Abstract
Impaired neural conductivity shown by delayed latency and reduced amplitude of characteristic peaks in somatosensory evoked potentials (SSEPs), has been used to monitor hypoxic-ischemic brain injury after Cardiac Arrest (CA). However, rather than characteristic peak deferral and suppression, the time jitter of the peak in SSEP related with time-variant neurological abnormalities is diminished by the commonly used ensemble average method. This paper utilizes the second order blind identification (SOBI) technique to extract characteristic peak information from one trial of SSEPs. Sixteen male Wistar rats were subjected to 7 or 9 minutes of asphyxial CA (n=8 per group). The SSEPs from median nerve stimulation were recorded for 4 hours after CA and then for 15 minute periods at 24, 48 and 72 h. Neurological outcomes were evaluated by Neurologic Deficit Score (NDS) at 72h post-CA. The SSEP signal was analyzed offline with SOBI processing in Matlab. The N10 feature of SSEP was compared between good (NDS≥50) and bad (NDS<50) outcomes. After processed by SOBI, the N10 detection rate was significantly increased (p<0.001) from 90 min post-CA. Statistical difference of the latency variance of the N10 between good and bad outcome groups existed at 24, 48 and 72 h post-CA (p<=0.001). Our study is the first application using SOBI detecting variance in neural signals like SSEP. N10 latency variance, related with neurophysiological dysfunction, increased after hypoxic-ischemic injury. The SOBI technique is an efficient method in the identification of peak detection and offers a favorable alternative to reveal the neural transmission variation.
Keywords: Somatosensory evoked potentials, Cardiac arrest, Second order blind identification, Variance, Electrophysiology
1. Introduction
There are about 450,000 cardiac arrests (CAs) that take place annually in the United States (Young, 2009). With advanced resuscitation techniques, only one third achieve resumption of spontaneous circulation (ROSC), and among those patients only one-third to one-half survive to hospital discharge (Meaney et al., 2010). Prolonged hypoxic-ischemic brain injury has been recognized as the main cause of high morbidity and mortality (Geocadin et al., 2008).
In recent years, it has been well established that somatosensory evoked potentials (SSEPs) give an early and accurate insight of neurological deficiency after CA, thus, such electrophysiological monitoring currently serves as an important prognosis measure when making clinical treatment regime of comatose patients (De Giorgio et al., 1993; Robinson et al., 2003; Rossetti et al., 2010; Rothstein, 2000; Young et al., 2004).
As demonstrated by electrophysiological studies, hypoxic-ischemic injury results in two types of abnormalities that represent the change of conduction velocity and dispersion of conduction due to electrophysiological derangement (Nowak et al., 1993). The first type of abnormality gives rise to the dissociation of excitation, which lengthens the latency and lowers the amplitude of the wave. Relative characteristic changes in magnitude and latency (Kang et al., 2009; Madhok et al., 2010), conduction velocity (Salami et al., 2003) and time-frequency distributions (Hu et al., 2001; Hu et al., 2002), have all been studied in the past decades and are commonly used as SSEP measurement parameters in both laboratory experiments and clinical situations (De Giorgio et al., 1993; Robinson et al., 2003; Rossetti et al., 2010; Rothstein, 2000; Young et al., 2004). The other type of abnormality is the altered or disturbed connection induced by the alterations to the neural signals and sources that occur after ischemic injury. This kind of alteration is always ignored or eliminated, even though it could represent the excessive noise induced by ischemic injury to the neural system (Freyschuss et al., 1994; Hagerman et al., 1996; Hagerman et al., 1993; Nowak et al., 1993). One possible means to assess this temporal electrical heterogeneity or time jitter is to analyze the variability of characteristic peak of SSEP before and after CA induced hypoxic-ischemic injury. Quantitatively defined by peak latency variance, time jitter represents the coherence among the SSEPs. Smaller time jitter means peaks stay more aligned around fixed time points.
SSEPs are only several microvolts in amplitude (Meissner et al., 2004) and are always accompanied by different kinds of noise, including biological noise from the subject and electrical noise from instrumentation and surrounding environment (Hu et al., 2010). The signal to noise ratio (SNR) of SSEP is especially low during the first two hours post-CA. Ensemble averaging (EA) is typically used to improve SNR through averaging waveforms in a large time window (20–30 trials). This method assumes all the SSEPs in the time window have identical temporal information. Consequently, the variability in waveforms would be canceled as the result of averaging (Turner et al., 2003). In this study, an independent component analysis (ICA) based on second order blind identification (SOBI) method is utilized to extract peak information from SSEPs recorded from rats that underwent asphyxial CA in order to preserve the peak variability alteration of SSEP. This method enables us to increase temporal resolution and pick up spatial or multichannel information instead of temporal information from ensemble averaging. The effectiveness of this method is quantitatively evaluated, and then the variances of characteristic peaks are compared with different neurological outcome groups at different recovery periods after CA. Thus, peak variability is a new parameter that can be used to ascertain neurological outcome.
