Abstract
In 2008, the National Malaria Control Program in Benin implemented a vector control intervention based on indoor residual spraying (IRS). Four districts of high resistance of Anopheles gambiae to pyrethroids were sprayed with bendiocarb. More than 350,000 inhabitants have been protected. Entomologic parameters in the control area were compared with those in intervention sites. The study has shown a drastic decrease in the An. gambiae biting rate in the sprayed areas. Results of an enzyme-linked immunosorbent assay were negative for Plasmodium falciparum antigen during the entire period of the intervention. No household members received infected bites (entomologic inoculation rate = 0 during January–July). Parous rates were low in areas covered by IRS because bendiocarb is not conducive to long-term mosquito survival. Bendiocarb was found to be a good alternative insecticide for IRS in Benin, in areas where An. gambiae has developed high resistance to pyrethroids.
Introduction
Malaria is a major public health problem in Africa. It is the leading cause of morbidity and mortality and of loss of work days.1–3 Two methods are currently used to control this disease: indoor residual spraying (IRS) of insecticides and use of insecticide treated nets (ITNs). In sub-Sahara Africa and southern Asia, these two methods have shown good results,4,5 but they have their drawbacks.
The main problem with ITNs and IRS is the development of insecticide resistance, particularly pyrethroid-resistance by several populations of Anopheles gambiae.6–9 In the past decade, the emergence of resistance in populations of An. gambiae to common classes of insecticides used in public health has been reported in many countries in Africa, including Côte d'Ivoire,6 Kenya,10 Benin,11,12 Niger,13 Burkina Faso,14 Mali,15 Nigeria,16 South Africa17 and Cameroon.18 In addition, N'Guessan and others19 have demonstrated a decrease in the efficacy of treated nets and IRS using pyrethroids against An. gambiae in a peri-urban area of Benin, where this species has developed high-level resistance to permethrin. In recent reports,20,21 widespread distribution of pyrethrinoid resistance in An. gambiae was shown in southern Benin, and there was a significant increase level of the kdr mutation, which remains the major resistance mechanism detected. A lowest frequency of Ace-1R was recorded during the same study and may be a sign of encouragement to use carbamates or organophosphates as alternative insecticides to pyrethroids for IRS in Benin.
In another study,22 which included four months evaluation of various insecticides in experimental huts, three insecticides (Sumithion 40 WP [Fenitrothion]; Master Quick ZC [mixture of chlorpyriphos, 250 g/L plus deltamethrin, 12 g/L]; and Ficam M [bendiocarb, 800 g/kg]) were effective against pyrethroid-resistant Anopheles. However, bendiocarb is the only product that the National Malaria Control Program (NMCP) has selected for the implementation of IRS in Benin because the Master Quick ZC formulation is not approved by the World Health Organization Pesticide Evaluation Scheme for its use. With regard to Sumithion 40 WP, doubts were raised on its safety in terms of its secondary effects and odor. This study was conducted at the experimental hut level and it is difficult to extrapolate from these results what might happen at a larger-scale community level.22
The present study aims to evaluate the entomologic impact after large-scale implementation of IRS with bendiocarb in areas of high resistance to pyrethroids in An. gambiae. The NMCP in Africa has usually implemented adapted strategies. However, it is widely recognized that evaluation and routine vector surveillance is a weak component of many national disease control programs. Therefore, it is important to assess the efficiency of the vector control strategies in areas where mosquitoes have developed high-level resistance to pyrethroids.
Materials and Methods
Intervention areas.
The study was conducted in four districts of Oueme Department (Adjohoun, Dangbo, Seme, and Misserete) in Benin (Figure 1). The first three districts are characterized by the presence of two types of environment. The first environment is a plateau zone situated far from flooding areas. In this study, this zone is referred to as the plateau area or IRS arm. In the plateau area, mosquito breeding sites are created, particularly during the rainy seasons; more than 90% of households have been treated with bendiocarb at a dose of 400 mg/m2. The second environment is represented by a swampy zone on the border of the Oueme River and Lake Nokoue. This zone is referred to as the swampy area or long-lasting insecticide-impregnated net (LLIN) arm.
Figure 1.
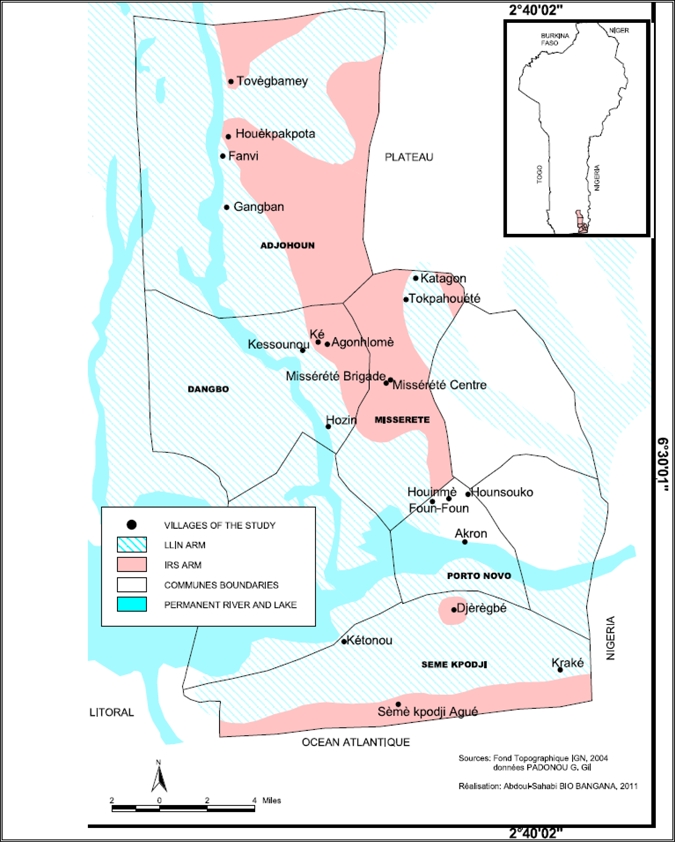
Map of the study area in Benin.
