Abstract
Background:
Maspin is a member of the serpin family of protease inhibitors and is thought to inhibit carcinoma invasion, metastasis, angiogenesis and induce apoptosis.
Aim:
The aim of this work is to investigate maspin expression in cutaneous basal and squamous cell carcinomas by means of immunohistochemistry.
Materials and Methods:
This study was carried out on 43 patients, 25 basal cell carcinoma (BCC) and 18 squamous cell carcinoma (SCC) together with ten apparently healthy volunteers as a control group.
Results:
There was a significant difference between the malignant and control groups regarding maspin expression since all control cases showed maspin expression compared to 60.5% (26/43) positivity in malignant cases. Maspin positive expression tended to be of higher percentage in SCC (77.8%) compared to BCC (48%) (P = 0.06) and the strong intensity of maspin was also significantly in favour of SCC compared to BCC (P = 0.02). The staining of both the cytoplasm and nuclei was seen in 27.7% of SCC and 12% of BCC and was significantly in favour of older age group (P = 0.02) and the adenoid variant (P = 0.04) of the latter.
Conclusions:
Maspin is associated with terminal squamous differentiation. Nuclear staining of maspin is seen in both BCC and SCC with a suggested tumour suppressor role in BCC.
KEYWORDS: Basal cell carcinoma, Maspin, Squamous cell carcinoma
INTRODUCTION
Non melanoma skin cancer is 18–20 times more frequent than cutaneous malignant melanoma (MM).[1] Although mortality from basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) is low but there may be a substantial morbidity from disfigurement as the lesions tend to be located on the skin of the head and neck.[2] According to the latest registry of Egyptian National cancer Institute, BCC and SCC represent 45.5% and 37.02% of total malignant skin cancer, respectively.[3]
Maspin is a member of the serpin family of protease inhibitors and is thought to inhibit carcinoma invasion, metastasis, angiogenesis and induce apoptosis.[4] It is synthesized by normal epithelial cells of a variety of mammalian organs such as mammary gland, prostate, skin and cornea,[5] but it is down-regulated during cancer progression.[6]
Maspin is expressed in several tumours such as pancreatic,[7] lung,[8] gastric[9] and breast carcinomas.[10] In spite of its tumour-suppressor activity, both a decrease,[7,11] and an increase of maspin levels,[12,13] have been described to parallel tumour progression. The available data considering maspin and epithelial skin tumours is limited and does not give clear idea about its possible role as a tumour suppressor or an oncogen.
The aim of this work is to investigate maspin expression in cutaneous basal and squamous cell carcinomas to throw light on its role in pathogenesis of these carcinomas by immunohistochemistry together with correlating its expression with the clincopathological features in these two types of skin carcinomas.
MATERIALS AND METHODS
This study was carried out on 43 patients, 25 BCC and 18 SCC together with 10 apparently healthy volunteers as a control group. Both patients and control subjects were selected randomly from the Outpatient of Dermatology and Surgery Clinics, during the period between May 2008 and April 2009.
Patient group was submitted to general and dermatological examinations. The patients were followed in Surgery Department where they underwent excision biopsy with safety margins.
All excision biopsies were processed in Pathology Department, Faculty of Medicine, Menofiya University, where they were fixed in 10% neutral buffered formalin, dehydrated in ascending grades of ethanol followed by immersion in xylene then impregnated in paraffin. Five micrometre (5 μm) thick sections from each block were taken. One to be stained with haematoxylin and eosin (H and E) for routine histopathological examination. Other sections were mounted on Superfrost Plus slides and stored at room temperature, one to be stained immunohistochemically for maspin and one negative control. Benign prostatic hyperplasia was used as a positive control.
Evaluation of haematoxylin and eosin stained sections for:
Histopahological diagnosis of cases.
Determination of the histopathological types of BCC.
Determination of the status of the surgical margins either involved or not.
Grading of SCC according to Broder's classification.[14]
Examination of the lymph nodes in cases of SCC and determination if they are involved or not.
