Abstract
Hematologic changes in acute and convalescent uncomplicated Plasmodium falciparum malaria have not been well studied, particularly in young children in Africa. Hematologic data were obtained for 3,044 children less than five years of age in seven randomized controlled trials at 14 sites. Using paired analysis between day 28 and baseline in patients without parasitologic failure as a proxy for malaria-induced effects, we found a statistically significant but clinically modest increase in leukocyte counts (5%) resulting from a larger increase in neutrophils (43%) than the decrease in lymphocytes counts (–16%); levels of hemoglobin and platelets decreased (–13% and –49%, respectively). Multivariate random effects analysis showed trends during follow-up (increased levels of hemoglobin, platelets and lymphocytes, and decreased levels of leukocytes and neutrophils) and identified explanatory variables. The risk of neutropenia increased with follow-up time independent of treatment outcome, and was lower with age, higher baseline parasitemia, and artemisinin combination treatment. These analyses provides information on hematologic variations caused by malaria.
Introduction
Hematologic changes in acute and convalescent Plasmodium falciparum malaria have not been well-studied, particularly in the most vulnerable population, children in malaria-endemic areas. Without such information, it is also difficult to appreciate the hematologic toxicity of antimalarial drugs.
To our knowledge, there is a general lack of data on hematologic parameters for malaria in children in Africa and on the clinical significance of variations of these parameters in patients treated with antimalarial drugs.1 Except for anemia, little is known about children in Africa with P. falciparum malaria; other hematologic parameters pre-antimalarial and post-antimalarial treatments have not been studied (or at least reported) systematically for this population. Most of the (limited) data available from the existing literature concerns adult malaria patients from areas of low malaria endemicity.2–5 Moreover, investigations have been conducted essentially on baseline values and have mostly ignored leukocyte differential counts and their changes post-treatment; the latter are reported in a fraction of the published randomized controlled trials (RCTs), and when they are, they concern changes of individual parameters without multivariate analysis.
The purpose of this study was to obtain and analyze hematologic parameters for children in Africa less than five years of age at presentation with acute uncomplicated P. falciparum malaria at the time of enrollment into RCTs and after antimalarial treatment. This was conducted to investigate how malaria affects hematologic parameters and how these parameters change post-antimalarial treatment (caused by recovery or treatment-induced adverse effects).
Methods
Data and patients.
Data on age, parasitemia, hematologic parameters (leukocyte total counts, neutrophil and lymphocyte counts, hemoglobin levels or hematocrit, platelet counts), treatment and treatment outcome were extracted from a database of seven RCTs conducted with 28-day follow-up at 14 sites in sub-Saharan Africa6–12 (Table 1). Data were censored for patients who did not complete follow-up or had recurrent P. falciparum infections. Details on these studies have been reported.13 Analyses were restricted to children less than five years of age for whom leukocyte counts were available at study enrollment (n = 3,044). The number of patients treated with an artemisinin-based combination therapy (ACT) was 2,459 (81%): artesunate plus amodiaquine (AS + AQ; n = 1,633, 54%) compared with artemether-lumefantrine (n = 396, 13%), artesunate plus sulfadoxine-pyrimethamine (n = 217, 7%), dihydroartemisinin-piperaquine (n = 211, 7%); and non-ACT (n = 585, 19%): AS (n = 228, 8%), AQ (n = 39, 1%), quinine plus clindamycin (n = 29, 1%), and AQ + sulfadoxine-pyrimethamine (n = 289, 9%). Parasite densities (number/μL) were computed by using the actual baseline leukocyte count instead of the standard approximation method.14
Table 1.
Admission values by study site for patients with uncomplicated Plasmodium falciparum malaria, sub-Saharan Africa
| Site | Reference | Age (years) | Parasitemia geometric mean (/μL) | Leukocytes (×109 cells/L) | Neutrophils (×109 cells/L) | Lymphocytes (×109 cells/L) | Hemoglobin (g/dL) | Platelets (×109cells/L) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | No. | Mean | SD | |||
