Abstract
A number of studies have demonstrated that co-exposure to low levels of melamine and cyanuric acid elicits renal toxicity due to the formation of melamine cyanurate crystals in the kidney nephrons. In this work, we investigated if co-exposure of rats to these compounds leads to alterations in the expression of the genes encoding kidney injury molecule 1 (KIM-1), metallopeptidase inhibitor 1 (TIMP1), clusterin, osteopontin, and neutrophil gelatinase-associated lipocalin/lipocalin 2 (NGAL), which have been proposed as urinary biomarkers for nephrotoxicity. Six-week-old male and female F344 rats were fed ad libitum a diet fortified with 0 (control), 7, 23, 69, 229, or 694 ppm melamine and cyanuric acid (co-exposure groups), 1388 ppm melamine, or 1388 ppm cyanuric acid for seven days. Histopathology and clinical chemistry examination indicated marked toxicity only in the animals exposed to the two highest combined doses of melamine and cyanuric acid. Consistent with these observations, quantitative real-time polymerase chain reaction analysis of kidney tissue indicated increased expression of all genes analyzed relative to the control in both male and female rats fed daily with 229 or 694 ppm melamine and cyanuric acid. Exposure to lower levels of both compounds or to the individual compounds did not induce gene expression changes. These data indicate that quantifying the expression levels of the selected biomarker genes constitutes a useful endpoint to assess the combined toxicity of melamine and cyanuric acid in both male and female rats.
Keywords: Melamine, Cyanuric Acid, Kidney, Gene expression, Biomarkers
Introduction
Nephrotoxicity has been traditionally assessed by histopathology and clinical chemistry. More recently, an effort has been made by the scientific community to identify biomarker proteins and genes, whose levels are specifically affected by nephrotoxicants (Davis and Kramer, 2006). This effort has been mainly driven by the evidence that urinary levels of proteins, such as KIM-1, osteopontin, NGAL, and clusterin, are often more sensitive and/or occur at earlier stages of toxicity than histopathological or clinical chemistry changes (Dieterle et al., 2010; Ozer et al., 2010). Given the non-invasive nature of these biomarkers, their potential use in the clinical environment has been considered by the U.S. Food and Drug Administration and the European Medicines Agency, which recently qualified several of these proteins as biomarkers for the detection of tubular or glomerular kidney injury in preclinical studies (Dieterle et al., 2010).
Several studies have also demonstrated renal expression changes in the genes encoding the proteins noted above in response to insult by a variety of nephrotoxicants (Ichimura et al., 2004; Kondo et al., 2009; Rached et al., 2008; Wang et al., 2008; Zhou et al., 2008). While the evaluation of the gene expression levels in kidney tissue is an invasive procedure, hampering, in principle, its translational potential, measuring changes in gene expression as a terminal endpoint presents great potential in animal studies. Compared to histopathology, gene expression assays offer several advantages, including the potential for being an earlier or more sensitive endpoint, quantitative over a wide dynamic range, suitable for high-throughput, and relatively inexpensive.
The triazines melamine and cyanuric acid, components of the industrial by-product “scrap melamine”, were found in adulterated wheat and rice gluten used in pet food that led to the illness and death of a large number of cats and dogs in 2007. While, individually, these triazines present very low toxicities, reports in the literature indicate that, when animals are exposed to mixtures of melamine and cyanuric acid, the compounds are absorbed in the GI tract, distributed systemically, precipitate in the kidney, and form nephrotoxic melamine cyanurate crystals (Dobson et al., 2008; Puschner et al., 2007); hence, the mechanism of nephrotoxicity due to co-exposure to melamine and cyanuric acid seems to be very distinct from those of other well characterized nephrotoxicants (Balakumar et al., 2010; Pabla and Dong, 2008; Dirheimer and Creppy, 1991). A correlation of the renal expression levels of known candidate biomarker genes of nephrotoxicity with the kidney toxic response induced by melamine and cyanuric acid would, thus, reinforce the usefulness of genomic biomarkers of nephrotoxicity and expand their application.
