Abstract
We report and explore changes in child mortality in a rural area of Kenya during 2003–2009, when major public health interventions were scaled-up. Mortality ratios and rates were calculated by using the Kenya Medical Research Institute/Centers for Disease Control and Prevention Demographic Surveillance System. Inpatient and outpatient morbidity and mortality, and verbal autopsy data were analyzed. Mortality ratios for children less than five years of age decreased from 241 to 137 deaths/1,000 live-births in 2003 and 2007 respectively. In 2008, they increased to 212 deaths/1,000 live-births. Mortality remained elevated during the first 8 months of 2009 compared with 2006 and 2007. Malaria and/or anemia accounted for the greatest increases in child mortality. Stock-outs of essential antimalarial drugs during a time of increased malaria transmission and disruption of services during civil unrest may have contributed to increased mortality in 2008–2009. To maintain gains in child survival, implementation of good policies and effective interventions must be complemented by reliable supply and access to clinical services and essential drugs.
Introduction
The Fourth Millennium Development Goal aims to achieve a two-thirds reduction in child mortality during 1990–2015.1 During the past decade, the Kenyan Ministry of Health introduced measures to improve child health and reduce mortality. Recent Demographic and Health Survey data show that under-five mortality has decreased in Kenya from 115/1,000 live births in 2003 to 74/1,000 live births in 2007.2
Since 2001, the Centers for Disease Control and Prevention and the Kenya Medical Research Institute have monitored childhood mortality as part of the KEMRI/CDC Health and Demographic Surveillance System (HDSS) in rural western Kenya. As part of the HDSS, data are collected from a variety of sources, including households, health facilities, and hospitals. We use these data to report the change in under-five mortality during 2003–2009, including age-specific and cause-specific mortality, and to explore factors that may have contributed to changes in mortality. Community-based and hospital-based mortality figures are explored and compared, and we explore the ecological relationship between changes in mortality and changes in the implementation and uptake of evidence-based large-scale interventions.
Materials and Methods
Ethics.
The KEMRI/CDC HDSS was approved by the ethical review boards of KEMRI (SSC #647) and CDC (IRB# 3308).
Setting.
Data were obtained from two contiguous areas of the KEMRI/CDC HDSS, Asembo and Gem in Nyanza Province. Asembo and Gem have a combined population of 140,581, of whom 16% are children < 5 years of age.2 The population is predominantly of Luo ethnicity and earn their living through subsistence farming and fishing.3 Within these areas of the HDSS, there are 18 government of Kenya or mission-run health facilities. These health facilities are not supplied or managed by KEMRI/CDC.
Malaria transmission is high and perennial with seasonal peaks in May–July and October–November. Malaria, pneumonia, and diarrhea are the leading causes of childhood illness and death,4 malnutrition is common,5 and HIV prevalence is high;6 the estimated HIV prevalence among children six weeks of age is 3% (KEMRI/CDC unpublished data).
A broad range of child survival interventions were initiated and scaled-up during the period of observation (Tables 1 and 2). These interventions were implemented by the government of Kenya or non-governmental organizations, independently of KEMRI/CDC. During the period of observation, there were no large-scale research studies that delivered interventions that may have impacted the observed mortality.
Table 1.
Public health interventions and developments affecting the Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System population, 2003–2008*
| Intervention | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|---|
| Subsidized insecticide-treated net distribution at health facilities | X | X | X | X | X | |
| Campaign distributing long-lasting insecticide treated nets, measles vaccine, and vitamin A | X | |||||
| New Kenyan Ministry of Health guidelines for the management of malaria introduced | X | X | X | |||
| First-line antimalarial drug recommended by MOH | ||||||
| Sulfadoxine-pyrimethamine | X | |||||
| Amodiaquine (interim recommendation by MOH until AL available) | X | X | X | |||
| Artemether-lumefantrine available in health facilities | X | X | X | |||
| First-line antibiotic for treatment of pneumonia recommended by MOH | ||||||
| Cotrimoxazole | X | |||||
| Amoxicillin | X | X | X | X | X | |
| Pentavalent vaccine, including Hib vaccine, included in routine childhood immunizations | X | X | X | X | X | X |
| In-home water treatment promoted in the community | X | X | X | X | X | X |
| Number of health facilities in the HDSS providing HIV care (cotrimoxazole and multivitamins) to adults through patient support centers | 1 | 2 | 2 | 6 | 8 | 11 |
| Number of health facilities in the HDSS providing ART to adults | 0 | 1 | 1 | 2 | 7 | 7 |
| Number of health facilities in the HDSS providing pediatric ART | 0 | 0 | 0 | 2 | 7 | 7 |
| Number of antenatal clinics offering ART for prevention of mother- to-child transmission in HDSS | 1 | 3 | 5 | 13 | 15 | 17 |
MOH = Ministry of Health; AL = artemether-lumefantrine; Hib = Haemophilus influenzae b; HDSS = Health and Demographic Surveillance System; HIV = human immunodeficiency virus; ART = antiretroviral therapy. “X” indicates intervention in place, there are 18 health facilities in the HDSS.
Table 2.