| Pouytenga, Burkina Faso | 12 | 2.0 | 1.2 | 18,966 | 582 | 11.5 | 4.8 | 567 | 4.1 | 2.7 | 582 | 6.1 | 3.4 | 582 | 5.1 | 1.0 | 582 | 177 | 112 |
| Mendong, Cameroon | 11 | 2.9 | 0.9 | 16,479 | 72 | 8.2 | 2.1 | 71 | 4.1 | 1.7 | 72 | 3.8 | 4.8 | 72 | 11.3 | 1.3 | |||
| Lambaréné, Gabon | 6 | 3.2 | 0.9 | 17,026 | 71 | 8.2 | 2.9 | 68 | 3.3 | 1.9 | |||||||||
| Migori, Kenya | 6 | 2.4 | 1.6 | 11,035 | 28 | 9.9 | 4.2 | 28 | 6.2 | 3.3 | 28 | 8.8 | 1.8 | ||||||
| Tsiroanomandidy, Madagascar | 11 | 3.3 | 0.8 | 21,942 | 55 | 7.7 | 2.9 | 50 | 4.2 | 2.6 | 55 | 2.9 | 1.2 | 55 | 9.8 | 2.2 | |||
| Bancoumana, Mali | 11 | 3.0 | 0.9 | 37,676 | 94 | 10.6 | 3.6 | 90 | 6.4 | 3.0 | 94 | 3.0 | 1.6 | 94 | 9.2 | 1.5 | |||
| Bougoula, Mali | 7 | 2.3 | 1.2 | 35,295 | 669 | 10.4 | 4.4 | 668 | 10.1 | 1.8 | 617 | 179 | 95 | ||||||
| Kicukiro, Rwanda | 9 | 2.9 | 1.0 | 14,151 | 174 | 6.8 | 2.6 | 174 | 3.7 | 1.9 | 174 | 10.7 | 1.9 | ||||||
| Mashesha, Rwanda | 9 | 2.8 | 1.0 | 27,402 | 218 | 5.4 | 1.6 | 218 | 3.0 | 1.3 | 218 | 10.2 | 1.5 | ||||||
| Rukara, Rwanda | 9 | 2.3 | 1.0 | 27,597 | 250 | 4.0 | 1.6 | 250 | 1.8 | 1.0 | 250 | 10.4 | 1.8 | ||||||
| Senegal | 6 | 3.1 | 0.9 | 15,285 | 204 | 11.2 | 4.6 | 189 | 5.8 | 3.4 | 189 | 4.4 | 2.3 | 200 | 8.8 | 2.1 | |||
| Kampala, Uganda | 23 | 3.2 | 0.8 | 54,353 | 249 | 9.1 | 3.8 | 247 | 4.7 | 2.9 | 249 | 10.9 | 1.3 | 243 | 228 | 107 | |||
| Kivunge, Zanzibar | 10 | 2.4 | 1.2 | 10,456 | 278 | 6.3 | 3.1 | 278 | 3.5 | 2.4 | 278 | 8.7 | 1.5 | ||||||
| Micheweni, Zanzibar | 10 | 2.0 | 1.2 | 23,813 | 100 | 6.5 | 1.5 | 100 | 3.1 | 1.2 | 100 | 8.0 | 1.4 | ||||||
| Total | 2.5 | 1.2 | 18,434 | 3,044 | 8.8 | 4.5 | 2,330 | 3.9 | 2.6 | 992 | 5.2 | 3.4 | 2,968 | 8.9 | 2.6 | 1,442 | 187 | 106 | |
Statistical analysis.
Baseline hematologic parameters (log-transformed) were analyzed by using a linear model including age (in years) and parasitemia (log-transformed) and their interaction. A random effect for each study site was included when the Lagrange multiplier (LM) test15 result was significant for heterogeneity between studies.
During the 28-day follow-up, the timing of post-treatment hematologic assessments varied across studies (Table 2). Therefore, an adjustment for time (day of observation) was included. A random intercept for each person was included in case of a significant LM test result. All available hematologic values for every patient were considered in linear multivariate analyses for each hematologic parameter (continuous, log-transformed) including time trends (in days), age (in years), parasitemia (log-transformed), potential effects of ACT (binary), parasitologic recurrence (binary), and interaction of age and parasitemia.
Table 2.
Follow-up duration per study for hematologic parameters for patients with uncomplicated Plasmodium falciparum malaria, sub-Saharan Africa
| Location (reference) | No. | Leukocytes | Neutrophils | Lymphocytes | Hemoglobin | Platelets |
|---|---|---|---|---|---|---|
| Multi-center (6) | 114 | Day 0 | Days 0, 28 | |||
| Multi-center (11) | 410 | Days 0, 7, 28 | Days 0, 7, 28 | Days 0, 7, 14, 28 | Days 0, 28 | |
| Burkina-Faso (12) | 582 | Days 0, 7, 14, 28 | Days , 7, 14, 28 | Days 0, 7, 14, 28 | Days 0, 7, 14, 28 | Days , 7, 14, 28 |
| Mali (7) | 669 | Days 0, 7, 14, 28 | Days 0, 7, 14, 28 | Days 0, 7, 14 | ||
| Rwanda (9) | 642 | Days 0, 14 | Days 0, 14 | Days 0, 14 | ||
| Uganda (23) | 249 | Days 0, 14 | Days 0, 14 | Days 0, 14 | Days 0, 14 | |
| Zanzibar (10) | 378 | Days 0, 7, 14, 21, D28 | Days 0, 7, 14, 21, 28 | Days 0, 7, 14, 21, 28 |
To illustrate trends in hematologic parameters, a descriptive analysis of the mean values weighted by site is presented for patients with data up to day 28 and a linear interpolation for missing values on day 21 (all except Zanzibar for neutrophils and hemoglobin) and day 14 in some sites (Table 2).
Neutropenia was defined16 as neutrophil counts < 1.2 × 109 cells/L. Mild-to-moderate neutropenia (grades 1 and 2) was defined as 0.4 to 1.2 × 109 cells/L, and severe neutropenia (grades 3 and 4) was defined as < 0.4 × 109 cells/L. A multivariate logistic regression with random effects for each person was included to assess the trend in neutropenia (analyzed as a binary variable) during follow-up and adjusting the risks (adjusted odds ratio [AOR]) by including the above-cited variables.