Determination of pathological stage of SCC which is performed according TNM staging system.[15] Then stages I and stage II are lumped to be an early stage while stages III and IV are lumped to represent advanced stage.
Immunostaining was performed by streptavidin-biotin amplified system. The primary antibody used was mouse monoclonal antibody (Cat No. MS-1767 - R7, clone EAW24) raised against maspin (Labvision, California, USA). It is received as 0.1 ml concentrated which is then diluted by phosphate buffer saline (PBS) in a dilution 1:100 according to instructions in the supplied pamphlet. Sections mounted on Superfrost Plus slides were submitted to subsequent steps of deparaffinization and rehydration in xylene and a graded series of alcohol, respectively. Antigen retrieval was performed by boiling in 10 mM citrate buffer (pH 6.0) for 20 mins followed by cooling at room temperature. The slides were incubated overnight at room temperature with maspin monoclonal antibody. The secondary antibody used was the ultravision detection system anti-polyvalent HRP/DAB (ready to use) (catalog #TP-015-HD, Labvision, USA). The reaction was visualized by an appropriate substrate/chromogen (Diaminobenzidine, DAB) reagent with Mayer's haematoxylin as a counter stain. The negative controls obtained by substitution of primary antibodies with PBS were included in the staining procedure.
Maspin immunoreactivity was evaluated as negative or positive, where the positive cases were assigned as long as cytoplasmic or nuclear expression in any percentage of cells were identified. The intensity of maspin expression was assigned a score of 0-3 with 0 indicating no staining, 1 indicating weak staining, 2 indicating moderate staining and 3 indicating strong staining. The extent of maspin expression is evaluated according to the percentage of positivity, which is divided into focal expression (less than 10%) and diffuse expression (more than 10%).[16]
Results were collected, tabulated, statistically analyzed by IBM personal computer and statistical package SPSS version 11. Fisher exact and Chi square tests were used in comparison between qualitative variables while student t test was used in comparison between quantitative variables. A P value of <0.05 was considered statistically significant.
RESULTS
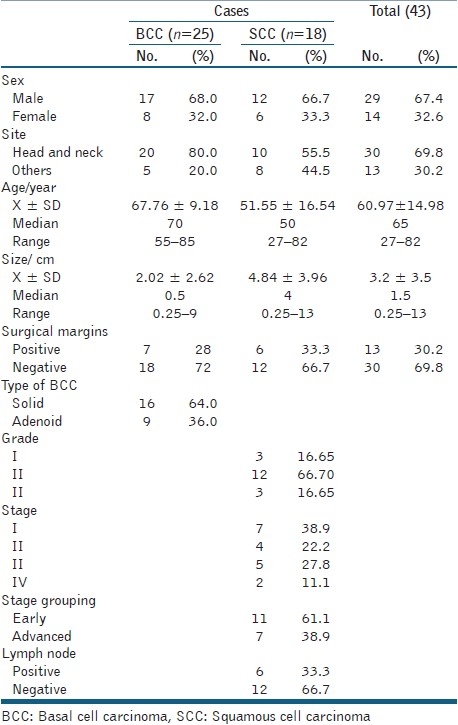
Clinical and pathological data of BCC and SCC are presented in Table 1.
Table 1.
Clinical and pathological characteristic of cutaneous carcinomas (BCCand SCC)
All control cases showed diffuse cytoplasmic maspin expression in the whole epidermal layers [Figure 1a] together with staining of hair follicles, sebaceous glands and sweat glands, but the latter showed nuclear staining [Figure 1b].
Figure 1.