In a previous study, we reported the effect of a seven-day combined exposure to melamine and cyanuric acid in F344 rats on body and kidney weights, kidney histopathology, and serum creatinine and blood urea nitrogen (BUN) levels (Jacob et al., 2011). We found that, while dietary exposure to 1388 ppm melamine or cyanuric acid failed to induce any significant changes on the endpoints analyzed, co-exposure to mixtures of 229 or 694 ppm melamine and cyanuric acid led to a decrease in body weight, enlarged and pale-yellow kidneys, multiple kidney tubular histopathological lesions, deposition of melamine cyanurate crystals in the renal tubules, and elevated serum creatinine and BUN levels compared to control animals. The no-observable adverse-effect level (NOAEL) was established at 69 ppm melamine and cyanuric acid, equivalent to 8.6 mg/kg bw/day of each compound. In the present study, we expanded the list of endpoints to include the analysis of the expression levels of biomarker candidate genes of nephrotoxicity. We aimed to 1) determine if these biomarker genes could be used to assess nephrotoxicity induced by a combined exposure to melamine and cyanuric acid, and 2) determine if the changes in gene expression levels are more sensitive endpoints than those previously assessed.
Material and methods
Experimental design
The kidney tissue used in the current study was obtained from the same rats used to analyze the endpoints reported in Jacob et al. (2011). Briefly, six-week-old F344 rats (6 males and 6 females per dose group) were fed ad libitum for seven days NIH-41 irradiated meal fortified with 0 (control), 7, 23, 69, 229, or 694 ppm melamine and cyanuric acid (co-exposure groups), 1388 ppm melamine only, or 1388 ppm cyanuric acid only. This dosing regimen resulted in the exposure of the animals to, respectively, ca. 0, 0.9, 2.8, 8.6, 17.6, or 29.8 mg melamine and cyanuric acid/kg bw/day, 123.7 mg melamine/kg bw/day, or 167.5 mg cyanuric acid/kg bw/day (Jacob et al., 2011). At necropsy, both kidneys were removed, weighed, sectioned longitudinally, and one half of each kidney was flash-frozen and stored at −80ºC until further processing. All procedures involving care and handling of animals were reviewed and approved by the National Center for Toxicological Research Institutional Animal Care and Use Committee.
Total RNA isolation and reverse-transcription
Total RNA was isolated from half of a kidney per animal. The frozen kidney tissues were macerated in liquid nitrogen using a mortar and pestle, and 10–20 mg samples of frozen tissue powder were used to isolate total RNA using an RNeasy Mini kit, with on-column DNase I digestion, following the manufacturer’s protocol (Qiagen, Valencia, CA). RNA purity and concentration were assessed using a Nanodrop 1000 spectrophotometer (Thermo Scientific, Waltham, MA) and the RNA integrity was assessed using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA). All RNA samples had an absorption 260 nm/280 nm ratio greater than 2.0 and the RNA integrity number (RIN) was 9.19 ± 0.59 (mean ± standard deviation (SD); n = 96). One microgram of total RNA was reverse transcribed using random hexamer primers and an Advantage RT-for-PCR kit (Clontech, Palo Alto, CA), following the manufacturer’s protocol. The cDNA was diluted 1:40 with nuclease-free water and stored at −20ºC.
Quantitative gene expression analysis
The gene expression levels of the biomarkers of nephrotoxiticy were quantified using quantitative real-time polymerase chain reaction (PCR). cDNAs were amplified in duplicate using TaqMan assays and a 7900HT real-time PCR detection system (Applied Biosystems, Foster City, CA), following the manufacturer’s protocol. The TaqMan assays used were Rn00597703_m1 (Kim-1/Havcr1, NM_173149; encodes KIM-1), Rn01430875_g1 (Timp1, NM_053819; encodes TIMP1), Rn01449972_m1 (Spp1, NM_012881; encodes osteopontin), Rn00562081_m1 (Clu, NM_053021; encodes clusterin), Rn00590612_m1 (Lcn2, NM_130741; encodes NGAL). A FastStart master mix (Roche Diagnostics, Indianapolis, IN) was used, and the quantitative real-time PCR cycling conditions were set at 95ºC for 10 min for the first cycle and 15 seconds at 95ºC followed by 1 min at 60ºC for the remaining 40 cycles. The coefficient of variation between technical replicates was < 1%. Data were normalized for the endogenous control Gapdh (NM_017008; assay part # 4352338E) and analyzed using the ΔΔCt method (Livak and Schmittgen, 2001). To express the relative gene expression levels as a %GAPDH, the cycle threshold (Ct) values of the gene of interest were subtracted with that of Gapdh (ΔCt ) and the 2-ΔCt × 100 value was calculated. Gapdh levels had been determined in preliminary absolute quantitation experiments to be unchanged with treatment (data not shown). Data are expressed as mean ± SD.