The influence of malaria on hematologic parameters was studied by using paired t-test analysis that compared convalescent-phase (day 28) and acute-phase (day 0) values in patients who cleared their parasites and in whom parasitemia did not recur (as a proxy for the P. falciparum-attributable fractional change). Changes are presented as paired mean relative difference (percentage, 95% confidence interval [CI] with day 28 as the reference).
The coefficient of correlation resulting from the multiple linear regressions with random effects was noted as r. P values < 0.05 were considered significant. The statistical program used was STATA version 10 (Stata Corp., College Station, TX).
Results
We analyzed data for 3,044 children and 8,295 measurements made at various time points for leukocyte counts: 6,195 for neutrophils, 2,518 for lymphocytes, 8,252 for hemoglobin levels, and 3,680 for platelets.
Relationship between baseline hematologic parameters, parasitemia, and age.
Heterogeneity was detected across sites in baseline hematologic parameters (LM, P < 0.001 for each parameter) and required random effect models. At presentation, leukocyte counts were higher in children with higher parasitemias (r = 0.20, P = 0.001), and there was no significant interaction between age and parasitemia (P = 0.057), i.e. the correlation between leukocyte count and parasitemia did not vary with age. Neutrophil counts were higher in children with higher parasitemias (r = 0.30, P = 0.001) irrespective of age (P = 0.80); no interaction was observed between age and parasitemia (P = 0.72). Lymphocyte counts showed no relationship with parasitemia or age, but there was a significant interaction between age and parasitemia; lymphocyte counts were lower in older children and those who had higher parasitemias (r = –0.06, P = 0.005). Age, parasitemia, and their interaction were not significantly related to hemoglobin levels or platelet counts.
Trends in hematologic parameters during post-treatment follow-up.
Linear regression models with a random effect (LM, P = 0.001, for each parameter) showed trends over time during the post-treatment follow-up for all hematologic parameters studied and identified contributing variables (Table 3). Leukocyte and neutrophil counts showed decreasing trends and lymphocytes show increasing trends. Patients with higher baseline parasitemias had higher leukocyte counts (neutrophils and lymphocytes) during follow-up than those with lower parasitemias. Treatment with an ACT was associated positively with leukocyte and neutrophil counts and negatively with hemoglobin levels and platelet counts; no association was found with lymphocyte counts. Age was positively associated only with an increased hemoglobin level. Recurrent parasitemia during follow-up was negatively associated with increases in lymphocyte counts (in trials with ACTs).
Table 3.
Post-treatment trends in hematology parameters, all patients and observations (linear multivariate analysis, random effect on individuals) for patients with uncomplicated Plasmodium falciparum malaria, sub-Saharan Africa*
| Parameter | Time trend | Significant predictor | Significant interactions |
|---|---|---|---|
| Leukocytes | Downward (P = 0.001) | Baseline parasitemia (+), ACT treatment (+) | None |
| Neutrophils | Downward (P = 0.001) | Baseline parasitemia (+), ACT treatment (+) | None |
| Lymphocytes* | Upward (P = 0.001) | Baseline parasitemia (+), parasitologic recurrence (–) | None |
| Hemoglobin | Upward (P = 0.001) | Age (+), ACT treatment (–) | None |
| Platelets | Upward (P = 0.001) | ACT treatment (–) | None |
Data were available only in trials of artemisinin-based combination drugs (ACTs).
To illustrate hematologic trends, a descriptive analysis was used (Table 4) for aggregate results for individual studies(Figure 1). Results were consistent with those of multivariate analysis. From baseline to day 7, leukocyte counts remained unchanged, then decreased through day 28 in a way that was statistically significant but of uncertain clinical significance. Neutrophil counts decreased from baseline to day 7 and thereafter remained constant through day 28, while lymphocyte counts showed the opposite trend (increased on day 7 and remained constant through day 28).
Table 4.
Evolution of hematologic parameters over time in randomized controlled trials with day 28 data for convalescent-phase patients with uncomplicated Plasmodium falciparum malaria, sub-Saharan Africa
| Parameter | Value | Day 0 | Day 7 | Day 14 | Day 21 | Day 28 |
|---|---|---|---|---|---|---|
| Leukocytes (×109 cells/L) | No. | 1,470 | 1,076 | 547 | NA | 841 |
| Mean | 9.8 | 9.9 | 9.5 | 9.3* | 9.1 | |
| SD | 1.6 | 1.8 | 1.4 | 1.3 | 1.2 | |
| Mean change % | 1% | −3% | NA | −7% | ||
| Neutrophils (×109 cells/L) | No. | 1,175 | 839 | 385 | 316 | 1,078 |
| Mean | 4.5 | 3.4 | 3.3 | 3.3 | 3.2 | |
| SD | 1.2 | 0.5 | 0.6 | 0.6 | 0.6 | |
| Mean change % | −24% | −27% | −27% | −29% | ||
| Lymphocytes (×109 cells/L) | No. | 877 | 532 | 111 | NA | 768 |
| Mean | 4 | 5.4 | 5.2 | 5.1* | 5.1 | |
| SD | 1.4 | 1.5 | 1.3 | 1.3 | 1.4 | |
| Mean change % | 35% | 30% | NA | 28% | ||
| Hemoglobin (g/dL) | No. | 1,730 | 1,410 | 914 | 317 | 1,642 |
| Mean | 8.9 | 8.7 | 9.2 | 9.7 | 10.2 | |
| SD | 1.1 | 0.9 | 0.8 | 0.8 | 0.9 | |
| Mean change % | −2% | 3% | NA | 15% | ||
| Platelets (×109 cells/L) | No. | 993 | 682 | 535 | NA | 459 |
| Mean | 178 | 321 | 297 | 303† | 309 | |
| SD | 0.8 | 32.5 | 37.6 | 42.4 | 47.2 | |
| Mean change % | 80% | 67% | NA | 74% |
Mean change is the relative difference with day 0 as the referent. NA = not applicable.