2. MATERIALS AND METHODS
2.1 Global hypoxic-ischemic rat model of CA
All use of rodents in the outlined experimental studies was approved by the Johns Hopkins School of Medicine Animal Care and Use Committee. Sixteen male Wistar rats (350g±25g) underwent asphyxia, resulting in CA and global cerebral ischemia, following the previously described model (Jia et al., 2008a; Jia et al., 2008b). Based on our previous research experience, 7 and 9 minutes of asphyxia time reflect survivable with moderate and severe brain injuries in rats respectively (Jia et al., 2008a; Jia et al., 2008b; Jia et al., 2006; Jia et al., 2008c, Madhok, 2010 #1868; Wu et al., 2010; Xiong et al., 2010). Eight of these rats were randomly selected and subjected to 7 min of asphyxia time while the other eight rats were subjected to 9 min of asphyxia time. On the day of experiment, rats were intubated and mechanically ventilated with 1.5% isoflurane carried by N2/O2 (50:50%) mixture. The femoral artery and vein distal to the inguinal ligament were cannulated so as to monitor arterial blood gases (ABG), observe blood pressure, and deliver drugs. During surgery, body temperature was maintained between 36.5–37.5°C by using a thermo-regulated heating pad (TCAT-2 Temperature Controller, Phyritemp, NJ, USA). The SSEP baseline was recorded for 15 min, followed by 5 min of isoflurane washout. 2mg/kg of vecuronium were injected 3 min before the end of isoflurane washout and the gas mixture was switched to room air. Then, CA was started by cessation of mechanical ventilation lasting for either 7 or 9 min as pre-determined. The time elapsed from the start of ventilation cessation to the time mean arterial pressure (MAP) went below 10 mmHg was recorded. Cardiopulmonary resuscitation (CPR) was performed with external chest compressions, mechanical ventilation, and intravenous epinephrine and NaHCO3 until ROSC. Keeping with animal care standards and alleviating pain and suffering associated with median nerve stimulation, isoflurane was used continuously starting from 45 min after ROSC to the end of the fourth hour, and for 15 min at 24, 48 and 72 hours after CA, restarting at 0.5% and titrating up to 1.5% as needed.
2.2 SSEP recording and stimulation protocol
One week before the experiment, 5 epidural screw electrodes were implanted above the rat's primary somatosensory cortex areas of the fore and hind limbs, and a ground electrode on the parasagittal right frontal lobe. All electrodes were carefully implanted so that they only made light contact with the dura matter and did not penetrate into the brain (Onifer et al., 2005). Dental cement was used to cover the electrodes, lead wires and exposed skull. SSEP signals were recorded using Tucker Davis Technologies (TDT) data acquisition systems and software (Alachua, FL). During the CA experiment, median nerve stimulation was delivered through subdermal needle electrodes placed in the right and left distal forelimbs. Stimulation with 200μs duration, 6 mA current and 0.5 Hz frequency was directly applied. The SSEP signals were recorded continuously in the first hour after CPR and every 15 min for the next 3 hours. Finally, SSEP at 24, 48, 72 hrs after CPR were recorded for 15 min. The signals were digitized with a sampling frequency of 6.1 KHz.
2.3 Neurologic Evaluation
Neurological function was evaluated by serial Neurologic Deficit Score (NDS) assessments at 24, 48, and 72 h after CPR (Jia et al., 2006). This scale has been previously validated as a measurement tool to assess neurologic outcome in rats after global hypoxic-ischemic injury (Geocadin et al., 2000a; Geocadin et al., 2000b; Jia et al., 2008b; Jia et al., 2006). The NDS scale is an amendment to several other examination tools used in human neurologic exams and to assess outcome scales for rats, dogs and piglets. The NDS ranges from 0 to 80, where 80 represents the best neurological outcome and 0 the worst. The 72-hr NDS was the primary outcome measure, as the neurological outcome will achieve a more steady state status after 72-hr according to our previous experiences (Jia et al., 2008a; Jia et al., 2008b; Jia et al., 2006; Madhok et al., 2010; Wu et al., 2010; Xiong et al., 2010). Rats were divided into good outcome (NDS≥50) and bad outcome (NDS<50) group according to NDS scores 72 hrs after CA (Wu et al., 2010; Xiong et al., 2010).
