SUMMARY
An activated intrarenal reninangiotensin system (RAS) plays a crucial role in the pathogenesis of hypertension and chronic kidney diseases (CKD). Angiotensinogen (AGT) is the only known substrate for renin, which is the rate-limiting enzyme of the RAS. Because the levels of AGT are close to the Michaelis-Menten constant for renin, AGT levels can also control the RAS activity, and upregulation of AGT may lead to elevated angiotensin peptide levels and increases in blood pressure. Recent studies on experimental animal models have documented the involvement of AGT in the intrarenal RAS activation and development of hypertension. Enhanced intrarenal AGT mRNA and/or protein levels occur in experimental models of hypertension and kidney diseases supporting important roles in the development and progression of hypertension and kidney diseases. Urinary excretion rates of AGT provide a specific index of intrarenal RAS status in angiotensin II-infused rats. Also, a direct quantitative method was recently developed to measure urinary AGT using human AGT ELISA. These data prompted us to measure urinary AGT in patients with hypertension and CKD, and investigate correlations with clinical parameters. This brief review will address the potential of urinary AGT as a novel biomarker of the intrarenal RAS status in hypertension and CKD.
Introduction
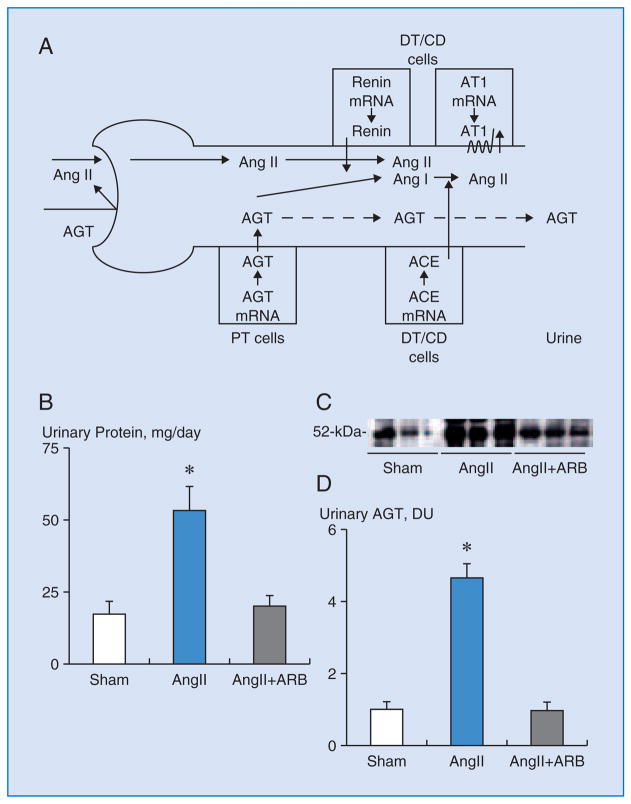
The activation of the renin-angiotensin system (RAS) plays a crucial role in the pathogenesis of hypertension and chronic kidney diseases (CKD) 1. In recent years, there has been increased emphasis on the role of the local/tissue RAS in specific tissues 2. Emerging evidence has demonstrated the importance of the tissue RAS in the brain 3, heart 4, adrenal glands 5, vasculature 6,7 and kidneys 1,8. Angiotensinogen (AGT) is the only known substrate for renin, which is the rate-limiting enzyme of the RAS. Although most of the circulating AGT is produced and secreted by the liver, the kidneys also produce AGT 9. Intrarenal AGT mRNA and protein have been localized to proximal tubular cells 10–13. The major fraction of angiotensin II present in renal tissues is generated locally from AGT delivered to the kidney as well as from AGT locally produced by proximal tubular cells 9,14. The AGT produced in proximal tubular cells appears to be secreted directly into the tubular lumen in addition to producing its metabolites intracellularly and secreting them into the tubular lumen 15,16. Because of its molecular size and protein binding, it seems unlikely that much of the plasma AGT filters across the glomerular membrane further supporting the concept that proximal tubular cells secrete AGT directly into the tubular lumen (Figure 1A) 1. Renin secreted by the juxtaglomerular apparatus cells into the renal interstitium and vascular compartment also provides a pathway for the local generation of angiotensin I 17. Angiotensin converting enzyme is abundant in the kidneys and is present in proximal tubules, distal tubules, and the collecting ducts 18. Angiotensin I delivered to the kidney can also be converted to angiotensin II 19. Therefore, all components necessary to generate intrarenal angiotensin II are present along the nephron 1,8.