Linear interpolation.
Figure 1.
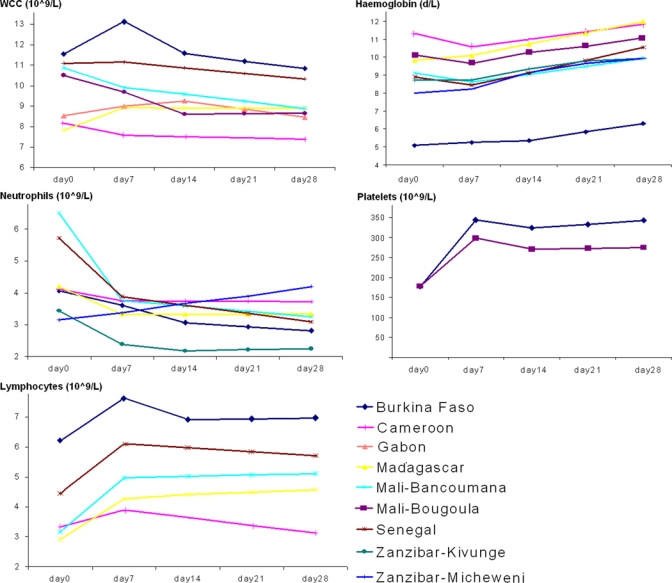
Mean hematologic values weighted by site for patients with data through day 28 and uncomplicated Plasmodium falciparum malaria, sub-Saharan Africa. WCC = white blood cells (leukocyte) counts. Units for leukocytes, neutrophils, lymphocytes, and platelets are ×109 cells/liter and units for hemoglobin are g/dL. A linear interpolation is shown for missing values on day 21.
Hemoglobin levels did not change significantly from baseline to day 7 and then increased through day 28. Platelet counts increased sharply by day 7, and showed a plateau thereafter.
Neutropenia.
The overall prevalence of neutropenia on admission was 7% (161 of 2,330), from 1% (2 of 247) in Uganda to 9% (86 of 642) in Rwanda. Using logistic regression with random effects on persons, we observed that risks for neutropenia increased with follow-up time (AOR = 1.04, 95% CI = 1.03–1.05, P = 0.001) independent of parasitologic outcome. Patients with higher parasitemias on admission (AOR = 0.69, 95% CI = 0.57–0.83, P = 0.001), older patients (AOR = 0.81, 95% CI = 0.73–0.91, P = 0.001) and patients treated with ACTs (AOR = 0.48, 95% CI = 0.33–0.68, P = 0.001) were at lower risk for neutropenia. Overall, severe neutropenia (neutrophil counts < 0.4 × 109 cells/L) was found in 33 patients: 21% (7 of 33) occurred at presentation and recovered during the follow-up, 6% (2/33) occurred on day 7, and 70% (23 of 33) occurred on day 28. All severe cases occurred only once per patient. Twenty-five of these 33 cases occurred in Burkina-Faso (6 at presentation and 19 on day 28).
Estimation of P. falciparum-attributable fractional changes in hematologic parameters.
The influence of acute uncomplicated malaria on hematologic parameters defined by estimation of P. falciparum-attributable paired fractional changes between day 0 (acute-phase malaria patients) and day 28 (convalescent-phase malaria patients) was assessed for patients who cleared parasites and did not show recurrent parasitemia within 28 days (Table 5). These findings represent 92% (1,157 of 1,254), 92% (1,061 of 1,153), 95% (768 of 806), 89% (1,642 of 1,841), and 96% (459 of 478) of patients with paired values for leukocyte, neutrophil, and lymphocyte counts; hemoglobin levels; and platelets counts, respectively. The estimated fractional changes were a slight increase in leukocytes counts (5%, 95% CI = 3–7%); a marked increase in neutrophil counts as absolute counts (43%, 95% CI = 26–35%) and as proportion of total leukocyte counts (from 34% to 45%); a decrease in lymphocytes both as absolute counts (–16%, 95% CI = –14% to –23%) and as a proportion of total leukocyte counts (59% to 47%); a decrease in hemoglobin levels (–13%, 95% CI = –14% to –11%); and a marked decrease in platelets counts (–49%, 95% CI –48% to –50%). The ratio of neutrophils to lymphocytes was 0.96 in patients with acute malaria and 0.58 in patients with convalescent malaria.
Table 5.