Changes in measures contributing to child survival in Nyanza Province and the Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System, 2003–2008*
| Factor | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | Source of data |
|---|---|---|---|---|---|---|---|
| Fertility and perinatal care | |||||||
| Total fertility rate for women 15–49 years of age, expressed per woman | 5.5 | 5.3 | 5.2 | 5.4 | 5.2 | 5.3 | HDSS |
| Sought antenatal care | 90% | 92% | KDHS | ||||
| Delivered by a health professional | 41% | 46% | KDHS | ||||
| Delivered in a health facility | 40% | 44% | KDHS | ||||
| Intermittent preventive treatment of malaria during pregnancy coverage (≥ 2 doses during pregnancy) | |||||||
| Nyanza Province | 5% | 6% | KDHS/MIS | ||||
| HDSS only | 21% | HDSS§ | |||||
| Maternal and child health | |||||||
| Child fully vaccinated¶ | 38% | 55% | KDHS | ||||
| Treatment sought from health facility for ARI or fever | 41% | 55% | KDHS | ||||
| Treatment sought from health facility for diarrhea | 23% | 57% | KDHS | ||||
| Oral rehydration solution used for diarrhea in children | 21% | 46% | KDHS | ||||
| Oral rehydration therapy used for diarrhea in children | 36% | 78% | KDHS | ||||
| Treated water use# | 40% | 46% | 43% | 49% | 52% | 50% | HDSS |
| Slept under an insecticide-treated net last night (children < 5 years of age) | |||||||
| Nyanza Province | 7% | 51% | KDHS | ||||
| HDSS** | 69% | 49% | 64% | HDSS | |||
| Women who were reportedly tested for HIV during pregnancy†† | 23% | 30% | 37% | 47% | 60% | Estimate 72% | HDSS |
| Estimated uptake of antiretroviral therapy for prevention of mother to child transmission of HIV (PMCT) among those who were HIV infected, assuming 50% of those tested received antiretroviral drugs peripartum‡‡ | 12% | 15% | 19% | 24% | 30% | 36% | HDSS |
| Calculated estimate of contribution of PMCT to annual reduction in under-five mortality in the HDSS§§ | 2% | 2% | 3% | 5% | 8% | 6% | HDSS |
| Nutritional status | |||||||
| Height for age (stunted) | |||||||
| Below –3 SD | 8% | 13% | KDHS | ||||
| Below –2 SD | 31% | 31% | KDHS | ||||
| Weight for height (wasted) | |||||||
| Below –3 SD | 0.1% | 2% | KDHS | ||||
| Below –2 SD | 2% | 4% | KDHS | ||||
| Weight for age (underweight indicating acute and chronic malnutrition) | |||||||
| Below –3 SD | 2% | 2% | KDHS | ||||
| Below –2 SD | 16% | 11% | KDHS | ||||
| Demographic features | |||||||
| Percentage of population in poorest socioeconomic stratum¶ | 23% | 22% | 20% | 20% | 18% | 17% | HDSS |
| Percentage of women of reproductive age who completed primary school | 41% | 42% | 41% | 42% | 42% | 41% | HDSS |
HDSS = Health and Demographic Surveillance System; KDHS = Kenya Demographic Health Survey; MIS; Malaria Indicator Survey, 2007; ARI = acute respiratory infection; HIV = human immunodeficiency virus; PMCT = prevention of mother-to-child transmission.
Ouma PO, Van Eijk AM, Hamel MJ, Sikuku E, Odhiambo F, Munguti K, Ayisi JG, Kager PA, Slutsher L, 2007. The effect of health care worker training on the use of intermittent preventive treatment of malaria in pregnancy in rural western Kenya. Trop Med Int Health 12: 953–961.
Fully vaccinated is defined as a child 12–23 months of age who received Bacillus Calmette-Guérin, measles, and three doses each of diphtheria, pertussis, and tetanus vaccine and polio vaccine (excluding polio vaccine given at birth) before the time of survey.
Treated water is defined as water that is boiled, filtered, or treated with chlorine.
Prevalence estimates based on reported net use and insecticide treatment (treatment within the prior nine months or long-lasting insecticide-treated net). Sample of insecticide-treated nets assessed by cone bioassay in 2009 indicated > 50% vector mortality among 77% of 563 nets randomly selected from households (Kenya Medical Research Institute/Centers for Disease Control, unpublished data).
Estimates are from an unpublished 2007 Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System community-based survey in Gem, which asked women whether they were tested for HIV their last pregnancy. The unmeasured 2008 estimate of pregnant women tested for HIV was projected to be 20% higher than that frequency measured in 2007 on the basis of the average annual increase in HIV testing during 2003–2007.
Assumed that 50% of those tested who are HIV infected receive antiretroviral therapy for PMCT (based on best guesses from clinical staff).
Assumed that 27% of pregnant women are HIV infected and have been so throughout period of observation, HIV-infected and HIV-uninfected women are equally likely to accept HIV testing, 50% of those found to be HIV infected will receive perinatal antiretroviral therapy (nevirapine during labor for the mother and within 72 hours post-partum for the neonate), 30% of children born to women do not receive antiretroviral therapy and 17% of children born to women who receive perinatal antiretroviral therapy will become HIV infected before their 5th birthday, and 80% of HIV-infected children will die before their fifth birthday.
Households were ranked into socioeconomic status quintiles for 2003 and movement between those quintiles in subsequent years was measured.
HDSS and verbal autopsy.
All houses in the HDSS were visited every four months; during each of these data collection periods, or rounds, births, deaths, pregnancies, and migrations were recorded. Detailed methods have been reported.4 Persons who were born into or who resided in the HDSS for at least four calendar months were registered as residents.
In the HDSS, deaths were captured through two systems. Designated community members reported deaths immediately. Additionally, deaths were recorded during HDSS rounds. After a grieving period, workers visited the deceased's home to administer a verbal autopsy questionnaire.7 The verbal autopsy form was independently reviewed by clinicians and the most likely cause of death assigned. Most deaths caused by anemia have an underlying cause of malaria in this area.8–10 Therefore, deaths caused by anemia and malaria were grouped together.
The HDSS captured information on adult education, treated water use, and socioeconomic indicators. In 2007, women residing in Gem were asked whether they had been tested for HIV during their most recent pregnancy. Principal component analysis was applied to socioeconomic indicators to obtain asset indices, a proxy for household socioeconomic status (SES).11 Using principal component analysis, we ranked households into SES quintiles on the basis of assets using data for 2003–2008; annual asset values were compared with the overall distribution to determine percentage in each SES category.
HDSS household surveys.
In 2003 a household-based survey of malaria parasitemia and anemia was conducted in the HDSS in Gem only. Methods have been described.12 During 2006–2009, annual HDSS household surveys were conducted to measure malaria parasite prevalence, anemia, and insecticide-treated net (ITN) use. A probability sample of compounds with at least one child < 5 years of age was randomly selected. All consenting compound members provided blood samples for diagnosis of malaria parasitemia and hemoglobin measurement. The surveys were conducted in April, typically after the rainy season began, but before the peak transmission season.
Health facility surveillance.
Siaya District Hospital (SDH) is located 12 km from the HDSS. Approximately 7% of pediatric admissions are Asembo and Gem HDSS residents. Blood for malaria microscopic examination was collected for all admitted children regardless of HDSS membership. Mortality data were transcribed from hospital charts. Lwak Mission Hospital is located in Asembo, within the HDSS. Beginning in mid-2006, blood smears for malaria microscopic examination were collected for all children with a history of or documented fever who came to the Lwak Mission Hospital. Blood smears were stained with Giemsa; 10% had quality control checks.
During 2003–2006, outpatient morbidity surveillance was conducted at five government of Kenya health facilities in Asembo and Gem. Thereafter, surveillance was conducted at one facility in Gem only.
Mortality rates and ratios.