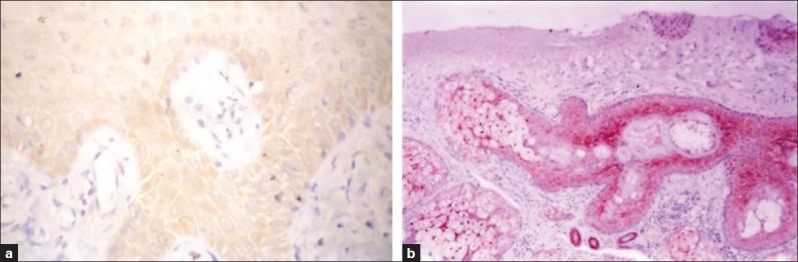
Maspin immunoreactivity in the epidermis (a) and in sebaceous glands, hair follicles and sweat glands in one of control cases (b) (Immunohistochemical staining ×200)
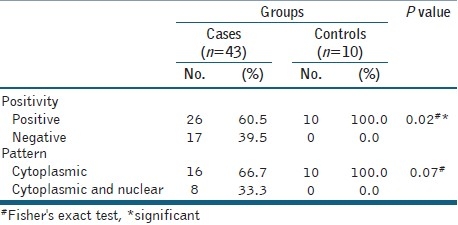
The positivity in malignant cases reached 60.5% (26/43), so there was a significant difference between the malignant and control cases regarding maspin expression but they did not differ as regards the pattern of maspin staining [Table 2].
Table 2.
Maspin expression among control and malignant cases
Maspin was expressed in 26/43 (60.5%) cases where it was expressed in 12 BCC (12/25, 48%) [Figure 2] and in 14 SCC (14/18, 77.8%) [Figure 3]. The intensity as well as the extent of staining varied among the positive cases, since weak staining was seen in 10 (38.5%) cases, moderate in 11 (42.3%) cases and strong in 5 (19.2%) cases. Diffuse expression constituted 17 cases (65.4%) in comparison to focal expression in 9 cases (34.6%). Most cases showed only cytoplasmic localization of maspin, which was seen in 18 cases while the presence of both cytoplasmic and nuclear staining was seen in 8 cases. The details of maspin status as regards positivity, pattern and intensity of expression in both BCC and SCC are presented in Table 3.
Figure 2.
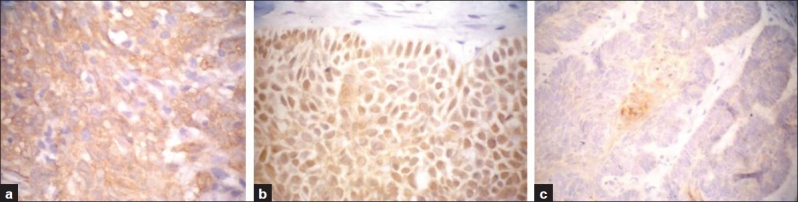
In BCC, maspin expression shows diffuse cytoplasmic staining (a), prominent nuclear postivity (b) or focal expression in center of BCC nests (hair follicle differentiation) (Immunohistochemical staining ×400 for a and b and ×200 for c)
Figure 3.
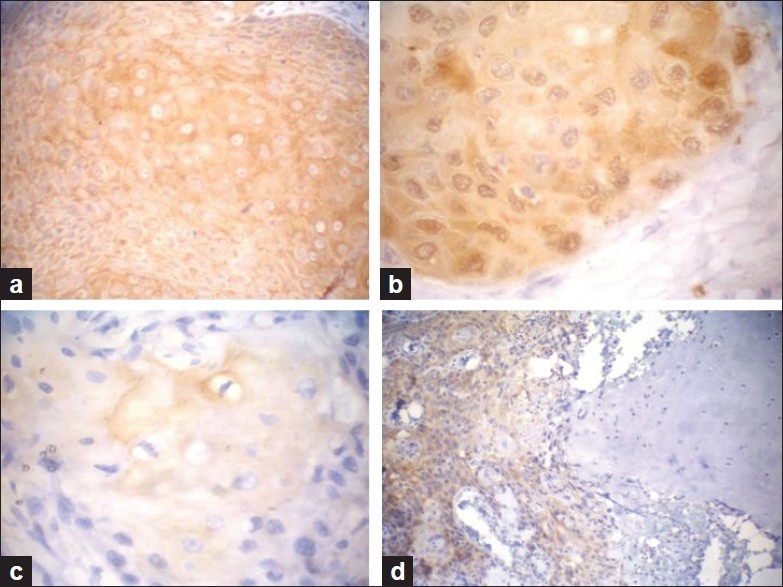
In SCC, maspin expression shows diffuse and strong cytoplasmic staining (a), nucleocytoplasmic immunoreactivity (b) and focal expression (c) Malignant squamous infiltrate invading the adjacent cartilage showing positive maspin expression (d) (Immunohistochemical staining ×400 for a, b and c and ×200 for d)
Table 3.