Benchmark dose modeling
Benchmark doses (BMDs) and the lower 95% confidence limits (BMDLs) were calculated using Environmental Protection Agency Benchmark Dose Software (version 2.1.1; http://www.epa.gov/ncea/bmds). The calculations were conducted using Hill, linear, polynomial, and power models to fit the relative gene expression levels of Kim-1/Havcr 1, Timp1, Clu, Lcn2, and Spp1 (mean ± SD; n = 6) and the mean doses of melamine and cyanuric acid from the entire seven-day study. The BMD was defined as the dose corresponding to a change in the mean response equal to one control standard deviation from the control mean.
Statistical analysis
Statistical significance between male and female control animals was assessed by t-tests. Statistical significance between dose groups within each sex was assessed by one-way ANOVA, followed by Dunnett’s test to compare treated groups to the matching control (SigmaStat v3.11). In order to maintain an equal variance and normal data distribution, the data were natural log transformed before conducting the analyses. A p-value < 0.05 was considered statistically significant.
Results and Discussion
Table 1 summarizes the results previously reported by our group in the same set of animals used for the current study (Jacob et al., 2011). All endpoints analyzed, which included body weight, kidney weight, histopathology, and blood chemistry, indicated that feeding six-week-old male and female F344 rats 229 or 694 ppm melamine and cyanuric acid for seven days resulted in alterations consistent with nephrotoxicity. Rats co-exposed to lower levels of melamine and cyanuric acid or exposed to melamine or cyanuric acid only showed no evidence of nephrotoxicity. Based on these endpoints, the NOAEL for kidney toxicity in the F344 rats was set at 69 ppm melamine and cyanuric acid in feed, which was equivalent to 8.6 mg/kg bw/day of each compound. BMD modeling was also conducted on these endpoints; kidney weights were the most sensitive endpoints modeled and afforded BMDLs of 8.4–10.9 mg/kg bw/day (Jacob et al., 2011).
Table 1.
Summary of the results reported in Jacob et al. (2011). Animals were exposed to feed supplemented with 0 (control), 7, 23, 69, 229, or 694 ppm melamine and cyanuric acid (co-exposure groups), 1388 ppm melamine, or 1388 ppm of cyanuric acid for seven days. All endpoints analyzed were affected by combined melamine and cyanuric acid, at a dose level greater than 69 ppm (NOAEL).
| Endpoint | Observations per dose group |
|---|---|
| Body Weight | ↔ 0, 7, 23, 69 ppm MEL + CYA |
| ↓ 229, 694 ppm MEL + CYA | |
| ↔ 1388 ppm MEL | |
| ↔ 1388 ppm CYA | |
|
| |
| Kidney Weight | ↔ 0, 7, 23, 69 ppm MEL + CYA |
| ↑ 229, 694 ppm MEL + CYA | |
| ↔ 1388 ppm MEL | |
| ↔ 1388 ppm CYA | |
|
| |
| Kidney Crystals | − 0, 7, 23, 69 ppm MEL + CYA |
| + 229, 694 ppm MEL + CYA | |
| + 1388 ppm MEL1 | |
| − 1388 ppm CYA | |
|
| |
| Urinary Bladder Uroliths and Crystals | − 0, 7, 23, 69 ppm MEL + CYA |
| + 229, 694 ppm MEL + CYA | |
| − 1388 ppm MEL | |
| − 1388 ppm CYA | |
|
| |
| Serum creatinine and BUN | ↔ 0, 7, 23, 69 ppm MEL + CYA |
| ↑229, 694 ppm MEL + CYA | |
| ↔ 1388 ppm MEL | |
| ↔ 1388 ppm CYA | |
Note. Abbreviations: ↔ No change; ↓ Decreased; ↑ Increased (relative to control). − Absent; + Present. MEL + CYA: co-exposure to melamine and cyanuric acid. MEL: exposure to melamine only. CYA: exposure to cyanuric acid only.
