Abstract
Malaria and severe pneumonia in hospitalized young children may show striking clinical similarities, making differential diagnosis challenging. We investigated ways to increase diagnostic accuracy in patients hospitalized with clinical symptoms compatible with malaria and severe pneumonia, in an area with high a prevalence of infection with human immunodeficiency virus. A total of 646 children admitted at the Manhiça District Hospital in Manhiça, Mozambique who met the World Health Organization clinical criteria for severe pneumonia and malaria were recruited for 12 months and thoroughly investigated to ascertain an accurate diagnosis. Although symptom overlap between malaria and severe pneumonia was frequent among hospitalized children, true disease overlap was uncommon. Clinical presentation and laboratory determinations were ineffective in reliably distinguishing between the two diseases. Infection with human immunodeficiency virus differentially influenced the epidemiology and clinical presentation of these two infectious diseases, further challenging their discrimination on clinical grounds, and having a greater impact on the current burden and prognosis of severe pneumonia.
Introduction
With an estimated 4.2 million annual child deaths,1 sub-Saharan Africa represents the paradigm of the devastating effects of infectious disease affecting child survival. Acute respiratory infections (ARIs), the leading cause of childhood mortality in the world, cause in this continent at least one million deaths annually, mostly caused by bacterial pneumonia,2,3 and malaria kills at least 750,000 children in Africa every year.1,4 Furthermore, both diseases impose a massive burden to the fragile health systems in Africa.
In rural settings of developing countries, diagnosis of the most common pediatric diseases such as malaria or ARIs generally depends on the recognition by primary health care workers of clinical symptoms. On the basis of this rationale, the World Health Organization (WHO) developed in the 1990s the Integrated Management of Childhood Illness (IMCI) guidelines,5 a set of highly sensitive clinical algorithms providing guidance for diagnosis and management of common pediatric conditions. Although at the peripheral level these highly sensitive clinical algorithms have contributed to saving thousands of lives,6 their poor specificity makes them insufficiently reliable at the hospital level unless complemented by laboratory determinations.7–9
There has been extensive reporting of the overlap in clinical presentation between malaria and ARI, both at the outpatient level10–14 and among children admitted to hospitals.15 The importance of this overlap will depend upon the epidemiology of both conditions in each particular setting, but also the severity of the presentation and the age of the patient. Resemblances in clinical presentation are particularly striking among young admitted patients, where severity from any of the two conditions often includes the appearance of respiratory signs and fever. In this specific subgroup of admitted patients, precise etiologic diagnosis without laboratory support is problematic.10–15 Diagnostic uncertainty often leads to overtreatment, wasting the limited existing resources and accelerating antibiotic/antimalarial drug resistance levels in the community, but more importantly, misdiagnosis may divert the specific treatment from the real etiology, putting in danger the patient's life. Because the quality of care and services in many referral hospitals in Africa is already notoriously poor and necessarily constrained by limited resources,16,17 distinguishing the real etiology causing their admission becomes critical to improve patient's survival chances.
Consequently, this report attempts to explore the diagnostic and management challenge represented by the large subgroup of children fulfilling simultaneously clinical criteria for severe pneumonia and malaria and to identify risk factors that could specifically lead to the recognition of either condition. It also attempts to give some insight on how the human immunodeficiency virus (HIV) pandemic may differentially impact the clinical presentation of malaria and severe pneumonia cases fulfilling these clinical criteria.
Materials And Methods
Study setting and population.
This prospective study was conducted during September 2006–September 2007 at the Manhiça District Hospital, the referral health facility for Manhiça District, a rural area in southern Mozambique. The Centro de Investigação em Saúde de Manhiça (CISM) has conducted a Demographic Surveillance System (DSS)18 in the area since 1996 and a morbidity surveillance system at Manhiça District Hospital,19 covering an area of approximately 500 km2, with 80,000 persons under permanent surveillance, 18% of whom are less than five years of age. In 2005, the prevalence of HIV among pregnant women attending the antenatal clinic was high (23.6%),20 and the estimated prevalence among the birth cohort was 3%.21
Malaria and pneumonia in the study area.
At the time of the study, malaria in Manhiça accounted for one-third of all outpatient visits,19 half of the pediatric admissions, and 19% of all in-hospital pediatric deaths.22 A total of 11.1% of all malaria admissions and 41.1% of severe malaria cases had severe respiratory findings.22 Severe pneumonia accounted for 16% of hospitalizations among children less than two years of age, and had an associated case-fatality rate (CFR) of 11%.23 Verbal autopsy studies confirmed from the community perspective that these two conditions are the leading causes of death in children less than five years of age.24
Procedures for recruited children and sample collection.