Paired attributable fractional difference induced by Plasmodium falciparum infection at days 0 and 28 in randomized controlled trials for convalescent-phase patients with uncomplicated Plasmodium falciparum malaria, sub-Saharan Africa*
| Parameter | No. | Day 28 | Day 0 | Paired fractional difference | Paired difference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | % | Lower 95% CI | Upper 95% CI | Mean | SD | P | ||
| Leukocytes (×109 cells/L) | 1,157 | 9.0 | 3.8 | 9.5 | 4.4 | 5 | 3 | 8 | 0.5 | 4.1 | 0.001 |
| Neutrophils (×109 cells/L) | 1,061 | 3.0 | 1.7 | 4.2 | 2.8 | 43 | 37 | 50 | 1.3 | 3 | 0.001 |
| Lymphocytes (×109 cells/L) | 768 | 6.1 | 3.1 | 5.1 | 3.3 | −16 | −20 | −11 | −0.9 | 3.5 | 0.001 |
| Hemoglobin (g/dL) | 1,642 | 9.4 | 2.4 | 8.2 | 2.7 | −13 | −14 | −11 | −1.2 | 1.5 | 0.001 |
| Platelets (×109 cells/L) | 459 | 338 | 163 | 172 | 102 | −49 | −50 | −48 | −165 | 174 | 0.001 |
CI = confidence interval.
Discussion
This study analyzed hematologic parameters of a relatively large sample of children less than five years of age with acute uncomplicated P. falciparum malaria living in countries in sub-Saharan Africa with moderate-to-intense malaria transmission. Results of analyses concur to show the direction of changes in hematologic parameters in patients with acute malaria and during recovery. However, quantitating the changes induced by malaria (on presentation) and the contribution of recovery and drug effects post-treatment proves challenging.
The overall purpose of these analyses was to obtain knowledge of an estimation of the natural history of hematologic parameters before and after treatment of uncomplicated P. falciparum malaria in young children in Africa. These analyses could help improve the understanding of basic biological phenomenon and better assess causal relationships between antimalarial drugs and laboratory-associated adverse events.
Baseline leukocyte and neutrophil counts were consistently associated with higher parasitemias across the entire age range. Platelet counts and hemoglobin levels were not related to parasitemia; lymphocyte counts varied with age and were lowest in older children with higher parasitemias. In adults in Thailand, no correlation was found between parasitemia and leukocyte counts.2,3 The first study reported a correlation between leukocyte count and temperature, and the second study reported an inverse correlation with platelet counts. Conversely, McKenzie and others17 found a positive correlation between leukocytes counts and parasitemias in adults in Burma and Peru (the strength of the correlation varied with the site and the year of study) but not with fever and duration of illness.
In an attempt to describe the influence of acute uncomplicated malaria on hematologic parameters on admission (i.e., the natural history of malaria), we used the values of convalescent-phase patients (day 28 values of those patients who cleared their initial parasitemias and did not show recurrence during follow-up) as a proxy for patients baseline; the direction and dimension of change was derived as the difference between this value and the baseline value (defined as P. falciparum-attributable fractional change).
We estimate that acute uncomplicated P. falciparum malaria induces a slight net increase (5%) in total leukocyte counts resulting from a large increase in neutrophils (43%) and a proportionally smaller decrease in lymphocytes (–16%). Hemoglobin levels were decreased by –13% and platelet counts were decreased by half (–49%).
Our method of estimation has limitations. One must assume that by day 28 values return to reference (normal) levels after acute malaria, that drug (adverse) effects on these parameters are cleared, and that, if a recurrence occurred post-day 28, it would have negligible effects on the recovery values. Furthermore, normality has relatively broad ranges and is affected by other factors, including frequency of malaria episodes (determined by intensity of transmission) and concomitant infections (e.g., gastrointestinal helminths, schistosomiasis).
In the absence of (site-specific and age-specific) reference laboratory ranges for children in Africa, it is difficult to quantitate the clinical significance of change induced by acute malaria. Quintó and others18 found that the local intervals in Mozambique were different from western standards. In children 1–5 years of age, hemoglobin levels increased from 6.8–12.4 g/dL to 8.0–13.5 g/dL; leukocyte counts decreased from 6.2–16.6 × 109 cells/L to 5.6–14.4 × 109 cells/L); and platelets counts remained stable (from 133–750 × 109 cells/L to 138–533 × 109 cells/L). Applying reference values of Quintò and others was not informative because 67% of the children who responded to treatment had baseline leukocyte counts within reference ranges compared with 65% of those who had a recurrence. The proportions of those who had values below and above reference ranges were 25% versus 28% and 8% versus 6%, respectively.