Neonatal mortality was defined as deaths in the first 28 days of life, post-neonatal mortality as deaths occuring during 29 days–11 months of age, infant mortality as deaths during 0–11 months of age, child mortality as deaths during 1–4 years of age, and under-five mortality as deaths during 0–4 years of age.
Mortality rates were calculated for all HDSS-resident children < 5 years of age at any time during January 1, 2003–August 31, 2009. (Data from the last round in 2009 were not yet available for this analysis.) Person-time was measured from date of birth or in-migration into the HDSS until date of death, out-migration, or fifth birthday. Mortality ratios, calculated using the synthetic cohort method,13 are also presented for ease of comparison with other areas lacking mortality rates.
Model of deaths averted from prevention of mother-to-child transmission.
Data on percentage of women who self-reported being tested for HIV during their last pregnancy were used to estimate the contribution of prevention of mother-to-child transmission (PMCT) to annual reduction in under-five mortality (Table 2). The unmeasured 2008 estimate of pregnant women tested for HIV was projected to be 20% higher than that measured in 2007 on the basis of the average annual increase in HIV testing during 2003–2007. An estimated 27% of pregnant women in the HDSS are HIV-infected.6,8 On the basis of best estimates from clinical staff, HIV-infected and HIV-uninfected women were considered equally likely to accept HIV testing, and 50% of women found to be HIV infected were conservatively estimated to have received perinatal antiretroviral therapy (ARV). Using data from other sites in Africa, we assumed 30% of children born to HIV-infected women who did not receive ARV and 17% of children born to HIV-infected women who received ARV became HIV infected by 5 years of age,14,15 and 80% of HIV-infected children died before their fifth birthday.16
Data collection and statistical analysis.
The HDSS data were entered by using Teleforms® (Teleforms, Inc., Winnipeg, Manitoba, Canada) or personal digital assistants. Artemether-lumefantrine (AL) and antibiotic stock-out information were obtained from the Siaya District Pharmacist, and reflect stock-outs reported in Siaya District, including Gem, during the study period. A drug was coded as stock-out during any given month if it was out of stock for at least one day of that month. Similar data were not available for Asembo, but because both districts received AL from the same central store, stock-out information should be similar. Monthly rainfall measurements are from Kisumu Airport (Kenya Meteorological Department, Kisumu), located 60 km from the HDSS. We used a Poisson regression model to measure the effect of rainfall in the prior 12 months and in the prior 3 months on community parasitemia. Analysis of cause-specific mortality rates for children < 5 years of age excluded neonates. Because mortality and hospital-based data were not from a sample, but rather are census data, no statistics were used in describing those data. Point estimates of malaria parasitemia and anemia were adjusted for clustering within compounds by using generalized estimating equations. Analysis was performed by using SAS version 9.1.3 (SAS Institute, Cary, NC).
Results
Mortality.
During the seven years of observation, 78,159 children contributed 158,681 person-years of observation. There were 8,113 deaths. Under-five mortality decreased annually from 2003–2007, followed by a marked increase in 2008 (Table 3 and Figure 1). Mortality rates during the first two rounds of 2009 decreased from 2008, although they remained elevated compared with 2007 rates (Table 3). Infant and child mortality rates decreased from 134 and 33 deaths per 1000 person-years, respectively, in 2003 to 76 and 17 deaths per 1,000 person-years, respectively, in 2007, a 43% reduction in infant mortality and 49% reduction in child mortality. Mortality rates increased in 2008 compared with 2007, with the greatest increase (45%) observed in children 1–4 years of age (Figure 1and Table 4). Notably, mortality rates in children 5–14 years of age and adults ≥ 15 years of age increased only 3% and 4%, respectively, during 2007–2008. This minimal increase followed four years of generally decreasing mortality in these age groups (KEMRI/CDC, unpublished data).
Table 3.
Under five mortality rates (deaths per 1,000 person-years) triannually, Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System, 2003–2009*
| Year | Overall | January–April | May–August | September–December | ||||
|---|---|---|---|---|---|---|---|---|
| No. deaths/person year follow-up | Rate | No. deaths/person year follow-up | Rate | No. deaths/person year follow-up | Rate | No. deaths/person year follow-up | Rate | |
| 2003 | 1,234/22,214 | 56 | 444/7,354 | 60 | 492/7,441 | 66 | 298/7,418 | 40 |
| 2004 | 1,203/22,022 | 55 | 369/7,380 | 50 | 534/7,323 | 73 | 300/7,319 | 41 |
| 2005 | 1,045/21,868 | 48 | 360/7,239 | 50 | 455/7,317 | 62 | 230/7,312 | 32 |
| 2006 | 873/22,249 | 39 | 266/7,262 | 37 | 436/7,438 | 59 | 171/7,549 | 23 |
| 2007 | 865/22,920 | 30 | 198/7,516 | 26 | 285/7,663 | 31 | 382/7,746 | 33 |
| 2008 | 1,702/24,124 | 48 | 472/8,037 | 42 | 760/8,112 | 63 | 470/7,975 | 39 |
| 2009 | NA | NA | 376/7,838 | 36 | 548/7,797 | 48 | NA | NA |
NA = not available.
Figure 1.
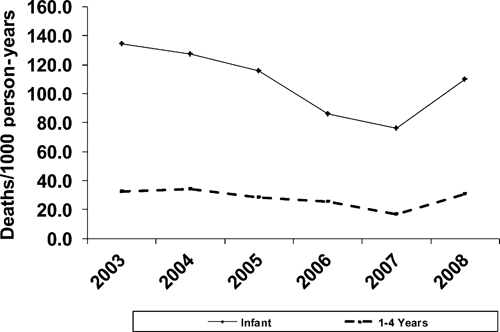
All cause childhood mortality in the Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System, 2003–2008.
Table 4.