During August–November, the Oueme River and Lake Nokoue, enlarged by rains, overflow their banks and invade the surrounding zones with floodwaters during three months (September–October) every year. In the swampy zone, IRS was not implemented because of the presence of the two bodies of water, which could be at risk for contamination by insecticides. Therefore, LLINs were distributed to these households in this area, and particular attention was given to children less then five years of age and pregnant women. More than five kilometers separate the plateau and swampy areas. This distance is sufficient to avoid the movement of mosquitoes on both sides. However, the human population density is high in both areas, and mosquitoes do not need to travel far to feed.
Control area.
In a context where universal access to LLINs was promoted, it was not easy to find a good control area. However, Akron, an area that presents the same ecological and geographic characteristics as the four districts mentioned above was chosen as the control area. Akron is a peripheral area of Porto-Novo, the administrative capital of Benin, also in Oueme Department (Figure 1). Before IRS implementation and the free distribution of LLINs, a baseline study was carried out in the four districts selected as intervention sites to collect entomologic parameters. The baseline data is shown for a comparison before and after interventions.
IRS with bendiocarb in the plateau areas (IRS arm).
Bendiocarb was selected for spraying on the walls. Studies have shown that this insecticide was efficient in phase II evaluations against malaria vectors.22,23 The application dose was 0.4 g/m2 of bendiocarb on walls of houses. Two rounds of IRS were carried out: the first in July 2008 and the second, eight months later, in March 2009. The two applications were completed by volunteers selected from the local community and trained by the Research Triangle Institute (RTI) team, the implementing partner of the U.S. Agency for International Development. According to RTI, the coverage rate was more than 90% for each of the two rounds.
LLINs distributed in the swampy areas (LLIN arm).
A total of 48,819 LLINs (Permanet 2.0; Vestergaard Frandsen, Lausanne, Switzerland) were distributed in October 2008 and May 2009 in the flood zones. They were distributed to 47,524 households. More than 90% of children less than five years of age and pregnant women received LLINs (RTI, unpublished data).
Mosquito sampling.
We evaluated anopheline and culicide biting rates inside and outside houses to identify the changes in mosquito biting behavior induced by the presence of bendiocarb on the walls or deltamethrin on the fibers of Permanet 2.0. In each district, two villages were selected per arm, and two houses were chosen per village for mosquito collection to monitor malaria transmission. Adult mosquitoes were collected twice a month by using human landing catches with one collector located inside and another outside in each village. Mosquito collections were carried out every month to monitor the dynamics of Anopheles vector density and to evaluate malaria transmission. In addition, we sampled mosquitoes using morning pyrethrum spray catches and window exit traps to evaluate the impact of interventions on exit induced by the presence of insecticides. In each intervention area, four bedrooms were selected for mosquito collection in the morning.
Laboratory processing of mosquitoes and parameters studied.
On the basis of morphologic characteristics in standard identification keys,24 all female mosquitoes belonging to An. gambiae complex were identified. Vector species were dissected by using a microscope to determine the physiological age grading (parous rates) according to the method of Détinova.25 The head and thoraxes of these females from human landing catches were tested by using an enzyme-linked immunosorbent assay according to Wirtz and others26 for the presence of circumsporozoite protein of Plasmodium falciparum. The abdomens of these females were used for identification of species and characterization of molecular forms within the An. gambiae complex. This analysis was performed using polymerase chain reaction–restriction fragment length [polymorphism analysis].27
Period of study.
The period was conducted during January 2008–December 2009 and spanned two rounds of IRS. To measure the impact of IRS on the biting rate, we compared the values indicated for the same periods, January–July 2008 before IRS and January–July 2009 after IRS. We excluded August–December 2008 and 2009 from the analysis because these two periods were those of IRS implementation, but there is no available database for these periods for before and after intervention.
Statistical analysis.
Data were analyzed by using with EPI Info version 6.0 (Centers for Disease Control and Prevention, Atlanta, GA) and SPSS version 16.0 (SPSS Inc., Chicago, IL) software. The efficiency of the intervention was tested by using analysis of variance. We calculated the percentage reduction of the human biting rate (HBR) and the entomologic inoculation rate (EIR) after intervention. The chi-square test was used to compare age grading and exit and blood feeding rates during periods before and after intervention. All tests were performed at the 5% significance level.
Ethical approval.
This study was approved by the Ministry of Health and the Center for Entomological Research of Cotonou. The voluntary mosquito collectors provided their consent before participating in the study. They were also subjected to regular medical check-ups, given preventive treatments for malaria, and vaccinated against yellow fever.
Results
Effect of IRS on HBR of An. gambiae.
During the study period, the An. gambiae HBR was 1 bite/person/night during the long dry season and 3.5–14 bites/person/night during the long rainy season in the control area. In intervention areas before IRS, the maximums HBRs (9.86 bites/person/night in Misserete2 and 15.11 bites/person/night in Seme) were observed in January–July. After IRS, the HBRs were reduced for the same period: 95.2% (2.93 to 0.14 bites/person/night) in Dangbo and 89.04% (9.86 to 1.89 bites/person/night) in Misserete2 (Table 1).