2.4 SOBI algorithm
ICA or blind source separation is a method that recovers and separates the source signals from the unknown mixed data (Lemm et al., 2006). Assuming that the responses to stimulation are independent from background noise, even if signals and noise are linearly mixed up, ICA can extract the target signal that overlap in some frequency range with the noises. Assume that the source components can be expressed by matrix s and the observed multichannel signals can be expressed by matrix x. Then the linear relationship between the sources and the observed data can be defined as x=As. The mixing matrix A could be calculated by ICA so that the source s could be estimated by A−1x. As one of the criteria to derive adaptive algorithms for A−1, SOBI has many advantages such as simplicity, reliability and robustness compared to other ICA algorithms (Sutherland and Tang, 2006; Ting et al., 2006). The effectiveness of SOBI in fast extraction of SSEPs has already been demonstrated (Liu et al., 2007). The biggest difference between SOBI and EA is that, other than incorporating the peak information of 20–30 trials, SOBI associates multi-channel information within one trial and achieves denoising and peak extraction through inter-channel coherence.
In this study, we recorded the electrical activities in the somatosensory cortex via four channels, which correspond to the sites for left forelimb, left hindlimb, right forelimb and right hindlimb respectively. Since only one side of the animal was stimulated at a given time, SSEPs only appeared at contralateral channels, and the ipsilateral channels provided the noise information in the experimental system. The obtained multi-channel information was decomposed into 4 source components using the SOBI method. The two source components that contained the characteristic SSEP peak were manually selected to reconstruct the SSEP signal while other source components were discarded as noise.
2.5 Signal processing and statistical analysis
After being processed by the SOBI algorithm, the SSEP signals were filtered by a low-pass filter with 1500 Hz cutoff frequency to prepare for the peak detection. Then, the SSEP's signature peak N10 was detected as the local maximum during 9 to 13 ms after the stimulation (Xiong et al., 2010). The number of undetectable N10 peaks was recorded to assist with further analysis. Latency was measured from the start of the stimulation to the N10 peak. In our study, time jitter of SSEPs was described by the N10 peak latency variance.
Parametric data (i.e. latency, latency variance, weight, and asphyxia time before CA) were reported as mean±SD and were analyzed by one-way analysis of variance (ANOVA) as a repeated measure with Student-Newman-Keuls (SNK) analysis used for multiple comparisons. To demonstrate the effectiveness of SOBI technique in different time segment, paired sample t-test was used to compare the N10 peak detection rate before and after processed by SOBI A level of p<0.05 was selected in order to consider the differences significant. All the statistical analyses were performed using PASW Statistics 18.0 software (SPSS, Inc., Somers, NY, USA).
3. Results
Sixteen rats were included in this study. Eight of them underwent 7 min asphyxia while the rest underwent 9 min asphyxia. There were 9 rats that had a good outcome, while the other 7 had a bad outcome. The mean time from the start of asphyxia to mean arterial pressure <10mmHg for good and bad outcomes were 184 ± 34 sec and 186 ± 34 sec and no significant difference was found (p-value 0.901). SSEP signals from all rats were processed by SOBI first. Through SOBI, the SSEP components were extracted, assuming statistical independence among the sources, and then used to reconstruct the SSEPs.
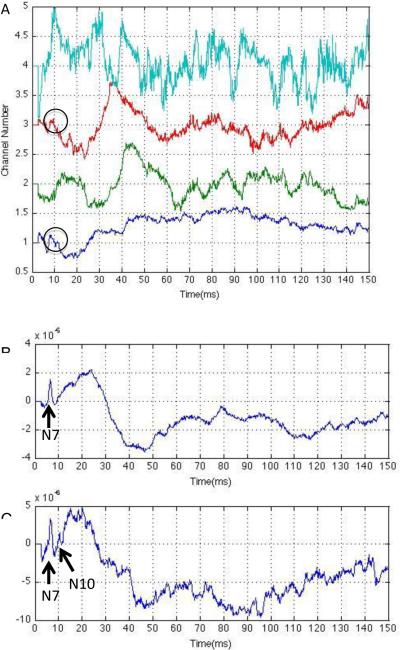
Fig. 1 is an illustration of the SOBI technique applied on SSEP signals from a randomly chosen representative rat 120 min after ROSC. Multi-channel SSEP recording from forelimb and hindlimb sensory area in both hemispheres were decomposed to four source components. It can be seen in Fig. 1 (A) that channel 1 and 3 contain SSEP N10 peak information around 10 msec while in channel 2 there is no appreciable characteristic information, and most of the signal in channel 4 is noise. Consequently, signals in channels 1 and 3 were selected to reconstruct the SSEP signals. Comparing the original waveform and the one reconstructed by SOBI from the stimulation contralateral side forelimb channel (Fig. 1(B) and Fig. 1(C)), it was seen that SOBI was able to discriminate the target N10 peak with substantial noise recorded from the ipsilateral channels.