Figure 1.
Figure 1A. Intrarenal renin-angiotensin (Ang) system (RAS) in proximal and distal nephron segments 1. Because of its molecular size, it seems unlikely that much of the plasma angiotensinogen (AGT) filters across the glomerular membrane. In Ang II-dependent hypertension, increased proximal tubular (PT) secretion of AGT spills over into the distal nephron and increases Ang II effects on distal tubular (DT) reabsorption.
CD, collecting ducts. ACE, Ang converting enzyme. AT1, Ang II type 1 receptors.
Figures 1B – 1D. Urinary protein and AGT excretion rates of each group 38.
B. Urinary excretion rates of total protein were greater in Ang II-infused animals. ARB treatment prevented this augmentation.
C. Representative western blot analysis of urinary AGT levels among groups showing the stimulation in Ang II-infused group.
D. Urinary excretion rates of AGT were increased by 4.7-times in Ang II-infused animals. ARB treatment prevented this augmentation.
ARB, AT1 blocker. DU, densitometric units. *, p < 0.05 compared to the sham group.
Because the levels of AGT are close to the Michaelis-Menten constant for renin, AGT levels can also control the activity of the RAS, and upregulation of AGT levels may lead to elevated angiotensin peptide levels and increases in blood pressure 20,21. Recent studies on experimental animal models and transgenic mice have documented the involvement of AGT in the activation of the intrarenal RAS and development of hypertension 22–30. Genetic manipulations that lead to overexpression of AGT have consistently been shown to cause hypertension 31,32. In human genetic studies, a linkage has been established between the AGT gene and hypertension 33–36. Enhanced intrarenal AGT mRNA and/or protein levels have also been observed in multiple experimental models of hypertension including angiotensin II-dependent hypertensive rats 13,37–41, Dahl salt-sensitive hypertensive rats 42,43, and spontaneously hypertensive rats 44 as well as in kidney diseases including diabetic nephropathy 45–50, IgA nephropathy 51,52, and radiation nephropathy 53. Thus, AGT upregulation plays an important role in the development and progression of hypertension and kidney diseases 1,8.
Previous reports have demonstrated that urinary excretion rates of AGT provide a specific index of intrarenal RAS status in angiotensin II-infused rats (Figures 1B – 1D) 13,38–41. Also, a direct quantitative method was recently developed to measure urinary AGT using human AGT enzyme-linked immunosorbent assay (ELISA) 54. The ELISA facilitated measurements of urinary AGT in patients with hypertension and CKD, and investigated correlations with clinical parameters. This brief review will address the potential of urinary AGT as a novel biomarker of the intrarenal RAS status in hypertension and CKD.
Urinary AGT in Hypertension
The important role of the activated intrarenal RAS in the pathogenesis of hypertension has been discussed in a recent review article 55.
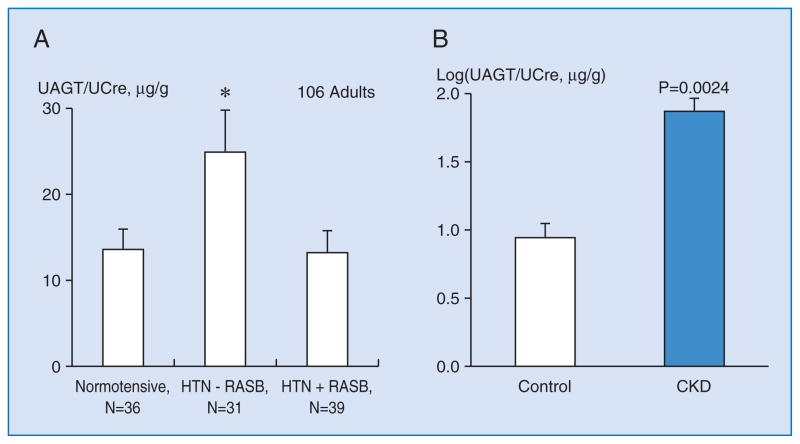
In a cross-sectional study, Kobori et al reported that urinary AGT levels are significantly greater in hypertensive patients not treated with RAS blockers compared with normotensive subjects (Figure 2A) 56. Moreover, patients treated with RAS blockers exhibit a marked attenuation of this augmentation. These data suggest that the efficacy of RAS blockade to reduce the intrarenal RAS activity can be assessed by measurements of urinary AGT 56.