Trends in childhood mortality ratios, Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System, 2003–2008
| Characteristic | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 |
|---|---|---|---|---|---|---|
| No. births | 5,180 | 4,977 | 4,937 | 5,163 | 5,125 | 5,531 |
| No. neonatal deaths | 134 | 125 | 93 | 79 | 96 | 140 |
| No. post-neonatal deaths (29 days–11 months) | 536 | 487 | 463 | 350 | 290 | 441 |
| No. infant deaths (< 1–11 months) | 670 | 615 | 561 | 429 | 386 | 582 |
| No. child deaths (1–4 years) | 564 | 588 | 484 | 444 | 300 | 574 |
| No. under-five deaths (< 1–4 years) | 1,234 | 1,203 | 1,045 | 873 | 686 | 1,156 |
| Neonatal mortality ratio (deaths/1,000 live births) | 19 | 21 | 15 | 13 | 16 | 20 |
| Post-neonatal mortality ratio (deaths/1,000 live births) | 109 | 102 | 97 | 70 | 57 | 88 |
| Infant mortality ratio (deaths/1,000 live births) | 126 | 121 | 111 | 82 | 72 | 105 |
| Child mortality ratio (deaths/1,000 live births) | 131 | 134 | 115 | 105 | 70 | 120 |
| Under-five mortality ratio (deaths/1,000 live births) | 240 | 239 | 213 | 178 | 137 | 212 |
As shown in Table 4, considerable increases were also observed in neonatal deaths, with initial increases first noted in 2007. Verbal autopsy data indicated that neonatal sepsis, stillbirths, and prematurity were the most common causes of neonatal mortality. Proportional neonatal mortality due to stillbirths increased in 2007 and those due to prematurity increased in 2008, while proportional neonatal mortality from sepsis decreased in both 2007 and 2008.
Malaria and/or anemia was the most common cause of post-neonatal mortality throughout the study period. Other common causes were pneumonia, malnutrition, HIV infection, diarrhea, and meningitis. Increases occurred in all common causes in 2008, except meningitis (Figure 2). The decrease observed in HIV-specific mortality from 2006–2007 likely reflect changes in verbal autopsy coding for HIV mortality.
Figure 2.
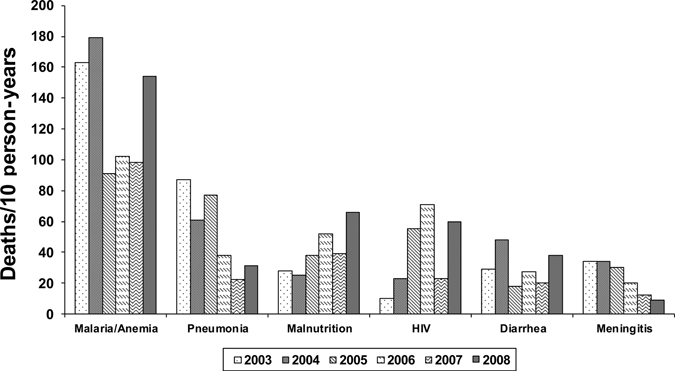
Verbal autopsy cause-specific under-five mortality rates, (excluding the neonatal period of 0–28 days of life), Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System, 2003–2008.
Triannual mortality rates show a markedly lower under-five mortality rate during the second round of 2007 compared with that in the second round in other years (Table 3). The second round of data collection was conducted over a four-month period during May–August, and typically encompassed the peak malaria transmission period. An increase in under-five mortality began in the third round in 2007, and showed a 31% increase over the third round in 2006.
Trends in SDH inpatient mortality are shown in Figure 3. During 2003–2007, the number of children who died of most common illnesses decreased. When we compared cause-specific inpatient mortality in 2007 to that in 2008, a modest increase was observed in most causes of childhood diseases, and substantial proportional increases were noted in malaria/anemia and malnutrition. The greatest increase in number of deaths was due to malaria/anemia. In-patient mortality decreased in 2009. However, malaria/anemia-specific mortality remained elevated when compared with that in 2007, and deaths from malnutrition increased.
Figure 3.
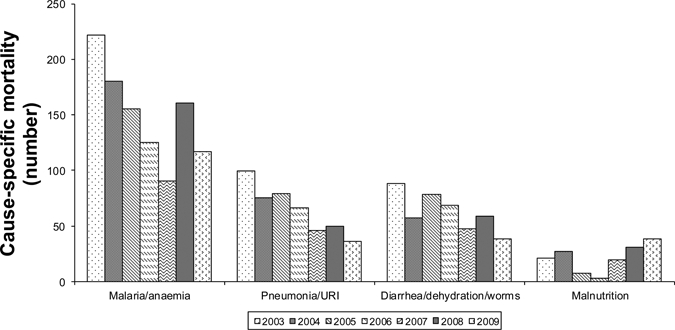
Under-five mortality Siaya District Hospital, Kenya, 2003–2009.
Possible contributing factors to changes in mortality.
Malaria.
Clinical data indicate an increase in blood smear–proven malaria cases beginning in 2007 after years of steady decreases. The number and percentage of children < 5 years of age admitted to SDH with malaria parasitemia decreased between 2004–2006, peaked in 2008, and decreased minimally in 2009 (Figure 4A). Data from Lwak Mission Hospital show increasing blood smear–proven malaria cases beginning in October 2007 (Figure 4B).
Figure 4.
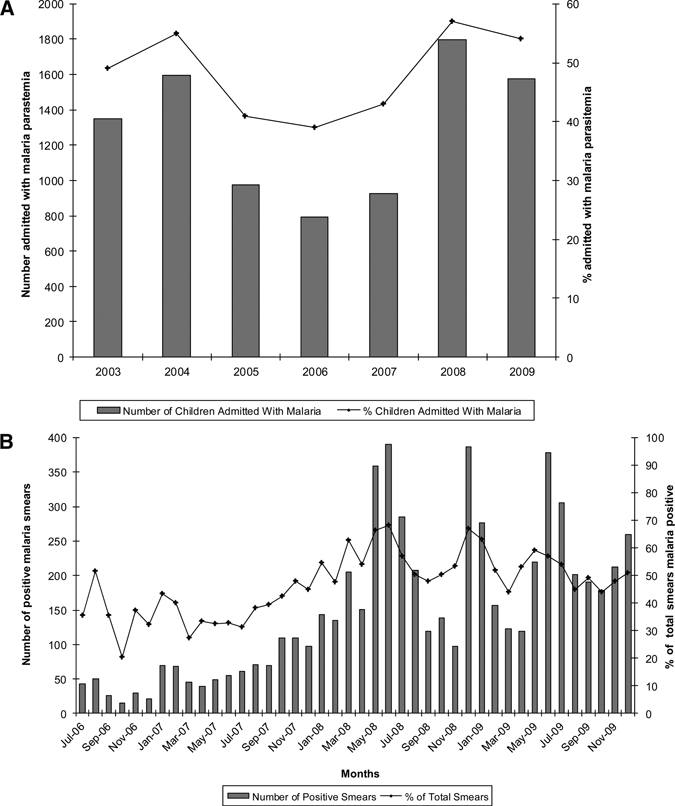
A, Children under-five admitted malaria blood smear positive, Siaya District Hospital, Kenya, 2003–2009. B, Children under-five presenting malaria blood smear positive, Lwak Mission Hospital, Kenya, July 2006–December 2009.