Fig. 1.
(A) Source components decomposed by SOBI from multi-channel SSEP. Channels 1 and 3 contain peak information around 10 msec which are recognized as N10, while channels 2 and 4 do not have short-latency components. (B) Original SSEP signal resulting from stimulation of contralateral side forelimb channel. Notice only N7 is visible. (C) SOBI reconstructed signal from stimulation of contralateral side forelimb channel. We see that now both the N7 and N10 peaks are visible after SOBI processing.
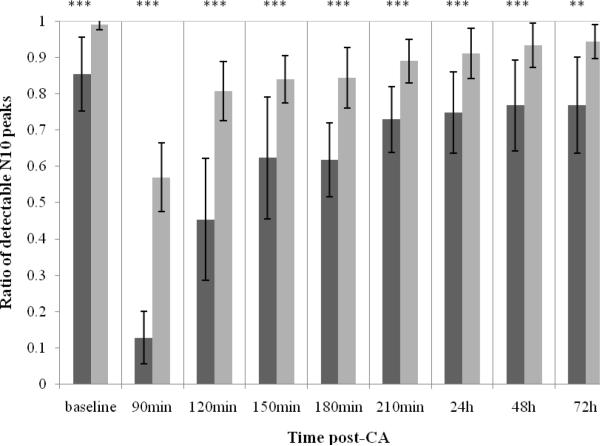
In this study, N10 was severely suppressed or even missing during the early stages of recovery (0–90 min). This was the same as in our previous study (Wu et al., 2010; Xiong et al., 2010), so the data in the first 90 min post-CA were excluded in our study. To evaluate the peak enhancement facility of SOBI, the peak detection rate -defined as ratio of detectable N10 peak over total sweeps number within 1 minute-was compared before and after SOBI processing, as shown in Fig. 2. The overall peak detection rate after processed by SOBI for all rats was significantly higher than the raw data before SOBI processing (repeated measures ANOVA, p<0.001). Combining all rats data together, consistent significantly higher detection rates were found with SOBI technique from 90 min to 48h (all p<0.001) and at 72h (p<0.01) post-CA. The significant improvement of peak detection rate remains during the whole time segment, when all rats are combined together, or stratified with good/bad outcome (all P <0.05), or 7 min/9min asphyxial time (all P <0.05 except p=0.075 for 9min CA group at 72h due to increased number of premature dead rats after 9min severe injury). After processing by SOBI, the peak detection rate remained above 80% after 120 min post-CA, thus all signals were maintained at a comparable level so that the following variability analysis was valid.
Fig. 2.
Ratio of detectable N10 peaks from raw data (dark grey bar) and data processed by SOBI (light grey bar) in baseline, at 90min, 120min, 150min, 180min, 210min, 24h, 48h and 72h post-CA (Mean ± SD). Statistically significant differences denoted by asterisks (**p<0.01, ***p<0.001).
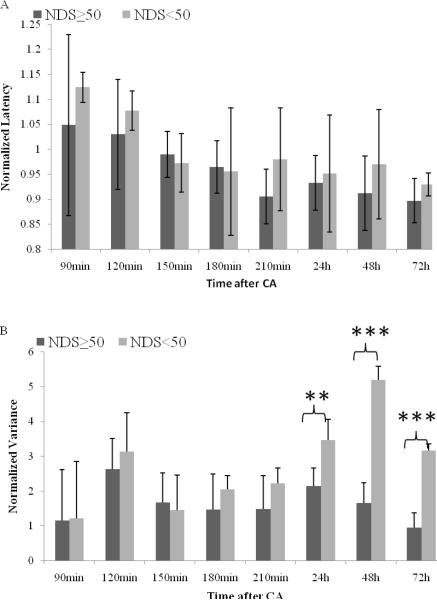
Based on the N10 peaks extracted by SOBI, the N10 peak latency and variance at 90, 120, 150, 180, 210 min, 24h, 48h and 72h of each rat were computed by MATLAB and normalized with respect to the baseline values (certain time value over baseline value) for the purpose of comparison. The result was averaged within good outcome and bad outcome groups (Fig. 3). There is significant difference of peak latency within different time segments (General Linear Model, p<0.001). The normalized latency significantly decreased from 90–120min post-CA (1.08–1.05) to 150min post-CA (0.98), continuously significantly decreased to 180min-72h post-CA (0.96–0.90) when rats gradually recovered from CA injury (all p <0.05), suggesting the early period (90–180 minutes) is critical period of peak latency recovery after cardiac arrest. Normalized N10 peak latencies of good outcome were slightly shorter than the bad outcome cases at most time points within 72h recovery, but that difference was not statistically significant (repeated measures of ANOVA, p=0.156).