Figure 2.
Figure 2A. UAGT/UCre levels were significantly increased in hypertensive patients not treated with RAS blockers (RASB) compared with normotensive subjects 56. Patients treated with RAS blockers exhibited a marked attenuation of this augmentation.
*, p < 0.05 vs. Normotensive. †, p < 0.05 vs. HTN - RASB.
Figure 2B. Log(UAGT/UCre) levels in chronic kidney disease (CKD) patients and in healthy volunteers 58. Log(UAGT/UCre) levels were significantly increased in CKD patients compared with that in control subjects.
In a population study, Kobori et al demonstrated that an activated intrarenal RAS is correlated with high blood pressure in humans 57. They recruited 251 subjects and collected a single random spot urine sample from each subject. Because urinary AGT levels are significantly increased in patients with diabetes 58 and the use of antihypertensive drugs affects urinary AGT levels 56, they excluded patients who had diabetes and/or were receiving antihypertensive treatment. Consequently, 190 samples were included for this analysis. Urinary AGT levels did not differ with race or gender, but were significantly correlated with systolic and diastolic blood pressure. Moreover, high correlations were shown in men, especially in Black men 57.
These data suggest that urinary AGT provides a novel reflection of the intrarenal RAS status in hypertension.
Urinary AGT in CKD
The important role of an activated intrarenal RAS in the pathogenesis of CKD is extensively discussed in a recent review article 59.
In order to test the hypothesis that urinary AGT levels are enhanced in CKD patients and correlated with some clinical parameters, eighty patients with CKD (37 women and 43 men, from 18 to 94 years old) and seven healthy volunteers (two women and five men, from 27 to 43 years old) were recruited 58. Plasma AGT levels showed a normal distribution; however, urinary AGT-to-creatinine ratios (UAGT/UCre) deviated from the normal distribution. When a logarithmic transformation was executed, Log(UAGT/UCre) levels showed a normal distribution. Therefore, Log(UAGT/UCre) levels were used for further analyses. Log(UAGT/UCre) levels were not correlated with age, gender, height, body weight, body mass index, systolic blood pressure, diastolic blood pressure, serum sodium levels, serum potassium levels, urinary sodium-creatinine ratios, plasma renin activity, or plasma AGT levels. However, Log(UAGT/UCre) levels were significantly correlated positively with urinary albumin-creatinine ratios, fractional excretion of sodium, urinary protein-creatinine ratios, and serum creatinine, and correlated negatively with estimated glomerular filtration rate. Log (UAGT/UCre) levels were significantly increased in CKD patients compared with control subjects (Figure 2B, 1.8801 +/− 0.0885 vs. 0.9417 +/− 0.1048; P = .0024). These data suggest that urinary angiotensinogen levels can be a potential biomarker of severity of CKD 58.