Table 1.
EIR and HBR before and after IRS intervention, Benin*
| Location | Before intervention | After intervention | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry season, January–March 2008 | Rainy season, April–July 2008 | January–July 2008 | Dry season, January–March 2009 | Rainy season, April–July 2009 | January–July 2009 | % Reduction | ||||||||
| 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | |||||||||
| Adjohoun | Total | 65 | 207 | 272 | 8 | 64 | 72 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 1.35 | –0.04 to 2.75 | 3.23 | –1.11 to 7.57 | 2.43 | 0.40–4.46 | 0.17 | –0.55 to 0.88 | 1 | –0.14 to 2.14 | 0.64 | 0.00–1.28 | 73.66 | |
| S% | 0 | 5.71 | 3.12 | 0 | 0 | 0 | ||||||||
| EIR | 0 | 0.18 | 0.07 | 0 | 0 | 0 | 100 | |||||||
| Dangbo | Total | 48 | 280 | 328 | 0 | 16 | 16 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 1 | 0.69–1.31 | 4.375 | 0.91–7.84 | 2.93 | 0.74–5.12 | 0 | 0.00–0.00 | 0.25 | –0.22 to 0.72 | 0.14 | –0.09 to 0.37 | 95.22 | |
| S% | 2.08 | 4.49 | 3.64 | 0 | 0 | 0 | ||||||||
| EIR | 0.02 | 0.19 | 0.10 | 0 | 0 | 0 | 100 | |||||||
| Misserete 1 | Total | 38 | 98 | 136 | 11 | 0 | 11 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 0.79 | 0.29–1.29 | 1.53 | 0.45–2.61 | 1.21 | 0.63–1.80 | 0.23 | –0.76 to 1.22 | 0 | 0.00–0.00 | 0.1 | –0.14 to 0.34 | 91.73 | |
| S% | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| EIR | 0 | 0 | 0 | 0 | 0 | 0 | – | |||||||
| Misserete 2 | Total | 160 | 944 | 1,104 | 0 | 121 | 121 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 3.33 | –1.37 to 8.04 | 14.75 | 3.68–25.82 | 9.86 | 2.54–17.18 | 0 | 0.00–0.00 | 1.89 | –0.40 to 4.18 | 1.08 | –0.25 to 2.41 | 89.04 | |
| S% | 0 | 1.07 | 0.71 | 0 | 0.84 | 0.84 | ||||||||
| EIR | 0 | 0.15 | 0.07 | 0 | 0.01 | 0.009 | 87.14 | |||||||
| Seme | Total | 100 | 1,592 | 1,692 | 29 | 507 | 536 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 2.08 | –1.34 to 5.50 | 24.88 | –2.11 to 51.86 | 15.11 | –0.72 to 30.9.3 | 0.6 | –0.33 to 1.54 | 7.92 | –6.65 to 22.49 | 4.79 | –2.21 to 11.78 | 68.29 | |
| S% | 1.92 | 3.5 | 3.01 | 0 | 0 | 0 | ||||||||
| EIR | 0.04 | 0.87 | 0.45 | 0 | 0 | 0 | 100 | |||||||
| Akron control | Total | 48 | 224 | 272 | 50 | 896 | 946 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 1 | –1.48 to 3.48 | 3.5 | 0.38–6.62 | 2.43 | 0.57–4.29 | 1.04 | –0.05 to 2.13 | 14 | –21.57 to 49.57 | 8.45 | –7.52 to 24.41 | –248.14 | |
| S% | 0 | 4.3 | 2.98 | 8.16 | 6.34 | 6.85 | ||||||||
| EIR | 0 | 0.15 | 0.07 | 0.08 | 0.88 | 0.57 | –7.14 | |||||||
EIR = entomologic inoculation rate; HBR = human biting rate; IRS = indoor residual spraying; CI = confidence interval; S% = sporozoite index; EIR is given as infected bites/person/night.
Effect of LLIN on HBR of An. gambiae.
The reduction in the HBR observed in the IRS arm was also observed after increasing the rate of coverage of LLINs by using additional Permanet 2.0. In Seme before distribution of LLINs, the An. gambiae HBR was relatively high: 24.43 bites/person/night in January–July 2008. This rate decreased to 6.11 bites/person/night after distribution of LLINs (75% reduction) (Table 2). A similar reduction (72.6%) was found in Dangbo. Data obtained in the two arms indicate a higher decrease in the HBR caused by IRS than by LLIN: a 73.66% reduction versus a 50.63% reduction in Adjohoun (P < 0.05) and a 95.22% reduction versus a 72.6% reduction in Dangbo (P < 0.05) (Tables 1 and 2).
Table 2.