Fig. 3.
Normalized N10 peak latency (3A) versus normalized N10 peak variance (3B) between good and bad outcome groups at 90 min, 120 min, 150 min, 180 min, 210 min, 24h, 48h and 72h post-CA (Mean ± SD). The dark grey bar represents the group with good outcome while the light grey bar represents bad outcome. Statistically significant differences denoted by asterisks (**p<0.01, ***p<0.001).
Increased variances of N10 peak latency after CA were found in both good and bad outcome groups. There are significant lower latency variances among good outcome animals comparing to the bad outcome animals (repeated measures of ANOVA, p<0.001) with significant differences at 24h (p=0.001), 48h (p<0.001) and 72h (p<0.001) after CA. It is also notable that the latency variances of good and bad outcome groups follow different recovery patterns. For good outcome group with NDS equal or above 50, the mean latency variance returned to the baseline level after 72 h post-CA recovery. In contrast, the latency variance remained at least 3 times larger than baseline during 72 h recovery period.
4. Discussion
This study demonstrates that hypoxic-ischemic injury causes increased N10 peak jitter in the somatosensory pathway. Unlike other averaging techniques, the SOBI technique is sensitive enough to detect such changes in SSEP signal. This was confirmed by the present results demonstrating that the SOBI technique clearly surpassed the performance of conventional methods. The discriminating capacity of the N10 peak detection rate was not blunted by treatment with stratifying the animals with good/bad outcome, or 7 min/9min asphyxial time. Rats with both good and bad outcome had an increase in the variance of SSEP N10 peak latency after CA. Moreover, correlation between neurological outcome and latency variance were found at the long-term recovery from hypoxic-ischemic injury (24–72 h). This correlation pattern differs from traditional patterns where the neurological outcomes are associated with peak latency and amplitude during acute recovery period (0–4h) after CA. This finding suggests that SSEP N10 peak variance is able to indicate the time-variant changes in sensory pathway induced by cardiac arrest.
The SOBI algorithm is necessary to detect the SSEP peak jitter in brain ischemia injury. As demonstrated by former studies, waveform distortion and suppression were observed in early recovery (Wu et al., 2010; Xiong et al., 2010). As Fig. 1(B) and Fig. 1(C) show, the N10 peak is so small after injury that it is more than often over-ridden by preceding peaks. As a consequence, local maxima approach to N10 peak detection will fail or be falsely detected in this scenario. Therefore, without the SOBI algorithm the high probability of false detection will invalidate the reliability of peak variances.
Comparing to traditional EA methods, the SOBI method makes full use of multi-channel information and manages to denoise SSEP by using one single trial instead of repetitive temporal experiments. We effectively increase the temporal resolution by capitalizing on the redundant spatial information present. This enables us to measure the peak variance in the first place. The SOBI method is also simple, reliable and robust compared to other ICA methods (Liu et al., 2007). Specifically, in SSEP extraction, there are two additional merits of the SOBI algorithm. First, the fast SSEP extraction capabilities of the SOBI method yield faster results with fewer SSEP trials. If used in clinical application, this method will distinctly shorten the signal acquisition time and neurological outcome prognostication time. The second advantage of the SOBI algorithm is that it could enhance certain desired characteristic peaks. In this study, we used an SSEP component in short-term latency responses, which are commonly considered as subcortical pathway activities (Wu et al., 2010; Koenig et al., 2006). Similarly, other SSEP characters, such as N30 and P60 in long-term latency responses, could be obtained by using different decomposed source components to reconstruct the signal. The drawback of SOBI technique lies in that amplitude measurements will be untenable after the signal reconstruction.