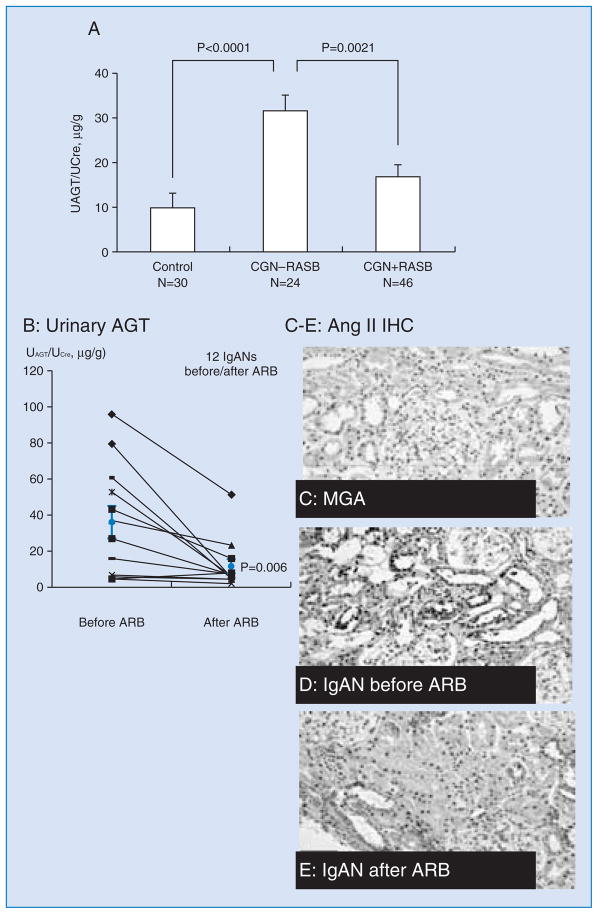
In order to test the hypothesis that urinary AGT levels are increased in chronic glomerulonephritis patients, 100 urine samples from 70 chronic glomerulonephritis patients (26 from IgA nephropathy, 24 from purpura nephritis, 8 from lupus nephritis, 7 from focal segmental glomerulosclerosis, and 5 from non-IgA mesangial proliferative glomerulonephritis) and 30 normal control subjects were analyzed 60. UAGT/UCre was correlated positively with diastolic blood pressure (p = 0.0326), urinary albumin-creatinine ratio (p < 0.0001), urinary protein-creatinine ratio (p < 0.0001) and urinary occult blood (p = 0.0094). UAGT/UCre was significantly increased in chronic glomerulonephritis patients not treated with RAS blockers compared with control subjects (Figure 3A, p < 0.0001). Importantly, patients with glomerulonephritis treated with RAS blockers had a marked attenuation of this augmentation (p = 0.0021). These data indicate that urinary AGT are increased in patients with chronic glomerulonephritis and treatment with RAS blockers suppressed UAGT. Thus, the efficacy of RAS blockade to reduce the intrarenal RAS activity can be confirmed by measurement of UAGT in chronic glomerulonephritis patients 60.
Figure 3.
Figure 3A. UAGT/UCre levels in chronic glomerulonephritis patients (CGN) with/without renin-angiotensin system blockade (RASB) and in control subjects 60.
*, P < 0.05 vs. Control subjects. †, P < 0.05 vs. CGN - RASB.
Figures 3B – 3E. Intrarenal Ang II and urinary AGT in patients with IgA nephropathy 61.
B. Urinary AGT levels were significantly suppressed by ARB treatments.
C–E. Representative images of immunostaining of Ang II in renal tissues from biopsy samples in patients with minor glomerular abnormality (MGA, C) and IgA nephropathy (D–E) are shown.
In the patient with IgA nephropathy, renal biopsy samples were also collected after treatment with an ARB, and Ang II immunostaining was determined. Each pair of the panels D and E comes from one IgA nephropathy patient (pretreatment; D vs. post treatment; E). Immunoreactivity of Ang II in tubules appeared to be higher in patients with IgA nephropathy than in patients with MGA. Furthermore, treatment with ARB decreased immunoreactivity of Ang II in tubules from patients with IgA nephropathy.
In order to test the hypothesis that urinary AGT provides a specific index of intrarenal RAS status in patients with IgA nephropathy, an observational study was performed 61. This paper is a survey of urine specimens from three groups: healthy volunteers, patients with IgA nephropathy and patients with minor glomerular abnormality. Patients with hypertension, diabetes, reduced glomerular filtration rate and/or who were under any medication were excluded from this study. Urinary AGT levels were not different between healthy volunteers and patients with minor glomerular abnormality. However, urinary AGT levels, renal tissue AGT expression and angiotensin II immunoreactivity were significantly higher in patients with IgA nephropathy than in patients with minor glomerular abnormality. Baseline urinary AGT levels were positively correlated with renal AGT gene expression and angiotensin II immunoreactivity but not with plasma renin activity or the urinary protein excretion rate (Figures 3B – 3E). In patients with IgA nephropathy, treatment with an ARB significantly increased renal plasma flow and decreased filtration fraction, which were associated with reductions in urinary AGT levels. These data indicate that urinary AGT is a powerful tool for determining intrarenal RAS status and associated renal derangement in patients with IgA nephropathy 61.