A very small number of dispersed crystals was observed in 5 of the 12 rats, when the kidneys were examined by wet mount, but not by histopathology (Jacob et al., 2011).
In the present study, we evaluated the use of quantitative expression analysis of known candidate biomarker genes as a tool to assess nephrotoxicity induced by a combined exposure to melamine and cyanuric acid. Kidneys from both male and female rats were analyzed. The use of five doses, two below the NOAEL and two above the NOAEL, allowed assessment of the shape of the dose-response curve. Furthermore, kidneys from rats treated with melamine only or cyanuric acid only were also analyzed to characterize further the effect of the individual compounds in the kidney.
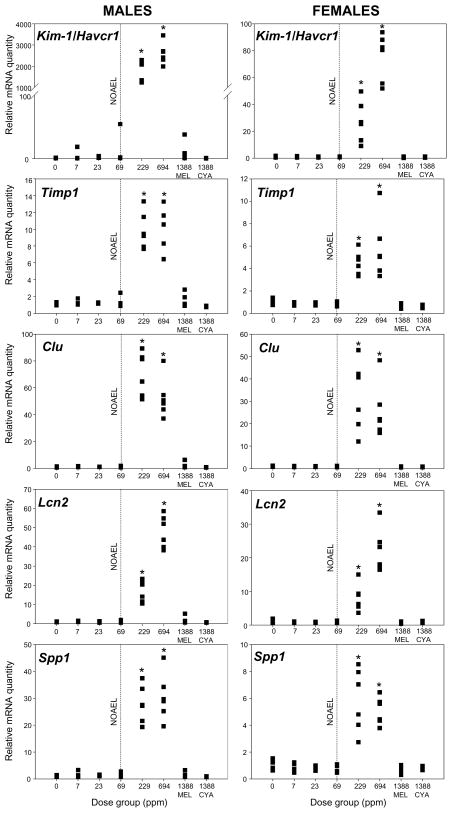
The expression of all genes analyzed, which included Kim-1/Havcr1, Timp1, Lcn2, Clu, and Spp1, was significantly up-regulated in the kidneys of both male and female rats exposed to 229 or 694 ppm melamine and cyanuric acid (Figure 1). There were no statistically significant gene expression changes, compared to the matching control rats, in either male or female rats treated with 69 ppm or less of combined melamine and cyanuric acid, or with 1388 ppm melamine only or cyanuric acid only. From these data, a NOAEL of 69 ppm (8.6 mg/kg bw/day) was established for combined melamine and cyanuric acid, which matched the previously reported NOAEL (Jacob et al., 2011). To investigate further the relative sensitivity of gene expression analysis versus the endpoints previously considered in this study, BMD modeling was also conducted, using the mean doses of melamine and cyanuric acid from the entire seven-day study and the resultant changes in relative gene expression (Table 2). The best fits were obtained with the Hill model, which led to BMDL values of 8.7–9.8 mg/kg bw/day, depending on the specific gene and sex of the animal. These values are in excellent agreement with the BMDLs for kidney weight (BMDL 8.4–10.9 mg/kg bw/day), the most sensitive endpoint previously reported. While other studies have reported gene expression changes to be a more sensitive endpoint than histopathology and/or clinical chemistry (Ichimura et al., 2004; Kondo et al., 2009; Rached et al., 2008; Zhou et al., 2008), we hypothesize that our observations may simply reflect the fact that, at exposures below 229 ppm melamine and cyanuric acid in feed, the threshold of crystallization of melamine cyanurate was not attained. Without crystal formation and consequent organ insult, alterations in gene expression levels, or any of the other endpoints, are not expected. Future studies, with a closer dose-spacing capable of affording a more continuous dose-effect, will be required to evaluate fully the relative sensitivity of these endpoints.
Figure 1.