Differences between BCC and SCC as regards maspin expression
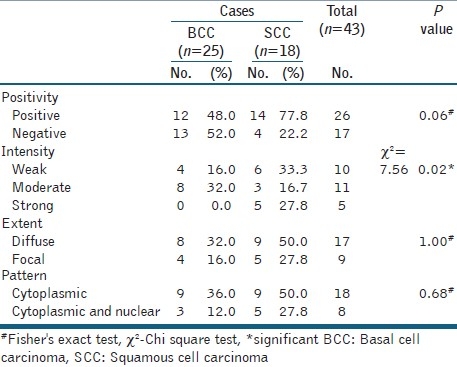
Maspin positive expression tended to be of higher percentage in SCC compared to BCC (P = 0.06) and the strong intensity of maspin was significantly in favour of SCC compared to BCC (P = 0.02), while the pattern did not show any significant differences between them [Table 3].
In BCC, maspin positive expression did not differ as regards the examined parameters except for its higher expression in a relatively younger age group (median = 62.5 versus 75, t-test = 2.7, P = 0.01). Diffuse maspin expression (>10%) tended to be associated with adenoid variant of BCC, since 100% of adenoid variant of BCC showed diffuse expression compared to 3/7 (42.9%) of solid variant (P = 0.08) [Figure 4]. The staining of both the cytoplasm and nuclei of BCC were significantly in favour of older age group (median = 72) (t-test = 2.26, P = 0.02) and the adenoid variant which showed this pattern (nuclear) in 60% of cases compared to only cytoplasmic-staining pattern in solid type (P = 0.04) [Figure 4].
Figure 4.
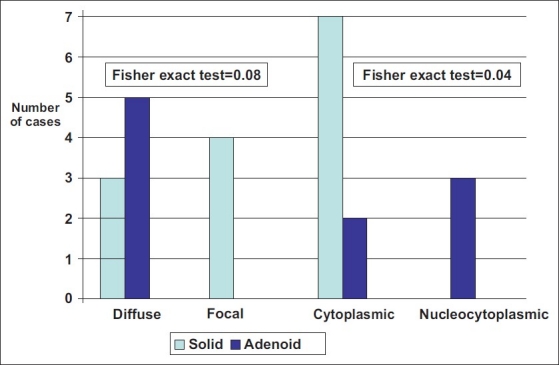
Adenoid variant of basal cell carcinoma showed greater tendency of diffuse maspin expression and nucleocytoplasmic pattern of maspin staining compared to solid variant
In SCC, either postivity, pattern or extent of maspin staining did not show any correlations with the studied clinical or pathological parameters. Regarding maspin intensity of expression, moderate and strong intensities were significantly in favour of female sex, since all females showed strong or moderate staining (100%) compared to 33% of males showing the same intensity degrees (P = 0.03).
DISCUSSION
Maspin expression is not universal but appears to be restricted to specific cell types, including myoepithelial cells of breast, basal cells of prostate, mucosal and glandular epithelia of gastrointestinal tract, and stratified layers of skin. In some cases, its expression levels can even vary between locations within the same tissue type.[17]
In the current study, maspin was expressed in the epidermis in normal control cases agreeing with Reis-Filho et al,[16] who showed that keratinized cutaneous structures expressed maspin. The expression in hair follicle seen in our cases and in others,[16] also confirms the same idea. Furthermore, Katz and Taichman,[18] reported that maspin is one of the serpins secreted by human keratinocytes.