Other studies estimated changes by comparing malaria-infected persons with healthy persons. A study in adults (n = 1,100 with malaria versus 979 negative controls; age range = 20–70 years) in Thailand found that leukocyte counts < 5,000 cells/μL, erythrocyte counts < 4,000,000 cells/μL, and platelet counts < 150,000 cells/μL were significantly more likely in patients with malaria (P. falciparum or P. vivax) than in controls.3 Another study of leukocyte counts in persons with P. falciparum or P. vivax malaria and uninfected persons (essentially adults from Myanmar and Peru) showed that leukocyte counts in persons with P. falciparum malaria were greater than those in persons with P. vivax malaria and healthy subjects.17 Differential cell counts were not reported in this study. In adults in India, no difference was detected in leukocyte and neutrophil counts between persons with P. vivax or those with P. falciparum malaria.19 In children in Kenya,20 leukocytosis resulting from high parasitemias was associated with severe outcomes and mortality, but in hyper-parasitemic children (≥ 10% parasitemia) in Nigeria, thrombocytopenia, although common, was not related to higher parasite counts.21
When we compared results of our study and previous studies, it is clear that there are increased leukocyte counts and decreased platelet counts in persons with acute uncomplicated malaria. However, whether these changes are related to parasitemia is controversial. Differences are likely influenced by the difference in age of the patients studied and the background intensity of infection between children from Africa and adults from low-transmission areas. Moreover, different from previous studies, our study analyzed differential (neutrophils and lymphocytes) cell counts.
Multivariate and descriptive analyses showed statistically significant changes during the 28-day follow-up, which enabled us to derive trends and identify predictors and interactions. However, if one considers all patients and observations, we cannot quantitate the changes in a manner that is easily transposed into net gains or losses. Regarding direction of changes, there was a net decrease in leukocyte counts resulting from a decrease in neutrophil counts (also reported by Adjei and others1) that was proportionally greater than the increase in lymphocyte counts. Hemoglobin levels and platelet counts also increased.
Our analysis identified predictors of the extent of these changes. Baseline parasitemia was positively associated with the trends in leukocyte and differential cell counts: the higher the parasitemia at baseline, the greater the decrease in neutrophil and leukocyte counts and the greater the increase in lymphocyte counts. Children who received ACT had a greater decrease in neutrophil and leukocyte counts (lymphocytes counts were not affected) and a smaller increase in hemoglobin levels and platelet counts. The increase in lymphocyte counts was less than in those who had a recurrent malaria episode. Older children within this population of persons less than five years of age had a greater increase in hemoglobin levels.
The main issues regarding the decrease in neutrophil counts during follow-up are 1) whether it is related to the natural history of (convalescent) malaria or drug-induced (thus, an adverse event), and 2) what the entity of the decrease is (occurrence and severity of neutropenia). In this dataset, the prevalence of neutropenia (neutrophil counts < 1.2 × 109 cells/L) varied greatly at admission and during follow-up across study sites and was lower for patients who were older, had higher baseline parasitemias, and received ACT. Concerns remain as to the potential risks of neutropenia associated with AQ-containing drug regimens, especially in children infected with human immunodeficiency virus, in whom the risk for neutropenia was significantly higher among those receiving antiretroviral drugs.22 The data in the present analysis do not specify human immunodeficiency virus status, but do not indicate differences in the risk for neutropenia post-treatment between those receiving AS + AQ and those receiving other antimalarial treatments.
Systematic reviews and individual patient analyses may generate new evidence. Pooling individual patient data offers advantages over aggregate meta-analysis because it enables defining and analyzing secondary outcomes and conducting subgroup and multivariate analyses that are not reported in publications and cannot be conducted for aggregate data. This finding is particularly true for safety in general and more specifically for laboratory data. However, data from different settings are likely to be heterogeneous (as was the case in our study), which indicates that appropriate analytical approaches must be applied and that results may not be universally applicable.
These analyses confirm the complexity of examining hematologic changes in children less than five years of age, a group that is particularly susceptible because they are more likely to have on presentation higher baseline parasitemias, be anemic, carry gametocytes, and have a higher risk of treatment failure compared with older children and adults, which is consistent with a lack of malaria-acquired immunity.23 Interpreting these results is not straightforward and will require confirmation on extended databases. For this reason, it is important that an effort be made to obtain and collate information from as many studies and sites as possible and analyze them systematically. Finally, establishing reference laboratory ranges that are locally relevant should be a priority. These values will be useful for clinical research.
Additional limitations of our study that may be sources of bias, including spectrum composition, are that it was restricted to RCTs up to August 2008 with available data for children less than five years of age; treatments were unevenly represented (AS + AQ constituting approximately half of all treatments); there were relatively few treatment failures, which makes it difficult to contrast changes for favorable and unfavorable outcomes; and hematologic data were often not presented in publications, making it difficult to identify all (additional) studies that could be included. Therefore, it is important to increase awareness of the pertinence of this research to expand the database. Furthermore, these analyses cannot distinguish between the changes occurring while patients recover from acute malaria and those resulting from drug adverse reactions. Again, a larger spectrum of treatments will help address this issue. Finally, these analyses show substantial heterogeneity of hematologic parameters in children across and within countries, a finding that illustrates the challenges faced when making drug policy decisions.
ACKNOWLEDGMENTS
We thank all patients and staff at all trial sites for participating in this study, and the principal investigators for sharing their datasets.
Disclaimer: The authors alone are responsible for the views expressed in this publication, which do not necessarily represent the decisions, policy, or views of the World Health Organization or the Centre de Recherche Publique.
Footnotes
Financial support: Julien Zwang was supported by a grant from the Drugs for Neglected Diseases Initiative for the analysis. This initiative is an independent, not-for-profit product developer in partnership working to research and develop new and improved treatments for neglected diseases. It sponsored one of the trials but had no role in the design and conduct of the analysis or interpretation of results reported in the article.