Relative gene expression levels of biomarkers of nephrotoxicity (n = 6 per treatment group per sex) in kidneys of male and female F344 rats. Six-week-old animals were exposed to feed supplemented with 0 (control), 7, 23, 69, 229, or 694 ppm melamine and cyanuric acid (co-exposure groups), 1388 ppm melamine, or 1388 ppm cyanuric acid for seven days. Data were normalized for the endogenous control Gapdh and for the mean expression level of the matching (same sex) control by the ΔΔCt method; the control mean expression was normalized to 1. Left panel: male rats, right panel: female rats. Dashed line indicates the NOAEL as determined in Jacob et al. (2011). * indicates a significant group difference relative to the matching control (p-value < 0.05, one-way ANOVA followed by Dunnett’s test). All genes analyzed were significantly up-regulated in both male and female rats at dose levels higher than 69 ppm combined melamine and cyanuric acid.
Table 2.
Results of benchmark dose modeling for the relative gene expression levels of biomarkers of nephrotoxicity in kidneys of seven-week-old male and female F344 ratsa.
| Gene | Sex | Model | AICb | GOFc | BMDd | BMDLd |
|---|---|---|---|---|---|---|
| Kim-1/Havcr1 | Male | Hill | 456.8 | >0.999 | 13.5 | 9.2 |
| Linear | 472.2 | 0.0007 | 4.3 | 3.5 | ||
| Polynomial | 467.4 | 0.006 | 7.2 | 4.8 | ||
| Power | 465.9 | 0.011 | 7.6 | 5.4 | ||
| Female | Hill | 201.2 | 0.997 | 16.2 | 9.7 | |
| Linear | 221.9 | <0.0001 | 5.0 | 4.0 | ||
| Polynomial | 201.0 | 0.601 | 11.6 | 8.3 | ||
| Power | 201.4 | 0.527 | 10.8 | 8.4 | ||
| Timp1 | Male | Hill | 66.9 | 0.967 | 10.2 | 8.7 |
| Linear | 99.3 | <0.0001 | 6.6 | 5.2 | ||
| Polynomial | 99.4 | <0.0001 | 4.5 | 3.1 | ||
| Power | 99.3 | <0.0001 | 6.6 | 5.2 | ||
| Female | Hill | 50.0 | 0.984 | 15.5 | 9.4 | |
| Linear | 58.5 | 0.031 | 6.9 | 5.5 | ||
| Polynomial | 60.2 | 0.015 | 8.0 | 4.8 | ||
| Power | 59.6 | 0.021 | 9.0 | 5.7 | ||
| Clu | Male | Hill | 207.3 | 0.006 | 10.3 | 8.7 |
| Linear | 249.7 | <0.0001 | 7.9 | 6.2 | ||
| Polynomial | 246.1 | <0.0001 | 3.9 | 2.7 | ||
| Power | 249.7 | <0.0001 | 7.9 | 6.2 | ||
| Female | Hill | 189.4 | 0.481 | 10.7 | 8.8 | |
| Linear | 208.5 | <0.0001 | 9.5 | 7.2 | ||
| Polynomial | 207.7 | 0.0001 | 5.3 | 3.5 | ||
| Power | 208.5 | <0.0001 | 9.5 | 7.2 | ||
| Lcn2 | Male | Hill | 139.7 | >0.999 | 15.9 | 9.6 |
| Linear | 178.7 | <0.0001 | 4.4 | 3.5 | ||
| Polynomial | 143.0 | 0.354 | 10.5 | 7.9 | ||
| Power | 143.8 | 0.248 | 9.5 | 7.7 | ||
| Female | Hill | 120.8 | 0.970 | 15.2 | 9.8 | |
| Linear | 137.7 | 0.0003 | 5.4 | 4.4 | ||
| Polynomial | 119.9 | 0.777 | 12.6 | 8.9 | ||
| Power | 120.2 | 0.691 | 11.7 | 8.9 | ||
| Spp1 | Male | Hill | 148.6 | 0.987 | 12.1 | 8.9 |
| Linear | 175.9 | <0.0001 | 5.6 | 4.5 | ||
| Polynomial | 177.4 | <0.0001 | 4.7 | 3.2 | ||
| Power | 177.8 | <0.0001 | 6.0 | 4.5 | ||
| Female | Hill | 44.2 | 0.506 | 10.6 | 8.8 | |
| Linear | 69.3 | <0.0001 | 8.3 | 6.4 | ||
| Polynomial | 69.1 | <0.0001 | 5.1 | 3.4 | ||
| Power | 69.3 | <0.0001 | 8.3 | 6.4 |
Benchmark doses (BMDs) and the lower 95% confidence limits (BMDLs) were calculated using Environmental Protection Agency Benchmark Dose Software (version 2.1.1; http://www.epa.gov/ncea/bmds). The calculations were conducted using Hill, linear, polynomial, and power models to fit the relative gene expression levels of Kim-1/Havcr1, Timp1, Clu, Lcn2, and Spp1 (mean ± SD; n = 6) and the mean doses of melamine and cyanuric acid from the entire seven-day study. The BMD was defined as the dose corresponding to a change in the mean response equal to one control standard deviation from the control mean.