These data suggest that urinary AGT levels can be used as a novel biomarker of the intrarenal RAS status in patients with CKD.
Summary and Conclusion
This brief review discussed the important role of the activated intrarenal RAS in the pathogenesis of hypertension and CKD. In addition, the potential of urinary AGT as a novel biomarker of the intrarenal RAS status in hypertension and CKD was also addressed. The complicated and pleiotropic roles of an activated RAS in pathogenesis of hypertension and CKD continue to receive recognition from emerging and ongoing studies. Clearly, the use of ARBs and angiotensin converting enzyme inhibitors has become common practice in treating patients with CKD. Since RAS activation plays such a central role in the development and progression of CKD, there has been extensive interest in the potential hope for reduction in morbidity and mortality by using agents that block one or more steps in the RAS. Accordingly, the assessment of urinary AGT as an early biomarker of the status of the intrarenal RAS may be of substantial importance. It may be particularly helpful in serving as a means to determine efficacy of the treatment to reduce intrarenal angiotensin II levels.
Acknowledgments
The authors’ laboratories are supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408), the National Center for Research Resources (P20RR017659), the National Heart, Lung, and Blood Institute (R01HL026371), and American Heart Association Grant-in-Aid (10GRNT3020018).
References
- 1.Kobori H, Nangaku M, Navar LG, et al. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–87. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–8. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 3.Baltatu O, Silva JA, Jr, Ganten D, et al. The brain renin-angiotensin system modulates angiotensin II-induced hypertension and cardiac hypertrophy. Hypertension. 2000;35:409–2. doi: 10.1161/01.hyp.35.1.409. [DOI] [PubMed] [Google Scholar]
- 4.Dell’Italia LJ, Meng QC, Balcells E, et al. Compartmentalization of angiotensin II generation in the dog heart evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–8. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzocchi G, Malendowicz LK, Markowska A, et al. Role of adrenal renin-angiotensin system in the control of aldosterone secretion in sodium-restricted rats. Am J Physiol Endocrinol Metab. 2000;278:E1027–30. doi: 10.1152/ajpendo.2000.278.6.E1027. [DOI] [PubMed] [Google Scholar]
- 6.Danser AH, Admiraal PJ, Derkx FH, et al. Angiotensin i-to-II conversion in the human renal vascular bed. J Hypertens. 1998;16:2051–6. doi: 10.1097/00004872-199816121-00029. [DOI] [PubMed] [Google Scholar]
- 7.Griendling KK, Minieri CA, Ollerenshaw JD, et al. Angiotensin II stimulates nadh and nadph oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–8. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 8.Navar LG, Harrison-Bernard LM, Nishiyama A, et al. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–22. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingelfinger JR, Pratt RE, Ellison K, et al. Sodium regulation of angiotensinogen mRNA expression in rat kidney cortex and medulla. J Clin Invest. 1986;78:1311–5. doi: 10.1172/JCI112716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richoux JP, Cordonnier JL, Bouhnik J, et al. Immunocytochemical localization of angiotensinogen in rat liver and kidney. Cell Tissue Res. 1983;233:439–51. doi: 10.1007/BF00238309. [DOI] [PubMed] [Google Scholar]
- 11.Ingelfinger JR, Zuo WM, Fon EA, et al. In situ hybridization evidence for angiotensinogen messenger rna in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–23. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terada Y, Tomita K, Nonoguchi H, et al. Pcr localization of angiotensin II receptor and angiotensinogen mrnas in rat kidney. Kidney Int. 1993;43:1251–9. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–9. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 15.Rohrwasser A, Morgan T, Dillon HF, et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–74. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 16.Lantelme P, Rohrwasser A, Gociman B, et al. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39:1007–14. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 17.Moe OW, Ujiie K, Star RA, et al. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–9. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casarini DE, Boim MA, Stella RC, et al. Angiotensin i-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol. 1997;272:F405–9. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 19.Komlosi P, Fuson AL, Fintha A, et al. Angiotensin i conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–9. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 20.Gould AB, Green D. Kinetics of the human renin and human substrate reaction. Cardiovasc Res. 1971;5:86–9. doi: 10.1093/cvr/5.1.86. [DOI] [PubMed] [Google Scholar]