The SSEP test is now widely used as an evaluation of the somatosensory pathway continuity. The sensory stimulus travels through the peripheral nerve, nerve root, spinal cord, and subcortical brain structures to the primary sensory cortex (Koenig et al., 2006). Unlike an electroencephalogram which represents spontaneous activity, the SSEPs represent a compound series of triggered electrophysiological activities. Thus, different temporal segments represent distinctive behaviors. For example, long latency responses in humans (like N70) denote complex corticocortical pathways, which are important for sensory functions (Madl et al., 2000). Furthermore, absence or distortion of short latency responses (like N20) increase the likelihood of thalamic and cortical injury (Ahmed, 1988). It is generally believed that the N10 peak in the rat model shares the same origin with the N20 potential in humans (Madl et al., 1993).
In our previous studies, using combined averaged N10 peak latency and amplitude technique without latency variance information, delayed latency and suppressed amplitude were correlated with neurological outcome in the acute recovery period (0–4h) after CA (Madhok et al., 2010; Wu et al., 2010; Xiong et al., 2010). In this study, significant differences of N10 peak variance between good and poor outcome groups were found at 24, 48 and 72 h post-CA. The fact that the correlations of neurological outcome with N10 peak latency and variance reside in different recovery period may suggest that peak latency and variance evaluate two different kinds of abnormalities in neural system. The neural conductivity is impaired immediately after CA, while the disturbed connection may have a prolonged effect on the sensory pathway. Furthermore, the peak latency and peak variance could act as complementary indicators to shed better insight on the neurophysiology deficit caused by CA induced hypoxic-ischemic injury.
Though there is no evident pathophysiological explanation for this phenomenon, the increasing jitter is thought to be a reflection of the transition ability of the neural system to transmit impulses (Tanhehco, 2003). As a measure of electrical instability due to ischemia, it may result from both structural and dynamic abnormalities (Nowak et al., 1993). The increased latency variance at the later time (24–72h) after cardiac arrest may indicate inconsistent recovery in latency associated with bad functional outcome. Spatial nonuniformities of recovery and local differences in recorded SSEP may also contribute to it (Nowak et al., 1993). The effectiveness of time jitter analysis has been demonstrated in the area of electrocardiography and electromyography (Nowak et al., 1993; Tanhehco, 2003). Our study is the first application using SOBI detecting variance in neural signals like SSEP with quite promising result. The results of our study indicate that SOBI technique is an efficient method in the identification of peak detection and offers a favorable alternative which may also be useful in neural system evaluation through revealing the neural transmission variation.
5. Conclusion
This study demonstrated the discriminating capacity of the N10 peak detection by SOBI technique clearly surpassed the performance of conventional methods. N10 latency variance, related with neurophysiological dysfunction, increased after hypoxic-ischemic injury. The SOBI technique is an efficient method in the identification of peak detection and offers a favorable alternative to reveal the neural transmission variation.
Highlight.
This study demonstrated the discriminating capacity of the N10 peak detection by SOBI technique clearly surpassed the performance of conventional methods. N10 latency variance, related with neurophysiological dysfunction, increased after hypoxic-ischemic injury. The SOBI technique is an efficient method in the identification of peak detection and offers a favorable alternative to reveal the neural transmission variation.
Acknowledgments
This work was supported by grants 09SGD2110140 from the American Heart Association and RO1 HL071568 from the National Institute of Health. The authors would like to thank Hongtao Liu from Hong Kong University for the helpful discussion about the algorithm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Ahmed I. Use of somatosensory evoked responses in the prediction of outcome from coma. Clin Electroencephalogr. 1988;19:78–86. doi: 10.1177/155005948801900209. [DOI] [PubMed] [Google Scholar]