The HDSS community parasitemia prevalence mirrored but lagged behind clinic-based morbidity and mortality data. Plasmodium falciparum parasitemia prevalence among children < 5 years of age in the HDSS decreased steadily from 60% (95% confidence interval [CI] = 0.56–0.65) in 2003 to 26% (95% CI = 0.20–0.35) in 2008, an overall 14% decrease in parasitemia annually (relative risk [RR] = 0.86, 95% CI = 0.82–0.90, by Poisson regression linear test for trend). Parasitemia prevalence among children < 5 years of age increased to 41% (95% CI = 0.37–0.46) in 2009, which was significantly higher than prevalence in 2008 (RR = 1.58, 95% CI = 1.16–2.20) (Figure 5). The community prevalence of moderate anemia (hemoglobin level < 8 g/dL) among children < 5 years of age decreased from 11% (95% CI = 0.09–0.13) in 2003 to 4% (95% CI = 0.02–0.08) in 2007, then increased nearly 5-fold to 19% (95% CI = 0.15–0.24), in 2009 (RR = 4.83, 95% CI = 2.30–10.34).
Figure 5.
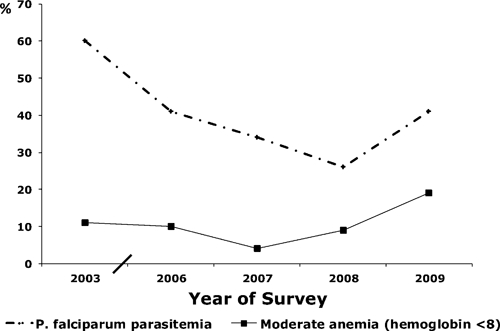
Trends in Plasmodium falciparum and anemia prevalence in children less than five years of age, Kenya Medical Research Institute/Centers for Disease Control and Prevention Health and Demographic Surveillance System, 2003–2009.
This increase in malaria parasite and anemia prevalence occurred despite relatively high ITN use. Reported ITN use the prior night among children < 5 years of age during 2006–2009 was 59% (95% CI = 0.52–0.67%) in 2006, 69% (95% CI = 0.62–0.76%) in 2007, 49% (95% CI = 0.41–0.59%) in 2008, and 64% (95% CI = 0.59–0.70) in 2009; the lowest ITN use was observed in 2008, the year with the lowest recorded malaria prevalence. Changes in ITN use were not statistically significant (linear trend over time = 1.01, 95% CI = 0.96–1.06).
Rainfall or change in entomologic inoculation rate (EIR) could not account for the increase in malaria parasitemia and anemia. Mean annual rainfall was 1,446 mm; annual rainfall was 1,366 mm in 2003, 1,376 mm in 2004, 1,671 mm in 2005, 1,789 mm in 2006, 1,123 mm in 2007, and 1,352 mm in 2008. Rainfall was not predictive of community parasite prevalence (RR = 1.00, 95% CI = 0.99–1.01 for every 100 mm of increase rainfall in the 12 months and RR = 1.03, 95% CI = 0.995–1.06 for every 100 mm of increase rainfall in the 3 months before the annual survey). There was no evidence that an increase in vector numbers or EIR contributed to the increase in malaria. The EIR remained < 10 throughout the study period4 (KEMRI/CDC, unpublished data).
Antimalarial drug stock-outs.
After its introduction in August 2006, AL stock-outs in child and adult dosages occurred in Siaya District during September and October 2007 and January, February, April, May, June, November and December 2008. Only 28% of children who came to the HDSS sentinel surveillance facility in Gem in 2008 with clinical malaria received AL (data not available for other years). The second-line treatment of malaria when AL was not available was quinine. The only other oral antimalarial drug included in standard government health facility formularies was sulfadoxine-pyrimethamine, which is indicated for presumptive intermittent treatment of malaria during pregnancy.17
Pneumonia and vaccinations.
Amoxicillin was introduced into health facilities in 2004. In 2008, 35% of children < 5 years of age seen in the HDSS surveillance health facility were prescribed amoxicillin or ampicillin. Surveillance data from all health facilities in the HDSS indicate there were no stock-outs of antibiotics during the period of observation. Full Expanded Program on Immunization vaccine coverage increased considerably in Nyanza Province (Table 2).
HIV.
A total of 47% (95% CI = 44.4–49.1%) of mothers who gave birth during 2003–2007 self-reported being tested for HIV during their last pregnancy (KEMRI/CDC, unpublished data). Among women who gave birth in 2003, 23% (95% CI = 0.15–0.31%) reported having been tested for HIV, and this value increased to 60% (95% CI = 0.54–0.62%) in 2007. We estimate that implementation of PMCT resulted in a 1–6% reduction in under-five mortality during 2003–2008 (Table 2).
Safe water, education, and SES.
Treated water use and SES among HDSS residents improved modestly (Table 2). There was no improvement in adult female education.
Discussion
During 2003–2007, a promising 43% reduction in infant and 48% reduction in child mortality were recorded in the KEMRI/CDC HDSS, which were coincident with scale-up of proven child survival interventions and modest improvement in SES. Unfortunately, a marked increase in mortality rates occurred in 2008.
The decrease in under-five malaria/anemia-specific mortality mirrors a decrease in community malaria and anemia prevalence, and in this area of high ITN use, coincides with the change in 2004 of first-line malaria treatment from sulfadoxine-pyrimethamine, an antimalarial drug that was failing as a treatment because of increased drug resistance, to amodiaquine, a more effective drug, and finally in 2006, to AL, a highly effective, rapidly acting drug. During August 2006–September 2007, when AL was reliably available in health facilities, triannual mortality rates were notably less compared with rates for the same periods in prior and subsequent years.
The steady decrease in pneumonia-specific mortality occurred concurrent with the change to a more effective first-line treatment for pneumonia,18 improved care-seeking, improved vaccination coverage,5 and a decrease in malaria, which may contribute to pneumonia incidence.19 Diarrhea-specific mortality decreased considerably during 2004–2005, coincident with hygiene and in-home water treatment promotion, and increased use of oral rehydration salts.5 Meningitis-specific mortality decreased as Haemophilus influenzae b vaccination coverage increased.