Disclosure: None of the authors have any conflicts of interest.
Authors' addresses: Piero Olliaro, Special Programme for Research and Training in Tropical Diseases, World Health Organization, Avenue Appia 20, 1211 Geneva 27, Switzerland, E-mail: olliarop@who.int. Abdoulaye Djimdé, Malaria Research and Training Center, Department of Epidemiology of Parasitic Diseases, Faculty of Medicine and Pharmacy, University of Bamako, Bamako, Mali, E-mail: adjimde@mrtcbko.org. Grant Dorsey, Division of Infectious Diseases Department of Medicine, University of California, San Francisco, San Francisco CA, E-mail: gdorsey@medsfgh.ucsf.edu. Corine Karema, National Malaria Control Programme, Kigali, Rwanda, E-mail: ckarema@gmail.com. Andreas Mårtensson, Infectious Diseases Unit, Karolinska Institutet, Karolinska University Hospital, Stockholm, Sweden, E-mail: andreas.martensson@ki.se. Jean-Louis Ndiaye, Department of Parasitology, Faculty of Medicine, University Cheikh Anta Diop, Dakar, Senegal, E-mail: jlndiaye@yahoo.com. Sodiomon B. Sirima, Centre National de Recherche et de Formation sur le Paludisme, Ministère de la Santé, Ouagadougou, Burkina Faso, E-mail: s.sirima.cnlp@fasonet.bf. Michel Vaillant, Clinical Epidemiology and Public Health Unit, Centre for Health Studies, Centre de Recherche Publique-Santé, 1A-B, Rue Thomas Edison L-1445, Strassen, Luxembourg, E-mail: michel.vaillant@crp-sante.lu. Julien Zwang, Drugs for Neglected Diseases Initiative, Geneva, Switzerland, 15 Chemin Louis-Dunant, 1202 Geneva, Switzerland, E-mail: zwang@free.fr.
Reprint requests: Piero Olliaro, Special Programme for Research and Training in Tropical Diseases, World Health Organization, Avenue Appia 20, 1211 Geneva 27, Switzerland, E-mail: olliarop@who.int.
References
- 1.Adjei GO, Kurtzhals JA, Rodrigues OP, Alifrangis M, Hoegberg LC, Kitcher ED, Badoe EV, Lamptey R, Goka BQ. Amodiaquine-artesunate vs artemether-lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow-up. Malar J. 2008;7:127. doi: 10.1186/1475-2875-7-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangpukdee N, Yew HS, Krudsood S, Punyapradit N, Somwong W, Looareesuwan S, Kano S, Wilairatana P. Dynamic changes in white blood cell counts in uncomplicated Plasmodium falciparum and P. vivax malaria. Parasitol Int. 2008;57:490–494. doi: 10.1016/j.parint.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Erhart LM, Yingyuen K, Chuanak N, Buathong N, Laoboonchai A, Miller RS, Meshnick SR, Gasser RA, Jr, Wongsrichanalai C. Hematologic and clinical indices of malaria in a semi-immune population of western Thailand. Am J Trop Med Hyg. 2004;70:8–14. [PubMed] [Google Scholar]
- 4.Taylor WR, Widjaja H, Basri H, Ohrt C, Taufik T, Tjitra E, Baso S, Fryauff D, Hoffman SL, Richie TL. Changes in the total leukocyte and platelet counts in Papuan and non Papuan adults from northeast Papua infected with acute Plasmodium vivax or uncomplicated Plasmodium falciparum malaria. Malar J. 2008;7:259. doi: 10.1186/1475-2875-7-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan SO, McGready R, Zwang J, Pimanpanarak M, Sriprawat K, Thwai KL, Moo Y, Ashley EA, Edwards B, Singhasivanon P, White NJ, Nosten F. Thrombocytopaenia in pregnant women with malaria on the Thai-Burmese border. Malar J. 2008;7:209. doi: 10.1186/1475-2875-7-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adjuik M, Agnamey P, Babiker A, Borrmann S, Brasseur P, Cisse M, Cobelens F, Diallo S, Faucher JF, Garner P, Gikunda S, Kremsner PG, Krishna S, Lell B, Loolpapit M, Matsiegui PB, Missinou MA, Mwanza J, Ntoumi F, Olliaro P, Osimbo P, Rezbach P, Some E, Taylor WR. Amodiaquine-artesunate versus amodiaquine for uncomplicated Plasmodium falciparum malaria in African children: a randomized, multicentre trial. Lancet. 2002;359:1365–1372. doi: 10.1016/s0140-6736(02)08348-4. [DOI] [PubMed] [Google Scholar]
- 7.Djimdé AA, Fofana B, Sagara I, Sidibe B, Toure S, Dembele D, Dama S, Ouologuem D, Dicko A, Doumbo OK. Efficacy, safety, and selection of molecular markers of drug resistance by two ACTs in Mali. Am J Trop Med Hyg. 2008;78:455–461. [PubMed] [Google Scholar]