- De Giorgio CM, Rabinowicz AL, Gott PS. Predictive value of P300 event-related potentials compared with EEG and somatosensory evoked potentials in non-traumatic coma. Acta neurologica Scandinavica. 1993;87:423–7. doi: 10.1111/j.1600-0404.1993.tb04128.x. [DOI] [PubMed] [Google Scholar]
- Freyschuss U, Hjelte L, Johannesson M, Nowak J, Sylvén C. Signal variance electrocardiogram: a test for early detection of myocardial involvement in cystic fibrosis? 1994. pp. 103–7. [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Ghodadra R, Kimura T, Lei H, Sherman DL, Hanley DF, Thakor NV. A novel quantitative EEG injury measure of global cerebral ischemia. Clin Neurophysiol. 2000a;111:1779–87. doi: 10.1016/s1388-2457(00)00379-5. [DOI] [PubMed] [Google Scholar]
- Geocadin RG, Koenig MA, Jia X, Stevens RD, Peberdy MA. Management of brain injury after resuscitation from cardiac arrest. Neurologic clinics. 2008;26:487–506. ix. doi: 10.1016/j.ncl.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geocadin RG, Muthuswamy J, Sherman DL, Thakor NV, Hanley DF. Early electrophysiological and histologic changes after global cerebral ischemia in rats. Mov Disord. 2000b;15(Suppl 1):14–21. doi: 10.1002/mds.870150704. [DOI] [PubMed] [Google Scholar]
- Hagerman I, Berglund M, Svedenhag J, Nowak J, Sylven C. Beat-to-beat QRS amplitude variability after acute myocardial infarction and coronary artery bypass grafting. The American journal of cardiology. 1996;77:927–31. doi: 10.1016/s0002-9149(96)00030-6. [DOI] [PubMed] [Google Scholar]
- Hagerman I, Nowak J, Sylven C. Diagnostic innovations in electrophysiology. Variance ECG. Nordisk medicin. 1993;108:184–8. 92. [PubMed] [Google Scholar]
- Hu Y, Liu H, Luk KD. Signal-to-noise ratio of intraoperative tibial nerve somatosensory-evoked potentials. J Clin Neurophysiol. 2010;27:30–3. doi: 10.1097/WNP.0b013e3181cb4257. [DOI] [PubMed] [Google Scholar]
- Hu Y, Luk KD, Lu WW, Holmes A, Leong JC. Comparison of time-frequency distribution techniques for analysis of spinal somatosensory evoked potential. Medical & biological engineering & computing. 2001;39:375–80. doi: 10.1007/BF02345294. [DOI] [PubMed] [Google Scholar]
- Hu Y, Luk KD, Lu WW, Leong JC. Comparison of time-frequency analysis techniques in intraoperative somatosensory evoked potential (SEP) monitoring. Computers in biology and medicine. 2002;32:13–23. doi: 10.1016/s0010-4825(01)00026-9. [DOI] [PubMed] [Google Scholar]
- Jia X, Koenig MA, Nickl R, Zhen G, Thakor NV, Geocadin RG. Early electrophysiologic markers predict functional outcome associated with temperature manipulation after cardiac arrest in rats. Critical care medicine. 2008a;36:1909–16. doi: 10.1097/CCM.0b013e3181760eb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Koenig MA, Shin HC, Zhen G, Pardo CA, Hanley DF, Thakor NV, Geocadin RG. Improving neurological outcomes post-cardiac arrest in a rat model: immediate hypothermia and quantitative EEG monitoring. Resuscitation. 2008b;76:431–42. doi: 10.1016/j.resuscitation.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Koenig MA, Shin HC, Zhen G, Yamashita S, Thakor NV, Geocadin RG. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;1111:166–75. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Koenig MA, Venkatraman A, Thakor NV, Geocadin RG. Post-cardiac arrest temperature manipulation alters early EEG bursting in rats. Resuscitation. 2008c;78:367–73. doi: 10.1016/j.resuscitation.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, Xiong W, Koenig M, Puttgen HA, Jia X, Geocadin R, Thakor N. Long-term assessment of post-cardiac-arrest neurological outcomes with somatosensory evoked potential in rats. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2196–9. doi: 10.1109/IEMBS.2009.5334918. [DOI] [PubMed] [Google Scholar]
- Koenig MA, Kaplan PW, Thakor NV. Clinical neurophysiologic monitoring and brain injury from cardiac arrest. Neurologic clinics. 2006;24:89–106. doi: 10.1016/j.ncl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Lemm S, Curio G, Hlushchuk Y, Muller KR. Enhancing the signal-to-noise ratio of ICA-based extracted ERPs. IEEE transactions on bio-medical engineering. 2006;53:601–7. doi: 10.1109/TBME.2006.870258. [DOI] [PubMed] [Google Scholar]
- Liu H, Hu Y, Chang CQ, Luk KD. Fast extraction of somatosensory evoked potential based on second order blind identification. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:5457–60. doi: 10.1109/IEMBS.2007.4353580. [DOI] [PubMed] [Google Scholar]