Regulation of tissue specificity of maspin expression may be related to the presence of a hormonal responsive element (HRE) in the maspin gene promoter which down-regulates the transcription of this gene.[19,20] It has been shown that activated androgen receptors (ARs) can bind to the HRE and block the transcription of maspin in prostate cancer cell lines.[19] ARs are ubiquitously distributed in the human body, and it would be tempting to speculate that ARs play a role in the regulation of maspin expression in cutaneous structures.[21–23] Interestingly, ARs were observed with a variable expression in sebaceous glands, hair follicles, eccrine and apocrine sweat glands and keratinocytes.[23] This means that ARs are co-expressed in the same sites of maspin expression observed in our normal cases which may suggest a possible regulatory role of ARs on maspin .
Cytoplasmic and sometimes membranous expression was the pattern of maspin staining of epidermis, hair follicles and mature sebaceous glands seen in our normal cases . Staining of sweat gland is also observed with cytoplasmic and nuclear staining, this nuclear staining may resemble maspin expression in breast lobules and ducts especially with its staining of basal cell layer similar to what is called myoepithelioma-like pattern.[10,16] Maspin RNA and protein expression in keratinocytes correlate with senescence in cell culture and with chronological age in skin samples,[24] maspin is reported as one of the proteins that show age dependent expression in rat colon.[25]
Subcellular cytoplasmic localization of maspin seen in our normal and most of malignant cases is stated by others, where they showed that maspin is predominantly a soluble cytoplasmic protein.[26] The occasional membranous staining is also reported where maspin can be present at the cell surface.[26] On the other hand, nuclear localization of maspin was also described such in ovarian carcinoma.[27] The nuclear expression of maspin was thought earlier as an artefact of staining,[28] but later on various studies demonstrated this pattern, the mechanism of presence of maspin in the nucleus is explained by passive diffusion through the nuclear membrane or it may be chaperoned to the nucleus.[29,30]
Our study demonstrated maspin expression in differentiated normal tissues such as skin and its down-regulation in epithelial skin tumours (60.5%).
In our study, there was a significant correlation between strong intensity of maspin expression and SCC compared to BCC which agreed with Reis-Filho et al.,[16] who found that maspin was strongly expressed in cells showing features of terminal squamous differentiation. It also agreed with Takanami et al.,[31] who found that the incidence of strong maspin expression was significantly higher in patients with squamous cell carcinoma of the lung than in patients with adenocarcinoma.
Maspin was expressed in 48% of our BCC cases while according to Reis –filho et al.,[16] it was expressed in 87.5%, the lower expression of maspin in our BCC cases compared to the previous study may be due to the presence of three metatypical BCC in their cases which shares intermediate features between SCC and BCC. Focal expression of maspin in the centre of BCC nests was seen in 16% of our cases, which was also observed by Reis–filho et al.,[16] this centre usually represents follicular differentiation, so maspin expression in BCC is also associated with cells of terminal squamous differentiation . On the other hand, maspin was expressed in 77.8% of our SCC compared to 85.7% of cutaneous SCC according to Reis –filho et al.,[16] and 58.9 % of oral SCC according to Marioni et al.,[32]
In the present study, the observation of nuclear expression in five SCC and three BCC cases was also reported by Reis-filho et al.,[16] and Marioni et al.,[23] This pattern of maspin distribution was also seen with other serpins, such as ovaalbumin,[33] and SCC antigen.[34]
The importance of maspin subcellular localization may be indicative of different functions,[30,35] some authors demonstrated that the less aggressive behaviour of laryngeal SCC was associated with nuclear maspin expression.[24] Our study showed that nuclear expression of maspin was in favour of older age group and adenoid variant in BCC. Adenoid variant of BCC is one of the non-aggressive subtypes since aggressive histological variants include the micronodular, infiltrative, basosquamous, morpheaform, and mixed subtypes.[36] Some studies demonstrated that BCCs might be more aggressive in younger than in older adults.[37,38] Poor prognosis of young age in BCC may be due to its association with aggressive histological type and incomplete excision because of cosmetic considerations.[39] So nuclear expression of maspin appears to be related to favourable prognosis in BCC. Maspin was originally considered a general tumour suppressor[34] and its expression is suppressed as the tumour progresses.[7,20] But a more detailed analysis revealed that nuclear expression of maspin correlates with a favourable outcome in several tumour types including breast cancer,[10] non-small cell carcinoma of the lung,[28] pancreatic ductal adenocarcinoma,[40] and laryngeal SCC.[26] While cytoplasmic localization of maspin has been related to a poor prognosis such in ovarian and breast carcinomas.[10,27,41] Furthermore Joensuu et al.,[42] showed that cytoplasmic expression of maspin is associated with early tumour relapse in the breast cancer suggesting a tumour suppressive role only for nuclear maspin.