EIR and HBR before and after LLIN intervention, Benin*
| Location | Before intervention | After intervention | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dry season, January–March 2008 | Rainy season, April–July 2008 | January–July 2008 | Dry season, January–March 2009 | Rainy season, April–July 2009 | January–July 2009 | % Reduction | ||||||||
| 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | 95% CI | |||||||||
| Adjohoun | Total | 59 | 837 | 896 | 64 | 378 | 442 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 1.23 | 0.23–2.22 | 13.08 | 0.66–25.49 | 8 | 0.23–15.77 | 1.33 | –1.79 to 4.45 | 5.91 | –0.55 to 12.36 | 3.95 | 0.40–7.50 | 50.63 | |
| S% | 0 | 1.17 | 0.87 | 0 | 0 | 0 | ||||||||
| EIR | 0 | 0.15 | 0.07 | 0 | 0 | 0 | 100 | |||||||
| Dangbo | Total | 384 | 1,520 | 1,904 | 42 | 480 | 522 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 8 | –17.82 to 33.82 | 23.75 | 0.71–46.79 | 17 | 3.54–30.46 | 0.875 | –1.59 to 3.34 | 7.5 | 0.94–14.06 | 4.66 | 0.39–8.94 | 72.59 | |
| S% | 0 | 4.70 | 2.86 | 0 | 2.20 | 1.80 | ||||||||
| EIR | 0 | 1.11 | 0.48 | 0 | 0.16 | 0.08 | 83.33 | |||||||
| Seme | Total | 288 | 2,448 | 2,736 | 11 | 673 | 684 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 6 | –13.37 to 25.37 | 38.25 | 13.65–62.85 | 24.43 | 5.10–43.76 | 0.23 | –0.32 to 0.77 | 10.52 | –2.45 to 23.48 | 6.11 | –1.26 to 13.47 | 74.99 | |
| S% | 0 | 4.83 | 3 | 0 | 0.9 | 0.84 | ||||||||
| EIR | 0 | 1.84 | 0.73 | 0 | 0.09 | 0.05 | 93.15 | |||||||
| Akron control | Total | 1,008 | 2,208 | 3,216 | 390 | 2,312 | 2,702 | |||||||
| Person night | 48 | 64 | 112 | 48 | 64 | 112 | ||||||||
| HBR | 21 | –10.35 to 52.35 | 34.5 | –7.27 to 76.27 | 28.71 | 9.10–48.33 | 8.13 | –7.74 to 23.99 | 36.13 | –5.53 to 77.78 | 24.13 | 1.85–46.40 | 15.95 | |
| S% | 2 | 3.33 | 3 | 1.53 | 3.57 | 2.92 | ||||||||
| EIR | 0.42 | 1.14 | 0.86 | 0.12 | 1.29 | 0.7 | 18.6 | |||||||
EIR = entomologic inoculation rate; HBR = human biting rate; LLIN = long-lasting insecticide-impregnated net (Permanet 2.0; Vestergaard Frandsen, Lausanne, Switzerland); CI = confidence interval; S% = sporozoite index; EIR is given as infected bites/person/night.
Effects of IRS and LLIN on EIR of An. gambiae.
Before IRS, the EIR in the control area was 0.07 infected bites/person/night (Table 1). In the IRS arm, similar EIRs were found: 0.07, 0.10, and 0.07 infected bites/person/night in Adjohoun, Dangbo, and Misserete2, respectively. These values represent 14.7 infected bites per person in Adjohoun, Misserete2, and Akron during January–July. In Dangbo and Seme, the rate is higher, respectively, 21 and 94.5 infected bites per human during the 7 months. After IRS, all ELISA results were negative for P. falciparum circumsporozoite antigen for all districts during the entire study period. However, during this period, each resident of the control area (Akron) received 0.57 infected bites per night (119.7 infected bites during January–July (Table 1).
In the LLIN arm, the mean EIR was 0.07, 0.48, and 0.73 infected bites/person/night in Adjohoun, Dangbo and Seme, respectively, during January–July 2008 (Table 2). After additional LLINs (January–July 2009), a decrease of 83.3% in the EIR was observed in Dangbo (Table 2).
Effects of IRS and LLIN on lifespan of An. gambiae.
Before IRS, the parous rate of An. gambiae was high (mean = 77.1% in areas selected for intervention, range = 70.7% in Misserete2 to 79.5% in Seme). After IRS, the parous rate decreased to 22.94% for the four districts (Table 3). This decrease was observed in all intervention sites (from 79.5% to 33.3% in Seme and from 75.5% to 26.3% in Misserete2). In Adjohoun and Dangbo, all An. gambiae dissected and microscopically examined were nulliparous. The IRS area was not conducive for mosquito survival. In the LLIN arm, the parous rate decreased from 73.58% before additional LLINs to 55.06% after additional LLINs (Table 3). However, in Adjohoun, the difference was not significant (P > 0.05).
Table 3.
Variation of parturity rate of Anopheles gambiae s.l. collected during the long dry season and the long rainy season in 2008 before IRS and LLIN interventions and in the same subsequent period, Benin*
| Location | IRS arm | Comparison of P rate % before and after IRS | LLIN arm | Comparison of P rate before and after LLIN | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before intervention, January–July 2008 | After intervention, January–July 2009 | Before intervention, January–July 2008 | After intervention, January–July 2009 | |||||||
| No. | P rate % | No. | P rate % | No. | P rate % | No. | P rate % | |||
| Adjohoun | 128 | 79.41 | 72 | 0 | P < 0.05 | 229 | 68.18 | 121 | 61.15 | P > 0.05 |
| Dangbo | 137 | 78.83 | 16 | 0 | P < 0.05 | 279 | 73.11 | 166 | 61.44 | P < 0.05 |
| Misserete 1 | 116 | 70.68 | 11 | 54.54 | P > 0.05 | |||||
| Misserete 2 | 139 | 75.53 | 118 | 26.27 | P < 0.05 | |||||
| Seme | 166 | 79.51 | 123 | 33.33 | P < 0.05 | 200 | 80.5 | 118 | 39.83 | P < 0.05 |
| Akron control | 134 | 67.16 | 175 | 80.57 | P < 0.05 | 200 | 80 | 205 | 80 | P > 0.05 |
| Total | 820 | 76.96 | 515 | 22.94 | P < 0.05 | 908 | 73.58 | 610 | 55.06 | P < 0.05 |
IRS = indoor residual spraying; LLIN = long-lasting insecticide-impregnated net (Permanet 2.0; Vestergaard Frandsen, Lausanne, Switzerland); No. = no. collected and dissected; P rate % = parturity rate %.