AIC, Akaike information criterion.
GOF, Goodness of fit.
Relative gene expression.
Kim-1/Havcr1 was the most up-regulated gene, following exposure to 694 ppm melamine and cyanuric acid, with the gene expression fold-change being >2500 in males and >75 in females, compared to the matching (same sex) control. Both Kim-1/Havcr1 and Lcn2 were up-regulated in a dose-dependent fashion. A similar dose-dependent pattern was reported for Kim-1/Havcr1 gene expression in male rats exposed to different dose-levels of known nephrotoxicants (Zhou et al., 2008; Chiusolo et al., 2010). On the other hand, the expression levels of Timp1, Clu, and Spp1 yielded a more dichotomous response, suggesting that an expression plateau had been reached for these genes at the 229 ppm melamine and cyanuric dose level. Rached et al. (2008) reported a dose-dependent up-regulation of Kim-1/Havcr1, Lcn2, Timp1, Clu, and Spp1 in male F344 rats, in response to a 90-day treatment with the nephrotoxicant ochratoxin A; however, the in vivo fold-changes were smaller than the ones reported here, suggesting that even the highest ochratoxin A dose level used in that study did not maximally induce expression of the biomarker genes.
To the best of our knowledge, the current study is the first to assess gene expression changes induced by a nephrotoxicant in both male and female rats. As shown in Figure 1, the up-regulation of genes in response to co-exposure to melamine and cyanuric acid is consistently higher in males than in female rats; however, this may not necessarily indicate that males are more sensitive than females to the melamine and cyanuric acid co-exposure. Comparison of the Gapdh-corrected, absolute transcript levels of control animals revealed that the constitutive expression of all biomarker genes studied was higher in females than in males (Table 3). This sex difference was most prominent for gene Kim-1/Havcr1 (>30-fold higher expression in females compared to males); thus, our data show that, unlike what was known for male rats (Ichimura et al. 1998; Vaidya et al. 2010), under our experimental conditions, seven-week-old female F344 rats constitutively express moderate levels of Kim-1/Havcr1 (Table 3). Differences in constitutive gene expression levels between males and females were also observed for Spp1 (ca. 5× more expression in females compared to males) and Timp1, Clu, and Lcn2 (all ca. 2× more expression in females compared to males). Due to this sex difference, even though Kim-1/Havcr1 was up-regulated >2500 times in males treated with 694 ppm melamine and cyanuric acid when compared with the male controls, and up-regulated >75 times in females treated with 694 ppm melamine and cyanuric acid when compared with the female controls, the maximum absolute amount of Kim-1/Havcr1 transcripts present per unit of tissue was similar in males and females. The same holds true for the other four genes analyzed; that is, the maximum absolute amount of transcript accumulated per cell, due to co-exposure to melamine and cyanuric acid, was similar in both male and female rats. These observations further suggest that an expression plateau of genes Timp1, Clu, and Spp1 was reached upon co-exposure to 229 ppm melamine and cyanuric acid, precluding an additional up-regulation upon co-exposure to higher levels of the mixture. The existence of a maximal level of expression of these genes had previously been speculated upon by Rached et al. (2008).
Table 3.