Mohsin et al.,[10] have found that staining of maspin was associated with estrogen and progesterone receptors positivity in breast cancer, which may explain the significant correlation between strong and moderate intensity of maspin expression and female gender in our SCC cases.
From this study, we can conclude that maspin is associated with terminal squamous differentiation manifested by its expression in normal skin, intense expression in SCC compared to BCC, which also partially showed maspin expression in areas of squamous differentiation. Nuclear staining of maspin is seen in both cutaneous BCC and SCC with a suggested tumour suppressor role in BCC.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(Suppl. 61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Dore JF, Autier P, Ringborg U. Cancer of the skin: A forgotten problem in Europe. Ann Oncol. 2004;15:5–6. doi: 10.1093/annonc/mdh032. [DOI] [PubMed] [Google Scholar]
- 3.Mokhtar N, Gouda I, Adel I. Cancer Pathology Registry (2003-2004): and time trend analysis. In: Mokhtar N, Gouda I, Adel I, editors. Malignant skin tumors, chapter (9) Cairo: Elsheraa press; 2007. pp. 83–86. [Google Scholar]
- 4.Kashima K, Ohike N, Mukai S, Sato M, Takahashi M, Morohoshi T. Expression of the tumor suppressor gene maspin and its significance in intraductal papillary mucinous neoplasms of the pancreas. Hepatobiliary Pancreat Dis Int. 2008;7:86–90. [PubMed] [Google Scholar]
- 5.Ngamkitidechakul C, Burke JM, O’Brien WJ, Twining SS. Maspin: Synthesis by human cornea and regulation of in vitro stromal cell adhesion to extracellular matrix. Invest Ophthalmol Vis Sci. 2001;42:3135–41. [PubMed] [Google Scholar]
- 6.Lee MJ, Suh CH, Li ZH. Clinicopathological significance of maspin expression in breast cancer. J Korean Med Sci. 2006;21:309–14. doi: 10.3346/jkms.2006.21.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maass N, Hojo T, Ueding M, Lüttges J, Klöppel G, Jonat W, et al. Expression of the tumor suppressor gene Maspin in human pancreatic cancers. Clin Cancer Res. 2001;7:812–7. [PubMed] [Google Scholar]
- 8.Kim S, Han J, Kim J, Park C. Maspin expression is transactivated by p63 and is critical for the modulation of lung cancer progression. Cancer Res. 2004;64:6900–5. doi: 10.1158/0008-5472.CAN-04-1657. [DOI] [PubMed] [Google Scholar]
- 9.Wang MC, Yang YM, Li XH, Dong F, Li Y. Maspin expression and its clinicopathological significance in tumorogenesis and progression of gastric cancer. World J Gastroenterol. 2004;10:6347. doi: 10.3748/wjg.v10.i5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohsin SK, Zhang M, Clark GM, Allred DC. Maspin expression in invasive breast cancer: Association with other prognostic factors. J Pathol. 2003;199:432–5. doi: 10.1002/path.1319. [DOI] [PubMed] [Google Scholar]
- 11.Czerwenka KF, Manavi M, Hosmann J, Jelincic D, Pischinger KI, Battistutti WB, et al. Comparative analysis of two-dimensional protein patterns in malignant and normal human breast tissue. Cancer Detect Prev. 2001;25:268–79. [PubMed] [Google Scholar]
- 12.Stathopoulou A, Mavroudis D, Perraki M, Apostolaki S, Vlachonikolis I, Lianidou E, et al. Molecular detection of cancer cells in the peripheral blood of patients with breast cancer: Comparison of CK-19, CEA and maspin as detection markers. Anticancer Res. 2003;23:1883–90. [PubMed] [Google Scholar]
