrin MJ. Spontaneous arterial lesions involving breaks in the internal elastic lamina in the rat: Effects of β-aminopropionitrile and familial distribution. Exp Mol Pathol. 1986;45:171–84. doi: 10.1016/0014-4800(86)90057-2. [DOI] [PubMed] [Google Scholar]
- 24.Hutchison KJ, Sanders EJ. Patterns of internal elastic lamina morphology in the canine common carotid artery. Blood Vessels. 1990;27:1–13. doi: 10.1159/000158791. [DOI] [PubMed] [Google Scholar]
- 25.Sims FH. Discontinuities in the internal elastic lamina: A comparison of coronary and internal mammary arteries. Artery. 1985;13:127–42. [PubMed] [Google Scholar]
- 26.Schwartz CJ, White TA. Stenosis of renal artery: An unselected necropsy study. Br Med J. 1964;2:1415–21. doi: 10.1136/bmj.2.5422.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wollenweber J, Sheps SG, Davis GD. Clinical course of atherosclerotic renovascular disease. Am J Cardiol. 1968;21:60–71. doi: 10.1016/0002-9149(68)90014-3. [DOI] [PubMed] [Google Scholar]
- 28.Baines AD. Nervous disorders of renal function. Clin Biochem. 1983;16:134–40. doi: 10.1016/s0009-9120(83)93900-0. [DOI] [PubMed] [Google Scholar]
- 29.Iaina A, Eliahou HE. The sympathetic nervous system in the pathogenesis of acute renal failure. Clin Exp Dial Apheresis. 1983;7:115–25. doi: 10.3109/08860228309076043. [DOI] [PubMed] [Google Scholar]