- 21.Brasier AR, Li J. Mechanisms for inducible control of angiotensinogen gene transcription. Hypertension. 1996;27:465–75. doi: 10.1161/01.hyp.27.3.465. [DOI] [PubMed] [Google Scholar]
- 22.Ding Y, Davisson RL, Hardy DO, et al. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem. 1997;272:28142–48. doi: 10.1074/jbc.272.44.28142. [DOI] [PubMed] [Google Scholar]
- 23.Kimura S, Mullins JJ, Bunnemann B, et al. High blood pressure in transgenic mice carrying the rat angiotensinogen gene. EMBO J. 1992;11:821–7. doi: 10.1002/j.1460-2075.1992.tb05119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukamizu A, Sugimura K, Takimoto E, et al. Chimeric renin-angiotensin system demonstrates sustained increase in blood pressure of transgenic mice carrying both human renin and human angiotensinogen genes. J Biol Chem. 1993;268:11617–21. [PubMed] [Google Scholar]
- 25.Bohlender J, Menard J, Ganten D, et al. Angiotensinogen concentrations and renin clearance: Implications for blood pressure regulation. Hypertension. 2000;35:780–6. doi: 10.1161/01.hyp.35.3.780. [DOI] [PubMed] [Google Scholar]
- 26.Smithies O. Theodore cooper memorial lecture. A mouse view of hypertension. Hypertension. 1997;30:1318–24. doi: 10.1161/01.hyp.30.6.1318. [DOI] [PubMed] [Google Scholar]
- 27.Merrill DC, Thompson MW, Carney CL, et al. Chronic hypertension and altered baroreflex responses in transgenic mice containing the human renin and human angiotensinogen genes. J Clin Invest. 1996;97:1047–55. doi: 10.1172/JCI118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobori H, Ozawa Y, Satou R, et al. Kidney-specific enhancement of ang II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293:F938–45. doi: 10.1152/ajprenal.00146.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sachetelli S, Liu Q, Zhang SL, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69:1016–23. doi: 10.1038/sj.ki.5000210. [DOI] [PubMed] [Google Scholar]
- 30.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol. 2004;286:F965–71. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 31.Smithies O, Kim HS. Targeted gene duplication and disruption for analyzing quantitative genetic traits in mice. Proc Natl Acad Sci U S A. 1994;91:3612–5. doi: 10.1073/pnas.91.9.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HS, Krege JH, Kluckman KD, et al. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci U S A. 1995;92:2735–9. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue I, Nakajima T, Williams CS, et al. A nucleotide substitution in the promoter of human angiotensinogen is associated with essential hypertension and affects basal transcription in vitro. J Clin Invest. 1997;99:1786–97. doi: 10.1172/JCI119343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeunemaitre X, Soubrier F, Kotelevtsev YV, et al. Molecular basis of human hypertension: Role of angiotensinogen. Cell. 1992;71:169–80. doi: 10.1016/0092-8674(92)90275-h. [DOI] [PubMed] [Google Scholar]
- 35.Zhao YY, Zhou J, Narayanan CS, et al. Role of c/a polymorphism at −20 on the expression of human angiotensinogen gene. Hypertension. 1999;33:108–15. doi: 10.1161/01.hyp.33.1.108. [DOI] [PubMed] [Google Scholar]
- 36.Ishigami T, Umemura S, Tamura K, et al. Essential hypertension and 5′ upstream core promoter region of human angiotensinogen gene. Hypertension. 1997;30:1325–30. doi: 10.1161/01.hyp.30.6.1325. [DOI] [PubMed] [Google Scholar]