Increased PMCT should have resulted in reductions in under-five mortality. However, verbal autopsy data showed no substantial sustained reductions in under-five HIV-specific mortality during 2003–2008, and a possible increase during 2007–2008. This finding could be attributed to a lack of specificity for verbal autopsy pediatric HIV diagnosis.20 However, if PMCT is not resulting in measurable reductions in under-five mortality, this finding is critical information that might indicate that perinatal antiretroviral therapy was not readily accessible or used in this area, where 80% of births occur at home,21 or that perinatal antiretroviral therapy might not be sufficient in an area where breast-feeding is nearly universal and mixed feeding practices are common.22 The increase in malnutrition deaths in the context of improving SES may be an indication of continued perinatal HIV transmission with nutritional decline or wasting among HIV infected children. Likewise, the increase in stillbirths and premature births that contributed to the rise in neonatal deaths could be a result of perinatal HIV infection. An alternative explanation might be increased malaria infections during pregnancy. It cannot be overlooked that increased use of routine antiretroviral therapy by mothers might have contributed to overall reductions in child mortality independent of PMCT. Both HIV-infected and HIV-uninfected children born to HIV-infected mothers with lower viral loads are less likely to die, perhaps because of greater functionality of mothers who are not sick.23
The increase in under-five mortality in 2008 is a robust finding; it was observed in community-based HDSS data, among inpatients at Siaya District Hospital, and in all rounds of 2008. Notably, there were no major changes in the methods of HDSS during 2007–2008, and increased mortality was not global; minimal increases in mortality among older children and adults were observed during this period.
Although increases were measured in most major causes of childhood mortality in 2008, a major contributor to increased under-five mortality was an increase in malaria-specific mortality. This occurred in the context of widespread antimalarial drug stock-outs at health facilities during a time when malaria infection and illness were increasing. The first stock-outs of children's dosages of AL were documented in October 2006, although adult AL was available. Stock-outs of both adult and pediatric dosages of AL began in September 2007 and became more frequent in 2008, when AL was unavailable for seven months of the year, including three months during peak malaria transmission season. Simultaneous stock-outs of pediatric and adult dosages precluded the common practice of dividing adult AL packets into appropriate child dosages.
During times of AL stock-out, antimalarial treatment options were limited, and children may have remained untreated or inadequately treated. Drawing from the standard health facility formulary, the health worker had the option of providing quinine, which is bitter and can be difficult to administer for the full seven-day treatment regimen, sulfadoxine-pyrimethamine, which is well tolerated and easily administered, but has poor treatment efficacy in Kenya because of high levels of resistance,24 or a prescription, which may not be filled as prescribed in this rural setting with few licensed pharmacies, and an impoverished population. Thus, the increase in malaria-specific mortality may have been caused by lack of access to any effective antimalarial drug during times of AL stock-out.
Community data showed decreasing malaria prevalence among children < 5 years of age until 2008 and anemia until 2007. The parasitemia data contrast with inpatient data from SDH and Lwak Mission Hospital that showed malaria admissions increased beginning in late 2007. The lag between increased parasitemia prevalence in health facilities and the community may indicate wide-spread antimalarial drug stock-outs resulted in inadequate treatment of malaria-infected children, who progressed to severe disease and hospitalization before the increase in community parasitemia. Notably, AL stock-outs were not particular to Nyanza Province, being documented nationwide in 2008.25,26 The increase in malaria prevalence and mortality occurred despite high ITN use, a worrisome finding that indicates that ITNs alone are not sufficient to prevent increases in malaria.
The increase in mortality in 2008 was not restricted to malaria, but occurred in other common causes of childhood mortality, including pneumonia, diarrhea, malnutrition, and infection with HIV. Studies from other parts of Africa have shown a decrease in all-cause and non-malaria-specific causes of mortality in parallel with a decrease in malaria mortality.27,28 To our knowledge, the simultaneous increase in pneumonia, diarrhea, and malnutrition mortality with increased malaria mortality has not been reported. It is possible that the increase in these other non–malaria-specific causes of mortality reflect a widespread change in vulnerability or access to health care of the population. Increasing malnutrition likely contributed to deaths from these common causes of childhood death. It might also be the case that these synchronous temporal changes in the cause of death in children was related to the impact that malaria has on children's nutritional and immune status, which puts them at greater risk for death from other infectious diseases.29 Alternatively, it might also be related to the non-specificity of verbal autopsy for the cause of death in children, particularly in malarious areas where it has been shown that malaria and pneumonia can be difficult to distinguish.30,31
Malnutrition can contribute to mortality directly or indirectly, increasing the risk of death from common childhood illnesses. Community-based malnutrition deaths in the HDSS first increased in 2005 during a time when mortality from other causes was decreasing. In-patient deaths increased in 2007 and 2008, in parallel with the other mortality increases described above.
Although the first increases in under-five mortality were measured during the last four months of 2007, some increase in child mortality observed in 2008 may be attributable to civil unrest associated with post-election violence that began December 29, 2007. The HDSS experienced a sudden population growth as internally displaced persons (IDPs) returned to their ancestral areas. The IDP status did not result in increased childhood mortality; IDPs living in the HDSS had lower under-five mortality rates than resident children.32 Moreover, IDP childhood deaths did not contribute to HDSS population-based estimates of mortality until after April 27, 2008, the earliest IDPs would become HDSS residents. However the consequences of post-election violence, including limited access to health care and essential drugs during the first 3–4 months of 2008, and an increased strain on already limited household resources, might have contributed to increased under-five deaths, particularly HIV-related and malnutrition-related deaths. Notably, increases in under-five mortality began before post-election violence, increased mortality rates continued well after disruptions in services normalized, and almost half the IDPs left the study area by July 2008,32 indicating that although post-election violence likely contributed to the observed increase in under-five mortality, it cannot explain it fully.
The analysis had limitations. As mentioned, verbal autopsy is an inexact method for determining causes of mortality, and can particularly confuse malaria and pneumonia in malarious areas.30,31 However, in resource-poor settings, where most deaths occur outside medical institutions, it is a useful tool for following mortality trends. Coding of the cause of death was conducted by the same clinical officers over time, providing longitudinal internal validity. HIV-specific mortality was affected by modifications in coding made in accordance with WHO recommendations, and implemented in the HDSS in 2006.7,33 Data on AL stock-outs in Siaya were not available and were assumed to be similar to Asembo. However, differences in consumption might have resulted in different stock-out dates. Factors that may have contributed to increased mortality are speculative, and cannot be proved as causal.