- 13.Bièche I, Girault I, Sabourin JC, Tozlu S, Driouch K, Vidaud M, et al. Prognostic value of maspin mRNA expression in ER alpha-positive postmenopausal breast carcinomas. Br J Cancer. 2003;88:863–70. doi: 10.1038/sj.bjc.6600812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broder AC. Practical points on the microscopic grading of carcinoma. N Y State J Med. 1932;32:667–71. quoted from Morgan MB, Purohit C, Anglin TR. Immunohistochemical distinction of cutaneous spindle cell carcinoma. Am J Dermatopathol 2008;30:228-32. [Google Scholar]
- 15.Sobin LH, Wittekind C, editors. TNM classification of malignant tumors. 6th ed. New york: Wiley-Liss; 2002. [Google Scholar]
- 16.Reis-Filho JS, Torio B, Albergaria A, Schmitt FC. Maspin expression in normal skin and usual cutaneous carcinomas. Virchows Arch. 2002;441:551–8. doi: 10.1007/s00428-002-0710-1. [DOI] [PubMed] [Google Scholar]
- 17.Pemberton PA, Tipton AR, Pavloff N, Smith J, Erickson JR, Mouchabeck ZM, et al. Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface. J Histochem Cytochem. 1997;45:1697–706. doi: 10.1177/002215549704501213. [DOI] [PubMed] [Google Scholar]
- 18.Katz AB, Taichman LB. A partial catalog of proteins secreted by epidermal keratinocytes in culture. J Invest Dermatol. 1999;112:818–21. doi: 10.1046/j.1523-1747.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Sheng S, Maass N, Sager R. mMaspin: The mouse homolog of a human tumor suppressor gene inhibits mammary tumor invasion and motility. Mol Med. 1997;3:49–59. [PMC free article] [PubMed] [Google Scholar]
- 20.Zou Z, Zhang W, Young D, Gleave MG, Rennie P, Connell T, et al. Maspin expression profile in human prostate cancer (CaP) and in vitro induction of Maspin expression by androgen ablation. Clin Cancer Res. 2002;8:1172–7. [PubMed] [Google Scholar]
- 21.Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: A sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426–31. doi: 10.1097/00000372-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Bayer-Garner IB, Smoller B. Androgen receptors: A marker to increase sensitivity for identifying breast cancer in skin metastasis of unknown primary site. Mod Pathol. 2000;13:119–22. doi: 10.1038/modpathol.3880021. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539–42. doi: 10.1038/modpathol.3880346. [DOI] [PubMed] [Google Scholar]
- 24.Nickoloff BJ, Lingen MW, Chang BD, Shen M, Swift M, Curry J, et al. Tumor suppressor maspin is up-regulated during keratinocyte senescence, exerting a paracrine antiangiogenic activity. Cancer Res. 2004;64:2956–61. doi: 10.1158/0008-5472.can-03-2388. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, Greeley GH, Englander EW. Age-associated changes in gene expression patterns in the duodenum and colon of rats. Mech Ageing Dev. 2001;122:355–71. doi: 10.1016/s0047-6374(00)00254-2. [DOI] [PubMed] [Google Scholar]
- 26.Marioni G, Blandamura S, Giacomelli L, Calgaro N, Segato P, Leo G, et al. Nuclear expression of maspin is associated with a lower recurrence rate and a longer disease-free interval after surgery for squamous cell carcinoma of the larynx. Histopathology. 2005;46:576–82. doi: 10.1111/j.1365-2559.2005.02141.x. [DOI] [PubMed] [Google Scholar]
- 27.Sood AK, Fletcher MS, Gruman LM, Coffin JE, Jabbari S, Khalkhali-Ellis Z, et al. The paradoxical expression of maspin in ovarian carcinoma. Clin Cancer Res. 2002;8:2924–32. [PubMed] [Google Scholar]