Gene expression level of candidate biomarkers of nephrotoxicity in the kidney of seven-week-old control (untreated) male and female F344 rats. Data are expressed as % Gapdh, except Gapdh, which is expressed as cycle threshold (Ct) value (mean ± SD).
| Gene | Females (F) | Males (M) | Fold-expression ratio (F/M) |
|---|---|---|---|
| Kim-1/Havcr1 | 1.21 ± 0.52 | 0.04 ± 0.01 | 34.53 * |
| Timp1 | 2.09 ± 0.51 | 0.94 ± 0.15 | 2.22 * |
| Clu | 10.79 ± 2.23 | 4.73 ± 1.39 | 2.28 * |
| Lcn2 | 0.02 ± 0.01 | 0.01 ± 0.00 | 2.28 * |
| Spp1 | 49.28 ± 17.97 | 9.45 ± 2.42 | 5.21 * |
| Gapdh | 22.97 ± 0.36 | 22.70 ± 0.56 | 1.01 |
indicates a significant difference between males and females (p-value < 0.05, t-test).
The very low levels of constitutive gene expression of Kim-1/Havcr1 and Lcn2 in male rats and Lcn2 in female rats may be advantageous when using these candidate genes as biomarkers of nephrotoxicity, since it may result in a higher sensitivity to detect expression changes; however, the extremely low basal expression of Lcn2 in F344 rats may be a strain-specific characteristic, since Rached et al. (2008) detected lipocalin-2/NGAL protein in the urine of untreated male Sprague-Dawley and Wistar rats, but failed to detect it in untreated male F344 rats.
Conclusions
In the current study, we investigated previously known candidate biomarker genes of nephrotoxicity to assess the renal injury induced by co-exposure to melamine and cyanuric acid in male and female F344 rats. Our results show that alterations in the expression of these genes afforded NOAEL and BMDL values consistent with those previously reported. These results demonstrate that the transcriptional analyses of KIM-1, TIMP1, clusterin, osteopontin, and NGAL constitute additional sensitive endpoints to assess nephrotoxicity induced by co-exposure to melamine and cyanuric acids in rats. In contrast with other previously studied biochemical nephrotoxicants, the putative mode of action of combined melamine and cyanuric acid is based on their ability to co-precipitate and physically block the lumen of the nephrons; hence, our results expand the range of nephrotoxicant mechanisms capable of being assessed by transcriptional analysis, reinforcing the usefulness of these complementary endpoints in toxicological studies. Future studies on the combined toxicity of melamine and cyanuric acid should include the quantification of the kidney gene expression of these biomarkers of nephrotoxicity, and investigate if the products of these candidate biomarker genes are altered in the urine upon co-exposure to melamine and cyanuric acid. Such analyses should further expand our knowledge on the use of non-invasive urinary biomarkers to assess renal toxicity and on the translational potential of these biomarkers in humans.
Highlights.
Rats fed more than 69 ppm melamine and cyanuric acid for 7 days show renal toxicity
Candidate biomarkers of nephrotoxicity genes were up-regulated at these same doses
Quantification of these transcripts is a sensitive endpoint to assess toxicity
Acknowledgments
This work was partly supported by Interagency Agreement No. 224-07-0007/Y1ES1027 between the National Center for Toxicological Research/Food and Drug Administration and the National Institute of Environmental Health Sciences/National Toxicology Program. K.P.K. was supported by an appointment to the Research Participation Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration. The opinions expressed in this paper do not necessarily represent those of the U.S. Food and Drug Administration.