- 8.Dorsey G, Staedke S, Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Dokomajilar C, Kamya MR, Rosenthal PJ. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA. 2007;297:2210–2219. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 9.Karema C, Fanello CI, van Overmeir C, van Geertruyden JP, van Doren W, Ngamije D, D'Alessandro U. Safety and efficacy of dihydroartemisinin/piperaquine (Artekin) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwandan children. Trans R Soc Trop Med Hyg. 2006;100:1105–1111. doi: 10.1016/j.trstmh.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Martensson A, Stromberg J, Sisowath C, Msellem MI, Gil JP, Montgomery SM, Olliaro P, Ali AS, Björkman A. Efficacy of artesunate plus amodiaquine versus that of artemether-lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clin Infect Dis. 2005;41:1079–1086. doi: 10.1086/444460. [DOI] [PubMed] [Google Scholar]
- 11.Ndiaye JL, Randrianarivelojosia M, Sagara I, Brasseur P, Ndiaye I, Faye B, Randrianasolo L, Ratsimbasoa A, Forlemu D, Moor VA, Traore A, Niawanlou Dara YD, Lameyre V, Diallo M, Djimde A, Same-Ekobo A, Gaye O. Randomized, multicentre assessment of the efficacy and safety of ASAQ – a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:125. doi: 10.1186/1475-2875-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sirima SB, Tiono AB, Gansane A, Diarra A, Ouedraogo A, Konate AT, Kiechel JR, Morgan CC, Olliaro PL, Taylor WR. The efficacy and safety of a new fixed-dose combination of amodiaquine and artesunate in young African children with acute uncomplicated Plasmodium falciparum. Malar J. 2009;8:48. doi: 10.1186/1475-2875-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zwang J, Olliaro P, Barennes H, Bonnet M, Brasseur P, Bukirwa H, Cohuet S, D'Alessandro U, Djimdé A, Karema C, Guthmann JP, Hamour S, Ndiaye JL, Mårtensson A, Rwagacondo C, Sagara I, Same-Ekobo A, Sirima SB, van den Broek I, Yeka A, Taylor WR, Dorsey G, Randrianarivelojosia M. Efficacy of artesunate-amodiaquine for treating uncomplicated falciparum malaria in sub-Saharan Africa: a multi-centre analysis. Malar J. 2009;8:203. doi: 10.1186/1475-2875-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olliaro P, Djimdé A, Karema C, Mårtensson A, Ndiaye JL, Sirima SB, Dorsey G, Zwang J. Estimated versus actual parasitaemia in acute uncomplicated Plasmodium falciparum malaria in African children under five. Trop Med Int Health. 2011;16:551–554. doi: 10.1111/j.1365-3156.2011.02738.x. [DOI] [PubMed] [Google Scholar]
- 15.Breusch TS, Pagan AR. The Lagrange multiplier test and its applications to model specification in econometrics. Rev Econ Stud. 1980;47:239–253. [Google Scholar]
- 16.NIAID . Division of Microbiology and Infectious Diseases Pediatric Toxicity Tables. 2007. http://www.niaid.nih.gov Available at. Accessed June 23, 2010. [Google Scholar]
- 17.McKenzie FE, Prudhomme WA, Magill AJ, Forney JR, Permpanich B, Lucas C, Gasser RA, Jr, Wongsrichanalai C. White blood cell counts and malaria. J Infect Dis. 2005;192:323–330. doi: 10.1086/431152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quintó L, Aponte JJ, Sacarlal J, Espasa M, Aide P, Mandomando I, Guinovart C, Macete E, Navia MM, Thompson R, Menéndez C, Alonso PL. Haematological and biochemical indices in young African children: in search of reference intervals. Trop Med Int Health. 2006;11:1741–1748. doi: 10.1111/j.1365-3156.2006.01764.x. [DOI] [PubMed] [Google Scholar]
- 19.Jadhav UM, Singhvi R, Shah R. Prognostic implications of white cell differential count and white cell morphology in malaria. J Postgrad Med. 2003;49:218–220. [PubMed] [Google Scholar]
- 20.Ladhani S, Lowe B, Cole AO, Kowuondo K, Newton CR. Changes in white blood cells and platelets in children with falciparum malaria: relationship to disease outcome. Br J Haematol. 2002;119:839–847. doi: 10.1046/j.1365-2141.2002.03904.x. [DOI] [PubMed] [Google Scholar]
- 21.Sowunmi A, Akindele JA, Balogun MA. Leukocyte counts in falciparum malaria in African children from an endemic area. Afr J Med Sci. 1995;24:145–149. [PubMed] [Google Scholar]
- 22.Gasasira AF, Kamya MR, Achan J, Mebrahtu T, Kalyango JN, Ruel T, Charlebois E, Staedke SG, Kekitiinwa A, Rosenthal PJ, Havlir D, Dorsey G. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin Infect Dis. 2008;46:985–991. doi: 10.1086/529192. [DOI] [PubMed] [Google Scholar]
- 23.Dorsey G, Gasasira AF, Machekano R, Kamya MR, Staedke SG, Hubbard A. The impact of age, temperature, and parasite density on treatment outcomes from antimalarial clinical trials in Kampala, Uganda. Am J Trop Med Hyg. 2004;71:531–536. [PubMed] [Google Scholar]