In conclusion, promising gains in child survival occurred in western Kenya over a five-year period during a time when the Kenyan Ministry of Health adopted numerous child-survival public health policies. Unfortunately, a setback occurred in 2008 in the context of good policy and established child survival programs. To sustain reductions in child mortality, good policy is not enough; reliable supply and access to effective essential drugs are critical. Anticipation of and concrete action plans to address potential disruptions in services are needed. The establishment of reliable mechanisms to ensure the availability of effective essential drugs, such as antimalarial drugs and antiretroviral drugs, and full scale-up of PMCT and pediatric anti-retroviral therapy could put this area of Kenya back on track to reaching the Fourth Millennium Development Goal.
ACKNOWLEDGMENTS
This paper is dedicated to the memory of our colleague and friend, the late Dr. Kubaje Adazu, who was instrumental in establishing the KEMRI/CDC HDSS. We thank Dr. Kim Lindblade for assisting with the development of the HDSS, Dr. Annemieke van Eijk for establishing the verbal autopsy system in the HDSS, Amek Nyaguara for contributing to the SES analysis, Sheila Ogwang for assisting with HDSS data analysis, the HDSS staff and the community for generously allowing us to collect this information for public health purposes, and the CDC Division of Global HIV/AIDSs Program in Kisumu for kindly assisting with the health facility HIV information used in this paper. KEMRI/CDC is a member of the INDEPTH Network. This paper is published with permission from the Director of KEMRI.
Disclaimer: The views expressed in this paper represent those of the authors and do not necessarily reflect those of the U.S. Centers for Control and Prevention.
Footnotes
Disclosure: None of the authors have any conflicts of interest.
Authors' addresses: Mary J. Hamel, Laurence Slutsker, and Daniel R. Feikin, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: mhamel@cdc.gov, lslutsker@cdc.gov, and dfeikin@jhsph.edu. David Obor and Maquins Sewe, KEMRI/CDC Research Station, Kisumu, Kenya, E-mails: dobor@ke.cdc.gov and msewe@ke.cdc.gov. John Vulule, Kenya Medial Research Institute, Kisumu, Kenya, E-mail: jvulule@ke.cdc.gov. John Williamson and Kayla Laserson, KEMRI/CDC Research Station, Unit 64112, E-mails: jwilliamson@ke.cdc.gov and klaserson@ke.cdc.gov.
References
- 1.United Nations . The Millenium Development Goals Report, 2009. New York: United Nations; 2009. [Google Scholar]
- 2.Laserson KF, Odhiambo F, Hamel MJ, Feikin DR, Bayoh N, Nyaguara AO, Sewe M, Ogwang S, Obor D. KEMRI/CDC Health and Demographic Surveillance System, Kisumu, Kenya, Fifth Annual Report, Sixth Year of Registration of Demographic Events, 2007. Kisumu: KEMRI/CDC; 2009. [Google Scholar]
- 3.Phillips-Howard PA, Nahlen BL, Alaii JA, ter Kuile FO, Gimnig JE, Terlouw DJ, Kachur SP, Hightower AW, Lal AA, Schoute E, Oloo AJ, Hawley WA. The efficacy of permethrin-treated bed nets on child mortality and morbidity in western Kenya I. Development of infrastructure and description of study site. Am J Trop Med Hyg. 2003;68:3–9. [PubMed] [Google Scholar]
- 4.Adazu K, Lindblade KA, Rosen DH, Odhiambo F, Ofware P, Kwach J, Van Eijk AM, Decock KM, Amornkul P, Karanja D, Vulule JM, Slutsker L. Health and demographic surveillance in rural western Kenya: a platform for evaluating interventions to reduce morbidity and mortality from infectious diseases. Am J Trop Med Hyg. 2005;73:1151–1158. [PubMed] [Google Scholar]
- 5.Kenya National Bureau of Statistics (KNBS) and ICF Macro . Kenya Demographic and Health Survey 2008–09. Calverton, MD: KNBS and ICF Macro; 2010. [Google Scholar]
- 6.Amornkul PN, Vandenhoudt H, Nasokho P, Odhiambo F, Mwaengo D, Hightower A, Buve A, Misore A, Vulule J, Vitek C, Glynn J, Greenberg A, Slutsker L, De Cock KM. HIV prevalence and associated risk factors among individuals aged 13–34 years in rural western Kenya. PLoS ONE. 2009;4:e6470. doi: 10.1371/journal.pone.0006470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anker M, Black RE, Coldham C, Kalter HD, Quigley MA, Ross D, Snow RW. A Standard Verbal Autopsy Method for Investigating Causes of Death in Infants and Children. WHO/CDS/CSR/ISR/99.4. Geneva: World Health Organization, Baltimore: Department of Communicable Disease Surveillance and Response: The Johns Hopkins School of Hygiene and Public Health, and London: London School of Hygiene and Tropical Medicine; 1999. [Google Scholar]
- 8.McElroy PD, Terk Kuile FO, Hightower AW, Hawley WA, Phillips-Howard PA, Oloo AJ, Lal AA, Nahlen BL. All-cause mortality among young children in western Kenya. VI: The Asembo Bay Cohort Project. Am J Trop Med Hyg. 2001;64:18–27. doi: 10.4269/ajtmh.2001.64.18. [DOI] [PubMed] [Google Scholar]
- 9.Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. J Nutr. 2001;131:636S–645S. doi: 10.1093/jn/131.2.636S. discussion 646S–648S. [DOI] [PubMed] [Google Scholar]
- 10.Akhwale WS, Lum JK, Kaneko A, Eto H, Obonyo C, Bjorkman A, Kobayakawa T. Anemia and malaria at different altitudes in the western highlands of Kenya. Acta Trop. 2004;91:167–175. doi: 10.1016/j.actatropica.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 12.Lindblade KA, Mwololo K, van Eijk AM, Peterson E, Odhiambo F, Williamson J, Slutsker L. Evaluation of the WHO Haemoglobin Colour Scale for diagnosis of anaemia in children and pregnant women as used by primary health care nurses and community health workers in western Kenya. Trop Med Int Health. 2006;11:1679–1687. doi: 10.1111/j.1365-3156.2006.01721.x. [DOI] [PubMed] [Google Scholar]