Effect of IRS and LLIN on exit of mosquitoes from treated houses after feeding.
The natural exit rate of An. gambiae varied from 26.7% in Misserete2 to 36.14% in Dangbo. A similar exit rate (38.12% in Dangbo and 39.05% in Adjohoun) was obtained in the LLIN arm. After IRS, the ext rate was high: 62.5% and 91.7% in Adjohoun and Dangbo, respectively. Compared with the natural exit rate of An. gambiae, the difference was significant in all study sites (Table 4). In Misserete1 and Misserete2, all mosquitoes were collected from window traps: the exit rate induced by bendiocarb in houses was 100%. The same result was found in the LLIN arm; exit rates were higher after distribution of additional LLINs (Table 4).
Table 4.
Exit rate for Anopheles gambiae from bendiocarb-treated walls and presence of LLINs in houses during the same period (long rainy season), Benin*
| Location | Before intervention (May–July 2008) | After intervention (May–July 2009) | Comparison of exit rates before and after intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | No. | Exit rate, % | Total | No. | Exit rate, % | ||||
| Mean | 95% CI | Mean | 95% CI | ||||||
| Adjohoun | |||||||||
| IRS arm | 84 | 26 | 30.17 | 20.17–40.92 | 32 | 20 | 62.50 | 39.53–85.47 | P < 0.05 |
| LLIN arm | 91 | 36 | 39.05 | 30.59–47.51 | 72 | 64 | 88.89 | 76.94–100.84 | P < 0.05 |
| Dangbo | |||||||||
| IRS arm | 84 | 31 | 36.14 | 24.26–48.01 | 48 | 44 | 91.67 | 91.67–91.67 | P < 0.05 |
| LLIN arm | 82 | 32 | 38.12 | 31.50–44.75 | 100 | 48 | 47.77 | 39.61–55.92 | P < 0.05 |
| Misserete 1 | |||||||||
| IRS arm | 84 | 32 | 37.88 | 29.09–46.66 | 5 | 5 | 100.00 | 100–100 | P < 0.05 |
| Misserete 2 | |||||||||
| LLIN arm | 288 | 78 | 26.7 | 20.35–33.04 | 4 | 4 | 100.00 | 100–100 | P < 0.05 |
| Seme | |||||||||
| IRS arm | 388 | 138 | 35.62 | 28.83–42.40 | 0 | 0 | |||
| LLIN arm | 90 | 35 | 38.62 | 29.42–47.81 | 216 | 142 | 67.15 | 61.68–72.44 | P < 0.05 |
| Akron control | |||||||||
| IRS control | 88 | 43 | 50.42 | 42.51–58.31 | 132 | 64 | 47.92 | 43.07–52.76 | P > 0.05 |
| LLIN control | 404 | 188 | 46.14 | 42.25–50.02 | 384 | 184 | 49.13 | 43.65–54.62 | P > 0.05 |
Exit rate is the estimated rate of the number of An. gambiae that have escaped treated walls and LLINs and are retained in the exit window traps. LLIN = long-lasting insecticide-impregnated net (Permanet 2.0; Vestergaaard Frandsen, Lausanne, Switzerland); No. = number that exited the window trap; CI = confidence interval; IRS = indoor residual spraying.
Effect of IRS and LLIN on blood feeding.
Despite IRS implementation, a non-negligible proportion of mosquitoes fed on humans. However, the blood feeding index was significantly lower after IRS than before IRS (Table 5). A similar result was observed in the LLIN arm. Conversely, in Sèmè, the blood feeding rates remained high after distribution of LLINs.
Table 5.
Percentage of blood-feeding Anopheles gambiae collected in IRS and LLIN arms by pyrethrum spray catch and in exit windows traps before and after interventions, Benin*
| Location | Before intervention (May–July 2008) | After intervention (May–July 2009) | Comparison of blood feeding rate before and after intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | No. fed | Blood-feeding rate, % | Total | No. fed | Blood-feeding rate, % | ||||
| Mean | 95% CI | Mean | 95% CI | ||||||
| Adjohoun | |||||||||
| IRS arm | 84 | 44 | 52.38 | 50.37–53.63 | 32 | 11 | 34.3 | 4.10–64.40 | P < 0.05 |
| LLIN arm | 91 | 61 | 66.7 | 61.90–71.44 | 72 | 20 | 33.3 | 19.79–46.88 | P < 0.05 |
| Dangbo | |||||||||
| IRS arm | 84 | 46 | 54.3 | 50.37–58.30 | 48 | 8 | 16.5 | –34.82 to 66.82 | P < 0.05 |
| LLIN arm | 82 | 57 | 70.2 | 62.05–78.28 | 100 | 22 | 22 | 18.35–25.30 | P < 0.05 |
| Misserete 1 | |||||||||
| IRS arm | 84 | 43 | 50.7 | 44.86–56.48 | 5 | 2 | 41.5 | –66.50 to 149.50 | P < 0.05 |
| Misserete 2 | |||||||||
| LLIN arm | 288 | 180 | 62.2 | 58.11–66.23 | 4 | 2 | 50 | 50.00–50.00 | P < 0.05 |
| Seme | |||||||||
| IRS arm | 388 | 213 | 54.3 | 43.74–64.93 | 0 | 0 | |||
| LLIN arm | 90 | 52 | 58.3 | 49.19–67.47 | 216 | 152 | 72.3 | 57.13–87.54 | P < 0.05 |
| Akron control | |||||||||
| IRS control | 88 | 52 | 61.7 | 49.86–73.48 | 132 | 80 | 60 | 50.20–69.80 | P > 0.05 |
| LLIN control | 404 | 264 | 64.3 | 56.69–71.98 | 384 | 200 | 54.7 | 43.87–65.46 | P > 0.05 |
IRS = indoor residual spraying; LLIN = long-lasting insecticide-impregnated net (Permanet 2.0, Vestergaard Frandsen, Lausanne, Switzerland); CI = confidence interval.