Abbreviations
- BMD
benchmark dose
- BMDL
benchmark dose (lower 95% confidence limit)
- BUN
blood urea nitrogen
- Ct
cycle threshold
- CYA
cyanuric acid
- KIM-1
kidney injury molecule 1
- MEL
melamine
- NGAL
neutrophil gelatinase-associated lipocalin/lipocalin 2
- NOAEL
no-observable-adverse-effect level
- PCR
polymerase chain reaction
- TIMP1
metallopeptidase inhibitor 1
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62:179–86. doi: 10.1016/j.phrs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Chiusolo A, Defazio R, Zanetti E, Mongillo M, Mori N, Cristofori P, Trevisan A. Kidney injury molecule-1 expression in rat proximal tubule after treatment with segment-specific nephrotoxicants: a tool for early screening of potential kidney toxicity. Toxicol Pathol. 2010;38:338–45. doi: 10.1177/0192623310362244. [DOI] [PubMed] [Google Scholar]
- Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, Sultana S, Gerhold DL, Phillips JA, Maurer G, Carl K, Laurie D, Harpur E, Sonee M, Ennulat D, Holder D, Andrews-Cleavenger D, Gu YZ, Thompson KL, Goering PL, Vidal JM, Abadie E, Maciulaitis R, Jacobson-Kram D, Defelice AF, Hausner EA, Blank M, Thompson A, Harlow P, Throckmorton D, Xiao S, Xu N, Taylor W, Vamvakas S, Flamion B, Lima BS, Kasper P, Pasanen M, Prasad K, Troth S, Bounous D, Robinson-Gravatt D, Betton G, Davis MA, Akunda J, McDuffie JE, Suter L, Obert L, Guffroy M, Pinches M, Jayadev S, Blomme EA, Beushausen SA, Barlow VG, Collins N, Waring J, Honor D, Snook S, Lee J, Rossi P, Walker E, Mattes W. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol. 2010;28:455–62. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- Dirheimer G, Creppy EE. Mechanism of action of ochratoxin A. IARC Sci Publ. 1991;115:171–86. [PubMed] [Google Scholar]
- Dobson RLM, Motlagh S, Quijano M, Cambron RT, Baker TR, Pullen AM, Regg BT, Bigalow-Kern AS, Vennard T, Fix A, Reimschuessel R, Overmann G, Shan Y, Daston GP. Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicol Sci. 2008;106:251–62. doi: 10.1093/toxsci/kfn160. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–63. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–42. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- Jacob CC, Reimschuessel R, Von Tungeln LS, Olson GR, Warbritton AR, Hattan DG, Beland FA, Gamboa da Costa G. Dose-response assessment of nephrotoxicity from a 7-day combined-exposure to melamine and cyanuric acid in F344 rats. Toxicol Sci. 2011;119:391–7. doi: 10.1093/toxsci/kfq333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo C, Minowa Y, Uehara T, Okuno Y, Nakatsu N, Ono A, Maruyama T, Kato I, Yamate J, Yamada H, Ohno Y, Urushidani T. Identification of genomic biomarkers for concurrent diagnosis of drug-induced renal tubular injury using a large-scale toxicogenomics database. Toxicology. 2009;265:15–26. doi: 10.1016/j.tox.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, Staedtler F, Mahl A, Grenet O, Roth DR, Wahl D, Legay F, Holder D, Erdos Z, Vlasakova K, Jin H, Yu Y, Muniappa N, Forest T, Clouse HK, Reynolds S, Bailey WJ, Thudium DT, Topper MJ, Skopek TR, Sina JF, Glaab WE, Vonderscher J, Maurer G, Chibout SD, Sistare FD, Gerhold DL. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol. 2010;28:486–94. doi: 10.1038/nbt.1627. [DOI] [PubMed] [Google Scholar]
- Pabla N, Dong Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- Puschner B, Poppenga RH, Lowenstine LJ, Filigenzi MS, Pesavento PA. Assessment of melamine and cyanuric acid toxicity in cats. J Vet Diagn Invest. 2007;19:616–24. doi: 10.1177/104063870701900602. [DOI] [PubMed] [Google Scholar]
- Rached E, Hoffmann D, Blumbach K, Weber K, Dekant W, Mally A. Evaluation of putative biomarkers of nephrotoxicity after exposure to ochratoxin A in vivo and in vitro. Toxicol Sci. 2008;103:371–81. doi: 10.1093/toxsci/kfn040. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–85. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EJ, Snyder RD, Fielden MR, Smith RJ, Gu YZ. Validation of putative genomic biomarkers of nephrotoxicity in rats. Toxicology. 2008;246:91–100. doi: 10.1016/j.tox.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Vaidya VS, Brown RP, Zhang J, Rosenzweig BA, Thompson KL, Miller TJ, Bonventre JV, Goering PL. Comparison of kidney injury molecule-1 and other nephrotoxicity biomarkers in urine and kidney following acute exposure to gentamicin, mercury, and chromium. Toxicol Sci. 2008;101:159–70. doi: 10.1093/toxsci/kfm260. [DOI] [PMC free article] [PubMed] [Google Scholar]