- 13.Kenya National Council for Population and Development, Central Bureau of Statistics Macro International Demographic and Health Survey of Kenya in 1993. Afr J Med Pract. 1994;1:41–48.. [PubMed] [Google Scholar]
- 14.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, Sherman J, Bakaki P, Ducar C, Deseyve M, Emel L, Mirochnick M, Fowler MG, Mofenson L, Miotti P, Dransfield K, Bray D, Mmiro F, Jackson JB. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 15.Dabis F, Msellati P, Meda N, Welffens-Ekra C, You B, Manigart O, Leroy V, Simonon A, Cartoux M, Combe P, Ouangre A, Ramon R, Ky-Zerbo O, Montcho C, Salamon R, Rouzioux C, Van de Perre P, Mandelbrot L. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. Diminution de la transmission mere-enfant. Lancet. 1999;353:786–792. doi: 10.1016/s0140-6736(98)11046-2. [DOI] [PubMed] [Google Scholar]
- 16.Preble EA. Impact of HIV/AIDS on African children. Soc Sci Med. 1990;31:671–680. doi: 10.1016/0277-9536(90)90249-r. [DOI] [PubMed] [Google Scholar]
- 17.Republic of Kenya Ministry of Health . National Guidelines for Diagnosis, Treatment and Prevention of Malaria. Nairobi, Kenya: Ministry of Health, Division of Malaria Control; 2006. [Google Scholar]
- 18.Straus WL, Qazi SA, Kundi Z, Nomani NK, Schwartz B. Antimicrobial resistance and clinical effectiveness of co-trimoxazole versus amoxicillin for pneumonia among children in Pakistan: randomised controlled trial. Pakistan Co-trimoxazole Study Group. Lancet. 1998;352:270–274. doi: 10.1016/s0140-6736(97)10294-x. [DOI] [PubMed] [Google Scholar]
- 19.Abdulla S, Oberholzer R, Juma O, Kubhoja S, Machera F, Membi C, Omari S, Urassa A, Mshinda H, Jumanne A, Salim N, Shomari M, Aebi T, Schellenberg DM, Carter T, Villafana T, Demoitié MA, Dubois MC, Leach A, Lievens M, Vekemans J, Cohen J, Ballou WR, Tanner M. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359:2533–2544. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- 20.Dowell SF, Davis HL, Holt EA, Ruff AJ, Kissinger PJ, Bijoux J, Boulos R, Boulos C, Halsey NA. The utility of verbal autopsies for identifying HIV-1-related deaths in Haitian children. AIDS. 1993;7:1255–1259. doi: 10.1097/00002030-199309000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Ouma PO, van Eijk AM, Hamel MJ, Sikuku ES, Odhiambo FO, Munguti KM, Ayisi JG, Crawford SB, Kager PA, Slutsker L. Antenatal and delivery care in rural western Kenya: the effect of training health care workers to provide “focused antenatal care”. Reprod Health. 2010;7:1. doi: 10.1186/1742-4755-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaai S, Baek C, Geibel S, McOdida P, Benson U, Muthumbi G, N'Katha-Matiko C. HIV Serostatus and Infant Feeding Counseling and Practice: Findings from a Baseline Study among the Urban Poor in Kenya. Washington, DC: Population Council; 2005. p. 6. [Google Scholar]
- 23.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, Sewankambo N, Kiduggavu M, Wawer M, Gray R. Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41:504–508. doi: 10.1097/01.qai.0000188122.15493.0a. [DOI] [PubMed] [Google Scholar]
- 24.Obonyo CO, Ochieng F, Taylor WR, Ochola SA, Mugitu K, Olliaro P, ter Kuile F, Oloo AJ. Artesunate plus sulfadoxine-pyrimethamine for uncomplicated malaria in Kenyan children: a randomized, double-blind, placebo-controlled trial. Trans R Soc Trop Med Hyg. 2003;97:585–591. doi: 10.1016/s0035-9203(03)80038-x. [DOI] [PubMed] [Google Scholar]
- 25.Tren R, Hess K, Bate R. Drug procurement, the Global Fund and misguided competition policies. Malar J. 2009;8:305. doi: 10.1186/1475-2875-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kangwana BB, Njogu J, Wasunna B, Kedenge SV, Memusi DN, Goodman CA, Zurovac D, Snow RW. Malaria drug shortages in Kenya: a major failure to provide access to effective treatment. Am J Trop Med Hyg. 2009;80:737–738. [PMC free article] [PubMed] [Google Scholar]
- 27.Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–597. doi: 10.1016/s1471-4922(01)02031-1. [DOI] [PubMed] [Google Scholar]
- 28.Sacarlal J, Nhacolo AQ, Sigauque B, Nhalungo DA, Abacassamo F, Sacoor CN, Aide P, Machevo S, Nhampossa T, Macete EV, Bassat Q, David C, Bardaji A, Letang E, Saute F, Aponte JJ, Thompson R, Alonso PL. A 10 year study of the cause of death in children under 15 years in Manhica, Mozambique. BMC Public Health. 2009;9:67. doi: 10.1186/1471-2458-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malaria. Snow RW, Omumbo JA. In: Disease and mortality in sub-Saharan Africa. 2nd edition. Jamison DT, Feachem RG, Makgoba MW, Bos ER, Baingana FK, Hofman KJ, Rogo KO, editors. Washington, DC: World Bank; 2006. Chapter 14. [PubMed] [Google Scholar]
- 30.English M, Punt J, Mwangi I, McHugh K, Marsh K. Clinical overlap between malaria and severe pneumonia in African children in hospital. Trans R Soc Trop Med Hyg. 1996;90:658–666. doi: 10.1016/s0035-9203(96)90423-x. [DOI] [PubMed] [Google Scholar]
- 31.Snow RW, Armstrong JR, Forster D, Winstanley MT, Marsh VM, Newton CR, Waruiru C, Mwangi I, Winstanley PA, Marsh K. Childhood deaths in Africa: uses and limitations of verbal autopsies. Lancet. 1992;340:351–355. doi: 10.1016/0140-6736(92)91414-4. [DOI] [PubMed] [Google Scholar]
- 32.Feikin DR, Adazu K, Obor D, Ogwang S, Vulule J, Hamel MJ, Laserson K. Mortality and health among internally displaced persons in western Kenya following post-election violence, 2008: novel use of demographic surveillance. Bull World Health Organ. 2010;88:601–608. doi: 10.2471/BLT.09.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.SAVVY Sample Vital Registration with Verbal Autopsy: Verbal Autopsy Certifier and Coder's Manual. 2007 http://www.cpc.unc.edu/measure/publications/ms-07-26-vac August. Available at. [Google Scholar]