Discussion
The indoor residual spraying strategy implemented by the NMCP in the Department of Oueme was successful. In all districts that used IRS, the density of An. gambiae (HBR) and the EIR showed a reduction of 94.4%. Anopheles gambiae positive for P. falciparum were not found during the evaluation period. This finding is justified by the scale of the campaign. Furthermore, because the four districts were contiguous, spraying covered a large area populated by nearly 350,000 persons. However, although positive results for CS protein were not found, this result does not indicate that malaria transmission was interrupted. Persons likely continued to receive a few infected An. gambiae bites. However, the proportion of infected mosquitoes was so low that it would have been necessary to analyze thousands of mosquitoes to find any positive for malaria parasites.
The first challenge for the NMCP, to identify a non-pyrethroid as alternative insecticide for IRS campaigns in Benin, has been achieved. Bendiocarb has emerged as a promising insecticide for the control of vector populations that are resistant to pyrethroids. Many countries, such as Mexico,28 Zimbabwe,29 the Philippines,30 and South Africa,31 have decreased malaria effectively by using this product. Previous studies in experimental huts showed that some organophosphates and carbamates were particularly effective on natural populations of vectors that are highly resistant to pyrethroids.32 This result is the reason why Zaim and Guillet33 have suggested that the search for control methods other than pyrethroids should be a priority for the NMCP in Africa.
The second challenge for the NMCP, to expand the successful findings for Oueme to other parts of Benin where An. gambiae has developed strong resistance to pyrethroids, has also been successful. This success was caused by several factors. In addition to the lethal effect of bendiocarb on mosquitoes that are resistant to pyrethroids,19,22 persons who had bed nets, especially children and pregnant women, used them. However, the proportion of consistent users was low. Conversely, IRS sites became inhospitable areas for mosquitoes to survive and most died before the end of the sporogonic cycle of Plasmodium. The unpleasant atmosphere created by the presence of bendiocarb on the walls inside houses is harmful to the mosquitoes. This atmosphere results in a decrease in endophily and an increase in the exit rate (Table 4). Thus, some Anopheles mosquitoes that managed to enter houses failed to obtain blood meals before exiting. However, some succeeded in obtaining blood meals inside houses (Table 5).
Despite the effectiveness of IRS, this method has its limitations. When mosquitoes enter the houses, even those houses that are treated, they go directly to their host to obtain a blood meal before resting on walls or seeking to escape if the houses are treated. This situation was found in experimental huts for many insecticides22 and at community level.34 This finding is why we have proposed that the NMCP always invite communities in Benin who are protected by IRS to add sleeping under LLINs to supplement malaria control efforts.
The IRS campaign in the Department of Oueme was an initial experience. The plan was to implement IRS strategy in other parts of Benin if initial results were encouraging. Fortunately, not only were the results good, but many communities have expressed appreciation for the strategy.
Implementation of IRS should not exclude use of LLINs by the community. The proportion of fed An. gambiae collected in soaked window exit traps was not significantly different from that observed before the IRS. Some families, particularly husbands and wives, usually sleep under bed nets. For these families, bed nets are considered an essential preventive measure. Despite implementation of IRS, these families should be encouraged to use their bed nets. The best strategy is the joint use of IRS plus LLIN. This combination not only protects homes from invasion by mosquitoes, it also prevents contact between humans and mosquitoes. However, such a strategy implicates an increase in the cost of malaria prevention and cannot be implemented everywhere. It must be reserved only for areas with highest levels of malaria transmission. For example, in areas where EIR is approximately 300 Anopheles infected bites per year, a 90% reduction is not sufficient to significantly reduce malaria prevalence.
In areas where persons were protected by LLINs, appreciable reductions in biting rates were also observed. The high coverage of LLINs was the major factor for this reduction. In the LLIN area, nets were used systematically because of the nuisance of Culicidae, particularly Mansonia spp. and Culex spp.35 If widely used, LLINs may be an effective means of vector control36–38 and significantly reduce malaria mortality.39
Success of LLINs, despite pyrethroid resistance, may also be justified by the role of mechanical barrier played by this tool.4 In addition, efficacy of LLINs could be justified by the results of Chandre and others,40 who showed that mosquito nets impregnated with permethrin or deltamethrin had long-lasting effectiveness against An. gambiae in west Africa with kdr-type resistance. These authors reported that pyrethroid-impregnated bed nets provided good levels of protection against kdr homozygous strains of An. gambiae. One explanation for this finding is that a high proportion of kdr females are killed by prolonged contact with pyrethroids through diminished sensitivity to the usual irritant and repellent effects, and relatively few kdr females take advantage of this prolonged contact to ingest a blood meal.40
After a meeting with all partners involved in malaria vector control in Benin, the NMCP decided to continue to implement this strategy in other regions of Benin. This strategy will now be implemented in the Department of Atacora.
ACKNOWLEDGMENTS
We thank the American people for supporting our shared goal to reduce the malaria burden in Africa, particularly in Benin, and the Center for Entomological Research of Cotonou team for technical assistance during laboratory bioassays and field collections. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Footnotes
Financial support: This study was supported by the President's Malaria Initiative of the U.S. Government. The American Society of Tropical Medicine and Hygiene assisted with publication expenses.