- Madhok J, Maybhate A, Xiong W, Koenig MA, Geocadin RG, Jia X, Thakor NV. Quantitative assessment of somatosensory-evoked potentials after cardiac arrest in rats: prognostication of functional outcomes. Critical care medicine. 2010;38:1709–17. doi: 10.1097/CCM.0b013e3181e7dd29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madl C, Grimm G, Kramer L, Yeganehfar W, Sterz F, Schneider B, Kranz A, Schneeweiss B, Lenz K. Early prediction of individual outcome after cardiopulmonary resuscitation. Lancet. 1993;341:855–8. doi: 10.1016/0140-6736(93)93061-5. [DOI] [PubMed] [Google Scholar]
- Madl C, Kramer L, Domanovits H, Woolard RH, Gervais H, Gendo A, Eisenhuber E, Grimm G, Sterz F. Improved outcome prediction in unconscious cardiac arrest survivors with sensory evoked potentials compared with clinical assessment. Critical care medicine. 2000;28:721–6. doi: 10.1097/00003246-200003000-00020. [DOI] [PubMed] [Google Scholar]
- Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Critical care medicine. 2010;38:101–8. doi: 10.1097/CCM.0b013e3181b43282. [DOI] [PubMed] [Google Scholar]
- Meissner W, Weiss T, Trippe RH, Hecht H, Krapp C, Miltner WH. Acupuncture decreases somatosensory evoked potential amplitudes to noxious stimuli in anesthetized volunteers. Anesthesia and analgesia. 2004;98:141–7. doi: 10.1213/01.ANE.0000096191.07929.44. table of contents. [DOI] [PubMed] [Google Scholar]
- Nowak J, Hagerman I, Ylen M, Nyquist O, Sylven C. Electrocardiogram signal variance analysis in the diagnosis of coronary artery disease--a comparison with exercise stress test in an angiographically documented high prevalence population. Clinical cardiology. 1993;16:671–82. doi: 10.1002/clc.4960160909. [DOI] [PubMed] [Google Scholar]
- Onifer SM, Zhang YP, Burke DA, Brooks DL, Decker JA, McClure NJ, Floyd AR, Hall J, Proffitt BL, Shields CB, Magnuson DS. Adult rat forelimb dysfunction after dorsal cervical spinal cord injury. Exp Neurol. 2005;192:25–38. doi: 10.1016/j.expneurol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Robinson LR, Micklesen PJ, Tirschwell DL, Lew HL. Predictive value of somatosensory evoked potentials for awakening from coma. Critical care medicine. 2003;31:960–7. doi: 10.1097/01.CCM.0000053643.21751.3B. [DOI] [PubMed] [Google Scholar]
- Rossetti AO, Oddo M, Logroscino G, Kaplan PW. Prognostication after cardiac arrest and hypothermia: a prospective study. Annals of neurology. 2010;67:301–7. doi: 10.1002/ana.21984. [DOI] [PubMed] [Google Scholar]
- Rothstein TL. The role of evoked potentials in anoxic-ischemic coma and severe brain trauma. J Clin Neurophysiol. 2000;17:486–97. doi: 10.1097/00004691-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Salami M, Itami C, Tsumoto T, Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc Natl Acad Sci U S A. 2003;100:6174–9. doi: 10.1073/pnas.0937380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Tang AC. Reliable detection of bilateral activation in human primary somatosensory cortex by unilateral median nerve stimulation. Neuroimage. 2006;33:1042–54. doi: 10.1016/j.neuroimage.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Tanhehco JL. Single-fiber electromyography. Physical medicine and rehabilitation clinics of North America. 2003;14:207–29. doi: 10.1016/s1047-9651(02)00123-7. [DOI] [PubMed] [Google Scholar]
- Ting KH, Fung PC, Chang CQ, Chan FH. Automatic correction of artifact from single-trial event-related potentials by blind source separation using second order statistics only. Med Eng Phys. 2006;28:780–94. doi: 10.1016/j.medengphy.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Turner S, Picton P, Campbell J. Extraction of short-latency evoked potentials using a combination of wavelets and evolutionary algorithms. Medical engineering & physics. 2003;25:407–12. doi: 10.1016/s1350-4533(03)00021-3. [DOI] [PubMed] [Google Scholar]
- Wu D, Anastassios B, Xiong W, Madhok J, Jia X, Thakor NV. Study of the origin of short- and long-latency SSEP during recovery from brain ischemia in a rat model. Neurosci Lett. 2010;485:157–61. doi: 10.1016/j.neulet.2010.08.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Koenig MA, Madhok J, Jia X, Puttgen HA, Thakor NV, Geocadin RG. Evolution of Somatosensory Evoked Potentials after Cardiac Arrest induced hypoxic-ischemic injury. Resuscitation. 2010;81:893–7. doi: 10.1016/j.resuscitation.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GB. Clinical practice. Neurologic prognosis after cardiac arrest. The New England journal of medicine. 2009;361:605–11. doi: 10.1056/NEJMcp0903466. [DOI] [PubMed] [Google Scholar]
- Young GB, Wang JT, Connolly JF. Prognostic determination in anoxic-ischemic and traumatic encephalopathies. J Clin Neurophysiol. 2004;21:379–90. [PubMed] [Google Scholar]