- 28.Lonardo F, Li X, Siddiq F, Singh R, Al-Abbadi M, Pass HI, et al. Maspin nuclear localization is linked to favorable morphological features in pulmonary adenocarcinoma. Lung Cancer. 2006;51:31–9. doi: 10.1016/j.lungcan.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Bailey CM, Khalkhali-Ellis Z, Seftor EA, Hendrix MJ. Biological functions of maspin. J Cell Physiol. 2006;209:617–24. doi: 10.1002/jcp.20782. [DOI] [PubMed] [Google Scholar]
- 30.Khalkhali-Ellis Z. Maspin: The new frontier. Clin Cancer Res. 2006;12:7279–83. doi: 10.1158/1078-0432.CCR-06-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takanami I, Abiko T, Koizumi S. Expression of maspin in non-small-cell lung cancer: correlation with clinical features. Clin Lung Cancer. 2008;9:361–6. doi: 10.3816/CLC.2008.n.052. [DOI] [PubMed] [Google Scholar]
- 32.Marioni G, Gaio E, Giacomelli L, Bertolin A, D’Alessandro E, Stramare R, et al. Maspin subcellular localization and expression in oral cavity squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2008;265:S97–104. doi: 10.1007/s00405-008-0583-2. [DOI] [PubMed] [Google Scholar]
- 33.Bird CH, Blink EJ, Hirst CE, Buzza MS, Steele PM, Sun J, et al. Nucleocytoplasmic distribution of the ovalbumin serpin PI-9 requires a nonconventional nuclear import pathway and the export factor Crm1. Mol Cell Biol. 2001;21:5396–407. doi: 10.1128/MCB.21.16.5396-5407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994;263:526–9. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]
- 35.Blandamura S, Giacomelli L, Leo G, Segato P, Ninfo V. Nuclear maspin detection in renal cell tumors: Possible diagnostic role and correlation with p53 status. Histopathol. 2006;49:274–82. doi: 10.1111/j.1365-2559.2006.02504.x. [DOI] [PubMed] [Google Scholar]
- 36.Batra RS, Kelley LC. Predictors of extensive subclinical spread in nonmelanoma skin cancer treated with Mohs micrographic surgery. Arch Dermatol. 2002;138:1043–51. doi: 10.1001/archderm.138.8.1043. [DOI] [PubMed] [Google Scholar]
- 37.Cox NH. Basal cell carcinoma in young adults. Br J Dermatol. 1992;127:26–9. doi: 10.1111/j.1365-2133.1992.tb14820.x. [DOI] [PubMed] [Google Scholar]
- 38.Leffel DJ, Headington JT, Wong DS, Swanson NA. Aggressive-growth basal cell carcinoma in young adults. Arch Dermatol. 1991;127:1663–7. [PubMed] [Google Scholar]
- 39.Milroy CJ, Horlock N, Wilson GD, Sanders R. Aggressive basal cell carcinoma in young patients: Fact or fiction? Br J Plast Surg. 2000;53:393–6. doi: 10.1054/bjps.1999.3267. [DOI] [PubMed] [Google Scholar]
- 40.Cao D, Zhang Q, Wu LS, Salaria SN, Winter JW, Hruban RH, et al. Prognostic significance of maspin in pancreatic ductal adenocarcinoma: Tissue microarray analysis of 223 surgically resected cases. Mod Pathol. 2007;20:570–8. doi: 10.1038/modpathol.3800772. [DOI] [PubMed] [Google Scholar]
- 41.Solomon LA, Munkarah AR, Schimp VL, Arabi MH, Morris RT, Nassar H, et al. Maspin expression and localization impact on angiogenesis and prognosis in ovarian cancer. Gynecol Oncol. 2006;101:385–9. doi: 10.1016/j.ygyno.2005.11.049. [DOI] [PubMed] [Google Scholar]
- 42.Joensuu KM, Leidenius MH, Andersson LC, Heikkilä PS. High expression of maspin is associated with early tumor relapse in breast cancer. Hum Pathol. 2009;40:1143–51. doi: 10.1016/j.humpath.2009.02.006. [DOI] [PubMed] [Google Scholar]