Authors' addresses: Martin Akogbeto and Gil Germain Padonou, Centre de Recherche Entomologique de Cotonou, 06 BP 2604 Cotonou, Benin, E-mails: akogbetom@yahoo.fr and pagergil@yahoo.fr. Honore Sourou Bankole, Ecole Polytechnique d'Abomey Calavi, Universite d'Abomey Calavi, Cotonou, Benin, E-mail: bahosour@yahoo.fr. Dorothee Kinde Gazard, Faculte des Sciences de la Sante, Universite d'Abomey Calavi, Cotonou, Benin, E-mail: kindegazard@gmail.com. Ghelus Louis Gbedjissi, Faculte des Sciences et Techniques, Universite d'Abomey Calavi, Cotonou, Benin, E-mail: ghelus/louis@yahoo.fr.
Reprint requests: Gil Germain Padonou, Centre de Recherche Entomologique de Cotonou, 06 BP 2604 Cotonou, Benin, E-mail: pagergil@yahoo.fr.
References
- 1.World Health Organization . World Malaria Report 2009. Geneva: World Health Organization; 2009. [Google Scholar]
- 2.Gentilini M, Caumes E, Danis M. Le Paludisme. Edition Flammarion. Paris: Médecine Tropicale; 1993. [Google Scholar]
- 3.Ministère de la Santé. Annuaire des Statistiques Sanitaires 2006. Cotonou, Benin: Direction de la Programmation et de la Prospective; 2007. [Google Scholar]
- 4.Curtis CF, Abraham E, Mnzava P. A comparison of use of a pyrethroid either for house spraying or for bednet treatment against malaria vectors. Trop Med Int Health. 1998;3:619–631. doi: 10.1046/j.1365-3156.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 5.Rowland M. Malaria control: bednets or spraying? Malaria control in the Afghan refugee camps of western Pakistan. Trans R Soc Trop Med Hyg. 1999;93:458–459. doi: 10.1016/s0035-9203(99)90336-x. [DOI] [PubMed] [Google Scholar]
- 6.Elissa N, Mouchet J, Rivière F, Meunier JY, Yao K. Resistance of Anopheles gambiae s.s. to pyrethroids in Côte d'Ivoire. Ann Soc Belg Med Trop. 1993;73:291–294. [PubMed] [Google Scholar]
- 7.Akogbéto M, Yakoubou S. Résistance des vecteurs du paludisme vis-à-vis des pyréthrinoïdes utilisés pour l'imprégnation des moustiquaires au Bénin, Afrique de l'Ouest. Bull Soc Pathol Exot. 1999;92:123–130. [PubMed] [Google Scholar]
- 8.Chandre F, Darriet F, Duchon S, Finot L, Manguin S, Carnevale P, Guillet P. Modifications of pyrethroid effects associated win kdr mutation in Anopheles gambiae. Med Vet Entomol. 2000;14:81–88. doi: 10.1046/j.1365-2915.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 9.Akogbeto M, Djouaka RF, Kinde-Gazard DA. Screening of pesticide residues in soil water samples from agricultural settings. Malar J. 2006;5:22. doi: 10.1186/1475-2875-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vulule JM, Beach RF, Atieli FK, McAllister JC, Brogdon WG, Roberts JM, Mwangi RW, Hawley WA. Elevated oxidase and esterase levels associated with permethrin tolerance in Anopheles gambiae from Kenyan villages using permethrin impregnated nets. Med Vet Entomol. 1999;13:239–244. doi: 10.1046/j.1365-2915.1999.00177.x. [DOI] [PubMed] [Google Scholar]
- 11.Corbel V, N'Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, Akogbeto M, Hougard JM, Rowland M. Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Trop. 2007;101:207–216. doi: 10.1016/j.actatropica.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Yadouleton AW, Asidi A, Djouaka RF, Braïma J, Agossou CD, Akogbeto MC. Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J. 2009;8:103. doi: 10.1186/1475-2875-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czeher C, Labbo R, Arzika I, Duchemin JB. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide longlasting insecticide-treated nets implementation. Malar J. 2008;7:189. doi: 10.1186/1475-2875-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diabate A, Baldet T, Chandre F, Guiguemde RT, Brengues C, Guillet P, Hemingway J, Hougard JM. First report of the kdr mutation in Anopheles gambiae M form from Burkina Faso, West Africa. Parassitologia. 2002;44:157–158. [PubMed] [Google Scholar]
- 15.Fanello C, Petrarca V, Della Torre A, Santolamazza F, Dolo G, Coulibaly M, Alloueche A, Curtis CG, Toure YT, Coluzzi M. The pyrethroid knock-down resistance gene in the Anopheles gambiae complex in Mali and further indication of incipient speciation within An. gambiae s.s. Insect Mol Biol. 2003;12:241–245. doi: 10.1046/j.1365-2583.2003.00407.x. [DOI] [PubMed] [Google Scholar]
- 16.Awolola TS, Brooke BD, Koekemoer LL, Coetzee M. Resistance of the malaria vector Anopheles gambiae s.s. to pyrethroid insecticides, in south-western Nigeria. Ann Trop Med Parasitol. 2002;96:849–852. doi: 10.1179/000349802125002581. [DOI] [PubMed] [Google Scholar]
- 17.Hargreaves K, Koerkemoer LL, Brooke B, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 18.Etang J, Manga L, Chandre F, Guillet P, Fondjo E, Mimpfoundi R, Toto JC, Fontenille D. Insecticide susceptibility status of Anopheles gambiae s.l. (Diptera: Culicidae) in the Republic of Cameroon. J Med Entomol. 2003;40:491–497. doi: 10.1603/0022-2585-40.4.491. [DOI] [PubMed] [Google Scholar]