- 37.Schunkert H, Ingelfinger JR, Jacob H, et al. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992;263:E863–9. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 38.Kobori H, Prieto-Carrasquero MC, Ozawa Y, et al. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–32. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobori H, Nishiyama A, Harrison-Bernard LM, et al. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–9. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–85. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–35. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobori H, Nishiyama A. Effects of tempol on renal angiotensinogen production in dahl salt-sensitive rats. Biochem Biophys Res Commun. 2004;315:746–50. doi: 10.1016/j.bbrc.2004.01.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobori H, Nishiyama A, Abe Y, et al. Enhancement of intrarenal angiotensinogen in dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–7. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobori H, Ozawa Y, Suzaki Y, et al. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–80. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson S, Jung FF, Ingelfinger JR. Renal renin-angiotensin system in diabetes: Functional, immunohistochemical, and molecular biological correlations. Am J Physiol. 1993;265:F477–86. doi: 10.1152/ajprenal.1993.265.4.F477. [DOI] [PubMed] [Google Scholar]
- 46.Nagai Y, Yao L, Kobori H, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–11. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh R, Singh AK, Leehey DJ. A novel mechanism for angiotensin II formation in streptozotocin-diabetic rat glomeruli. Am J Physiol Renal Physiol. 2005;288:F1183–90. doi: 10.1152/ajprenal.00159.2003. [DOI] [PubMed] [Google Scholar]
- 48.Suzaki Y, Ozawa Y, Kobori H. Intrarenal oxidative stress and augmented angiotensinogen are precedent to renal injury in zucker diabetic fatty rats. Int J Biol Sci. 2007;3:40–6. doi: 10.7150/ijbs.3.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyata K, Ohashi N, Suzaki Y, et al. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008;35:922–7. doi: 10.1111/j.1440-1681.2008.04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leehey DJ, Singh AK, Bast JP, et al. Glomerular renin angiotensin system in streptozotocin diabetic and zucker diabetic fatty rats. Transl Res. 2008;151:208–16. doi: 10.1016/j.trsl.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Kobori H, Katsurada A, Ozawa Y, et al. Enhanced intrarenal oxidative stress and angiotensinogen in IgA nephropathy patients. Biochem Biophys Res Commun. 2007;358:156–63. doi: 10.1016/j.bbrc.2007.04.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takamatsu M, Urushihara M, Kondo S, et al. Glomerular angiotensinogen protein is enhanced in pediatric IgA nephropathy. Pediatr Nephrol. 2008;23:1257–67. doi: 10.1007/s00467-008-0801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kobori H, Ozawa Y, Suzaki Y, et al. Young scholars award lecture: Intratubular angiotensinogen in hypertension and kidney diseases. Am J Hypertens. 2006;19:541–50. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katsurada A, Hagiwara Y, Miyashita K, et al. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293:F956–60. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Navar LG, Kobori H, Prieto MC, et al. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57:355–62. doi: 10.1161/HYPERTENSIONAHA.110.163519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobori H, Alper AB, Shenava R, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–50. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobori H, Urushihara M, Xu JH, et al. Urinary angiotensinogen is correlated with blood pressure in men (bogalusa heart study) J Hypertens. 2010;28:1422–28. doi: 10.1097/HJH.0b013e3283392673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobori H, Ohashi N, Katsurada A, et al. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–54. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiyomoto H, Kobori H, Nishiyama A. Chapter 4: Renin-angiotensin system in the kidney and oxidative stress: Local renin-angiotensin-aldosterone system and nadph oxidase-dependent oxidative stress in the kidney. In: Miyata T, Eckardt K-U, Nangaku M, editors. Studies on renal disorders, oxidative stress in applied basic research and clinical practice. 1. Boston, MA: Springer Science+Business Media, LLC; 2011. pp. 71–91. [Google Scholar]
- 60.Urushihara M, Kondo S, Kagami S, et al. Urinary angiotensinogen accurately reflects intrarenal renin-angiotensin system activity. Am J Nephrol. 2010;31:318–25. doi: 10.1159/000286037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishiyama A, Konishi Y, Ohashi N, et al. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–7. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]