- 19.N'Guessan R, Corbel V, Akogbeto M, Rowland M. Reduced efficacy of insecticide treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis. 2007;13:99–206. doi: 10.3201/eid1302.060631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadouleton AW, Padonou G, Asidi A, Moiroux N, Banganna S, Corbel V, N'Guessan R, Gbenou D, Yacoubou I, Gazard K, Akogbeto MC. Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J. 2010;9:83. doi: 10.1186/1475-2875-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djogbénou L, Pasteur N, Bio-Bangana S, Baldet T, Irish SR, Akogbeto M, Weill M, Chandre F. Malaria vectors in the Republic of Benin: distribution of species and molecular forms of the Anopheles gambiae complex. Acta Trop. 2010;114:116–122. doi: 10.1016/j.actatropica.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Akogbéto MC, Padonou GG, Gbénou D, Irish S, Yadouleton A. Bendiocarb, a potential alternative against pyrethroid resistant Anopheles gambiae in Benin, West Africa. Malar J. 2010;9:204. doi: 10.1186/1475-2875-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mabaso ML, Sharp B, Lengeler C. Historical review of malaria control in southern Africa with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 24.Gillies M, De Meillon B. The Anophelinae of Africa South of the Sahara. Johannesburg: South African Institute of Medical Research; 1968. p. 54. [Google Scholar]
- 25.Détinova TS. Méthode à Appliquer pour Classer par Groupe d'Âge les Diptères Présentant une Importance Médicale. Geneva: Organisation Mondiale de la Santé Série Monogr; 1963. p. 47. [Google Scholar]
- 26.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot T, Schneider I, Esser KM, Beaudoin RL, Andre RG. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 27.Favia G, Lanfrancotti A, Spanos L, Siden Kiamos I, Louis C. Molecular characterization of ribosomal DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 28.Arredondo JI, Bow DN, Vaca MA. Effect of indoor residual spraying of DDT and bendiocarb on the feeding patterns of Anopheles pseudopunctipennis in Mexico. J Am Mosq Control Assoc. 1990;6:635–640. [PubMed] [Google Scholar]
- 29.Mpofu SM, Kanyimo KH, Masendu H. Potential use of bendiocarb (Ficam VC) for malaria control in an area of Zimbabwe. J Am Mosq Control Assoc. 1991;7:53–542. [PubMed] [Google Scholar]
- 30.Asinas CY, Hugo CT, Boase CJ, Evans RG. Evaluation of selective spraying of bendiocarb (Ficam VC) for the control of Anopheles flavirostris in the Philippines. J Am Mosq Control Assoc. 1994;10:496–500. [PubMed] [Google Scholar]
- 31.Hargreaves K, Koekemoer LL, Brooke BD, Hunt RH, Mthembu J, Coetzee M. Anopheles funestus resistant to pyrethroid insecticides in South Africa. Med Vet Entomol. 2000;14:181–189. doi: 10.1046/j.1365-2915.2000.00234.x. [DOI] [PubMed] [Google Scholar]
- 32.Padonou GG. Evaluation en Cases Expérimentales de l'Efficacité de Quelques Insecticides Pyréthrinoïdes, Organophosphorés et Carbamate en Pulvérisation Intradomiciliaire. Mémoire de Master. Coutomou, Benin: Université d'Abomey Calavi; 2008. [Google Scholar]
- 33.Zaim M, Guillet P. Alternative insecticides: an urgent need. Trends Parasitol. 2002;18:161–163. doi: 10.1016/s1471-4922(01)02220-6. [DOI] [PubMed] [Google Scholar]
- 34.Georgia BD, Djènontin A, Rogier C, Corbel V, Bio-Bangana S, Chandre F, Akogbéto M, Kindé-Gazard D, Massougbodji A, Henry MC. Malaria infection and disease in an area with pyrethroid-resistant vectors in southern Benin. Malar J. 2010;9:380. doi: 10.1186/1475-2875-9-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akogbéto M, Feliho R. Connaissances et attitudes pratiques concernant l'utilisation des moustiquaires à Ganvié. République du Bénin. Bull OCCGE Info. 1996;105:10–20. [Google Scholar]
- 36.Robert V, Carnevale P. Influence of deltamethrin treatment of bed nets on malaria transmission in the Kou Valley, in Burkina Faso. Bull Wrld Hlth Org. 1991;69:735–740. [PMC free article] [PubMed] [Google Scholar]
- 37.Mouchet J. Pyrethroid impregnated bed nets in malaria control strategy at community level. Acta Trop. 1991;46:267–268. doi: 10.1016/0001-706x(89)90027-2. [DOI] [PubMed] [Google Scholar]
- 38.Lengeler C, Cattani J, De Savigny D. Net Gain, a New Method for Preventing Malaria Deaths. Geneva: International Development Research Center/World Health Organization; 1996. [Google Scholar]
- 39.Lengeler C. Insecticide Treated Bednets and Curtains for Malaria Control: A Cochrane Review. Oxford, United Kingdom: Oxford Update Software Ltd; 2005. The Cochrane Library, Issue 3. [Google Scholar]
- 40.Chandre F, Darriet F, Duchon S, Finot L, Manguin S, Carnevale P, Guillet P. Modifications of pyrethroid effects associated with kdr mutation in the malaria vector Anopheles gambiae. Med Vet Entomol. 2000;14:81–88. doi: 10.1046/j.1365-2915.2000.00212.x. [DOI] [PubMed] [Google Scholar]