This study was part of a larger project designed to describe the epidemiology and clinical characteristics of children less than five years of age admitted with respiratory distress. However, this analysis includes only patients simultaneously fulfilling at admission IMCI-defined clinical criteria for malaria (fever or a history of fever in the preceding 24 hours) and severe pneumonia (cough and/or breathing difficulties, plus chest indrawing with or without increased respiratory rate according to age group). Children fulfilling inclusion criteria and whose parents had signed an informed consent form underwent a series of standardized procedures. Antero-posterior chest radiographs were obtained on admission or otherwise within the first 48 hours of hospitalization according to severity. Pulse oximetry (Nellcor, Boulder, CO) was used to determine oxygen saturation, and nasopharyngeal aspirates were performed for diagnosis of respiratory viruses by using NPAK® Kits (MPRO, Farmington Hills, MI). Venous blood was obtained at admission for malaria diagnosis, blood culture, full blood cell count, and biochemical determinations.
HIV-specific procedures.
Recruited study children residing in the DSS area were referred for HIV counseling and testing, which required for study purposes an additional parental consent. HIV-1 serodiagnosis was performed by using a sequential testing algorithm with two rapid HIV-1 antibody tests (Determine®; Abbott Laboratories, Abbott Park, IL and Unigold®; Trinity Biotech, Plc., Bray, Ireland). HIV infection was confirmed when necessary by using an HIV-1 DNA Amplicor Test (version 1.5; Roche Molecular Systems, Inc., Branchburg, NJ). Children identified as HIV positive were followed-up according to national guidelines.
Definitions and clinical groups.
For the purpose of this analysis, a set of specific (gold-standard) definitions was used (Table 1), and four mutually exclusive outcome groups created: severe pneumonia, malaria, co-existing severe pneumonia and malaria, and all other uncertain diagnoses. The etiology of those cases with an uncertain diagnosis is the object of another analysis.
Table 1.
Specific (gold-standard) definitions used for outcome groups (the groups are self-excluding) for persons with malaria or pneumonia, Mozambique
| Disease | Specific definitions (study gold standard) |
|---|---|
| Malaria | Clinical entry criteria to the study* AND malaria as one of the final diagnoses in the admission questionnaire and any asexual Plasmodium falciparum parasitemia > 500 parasites/μL (irrespective of age) without radiologically confirmed pneumonia |
| Severe pneumonia | Clinical entry criteria to the study* AND radiologically confirmed pneumonia without malaria being one of the final diagnoses in the admission questionnaire and without detection of asexual P. falciparum parasitemias exceeding > 500 parasites/μL (irrespective of age) |
| Malaria and severe pneumonia | Clinical entry criteria to the study* AND radiologically confirmed pneumonia AND malaria as one of the final diagnoses in the admission questionnaire with asexual P. falciparum parasitemia > 500 parasites/μL (irrespective of age) |
| Others | Clinical entry criteria to the study* BUT not fulfilling any of the previous 3 definitions (without radiologically confirmed pneumonia OR malaria as one of the final diagnoses in the admission questionnaire and asexual P. falciparum parasitemia > 500 parasites/μL (irrespective of age)) |
Clinical entry criteria to the study were: Admitted children with fever or a history of fever in the preceding 24 hours AND clinically suspected severe pneumonia (cough or difficulty in breathing + IRR + indrawing).
All case definitions were based on data obtained on admission from standardized study questionnaires. An increased respiratory rate was defined according to age following standard WHO definitions.25 Hypoxemia implied an oxygen saturation < 90%. Mild anemia was defined as a packed cell volume (PCV) 25 to < 33%, moderate anemia as a PCV 15–< 25% and severe anemia as a PCV < 15%. Fever was defined as an axillary temperature of ≥ 37.50°C, and hyperpyrexia implied a temperature ≥ 39°C. Calibrated digital thermometers providing two-decimals readings were used. Nutritional status was based on weight-for-age Z scores, which were calculated by using the least mean square method and the 2000 CDC Growth Reference (Centers for Disease Control and Prevention, Atlanta, GA).
A case of bacteremia was defined as the isolation of at least one non-contaminant bacteria from the admission blood culture. Coagulase-negative staphylococci, Bacillus species, or Micrococcus species were considered contaminants.
Case-fatality rates were calculated for the different diagnoses as the number of patients who died with that diagnosis divided by the total number of patients with known outcome admitted with that diagnosis. These CFRs represent in-hospital mortality and do not include patients who did not return for follow-up or were transferred. Mortality occurring within 21 days after hospital discharge (delayed mortality) was investigated with DSS data for children residing in CISM study area.
The rainy season was defined as November–April, and the dry season was defined as May–October.
Case management and treatment.
Treatment of malaria or severe pneumonia was conducted according to national guidelines, and has been described.23,26 Antibiotic therapy was reassessed according to culture results and sensitivity patterns. Children requiring specialized care were transferred to Maputo Central Hospital. By the time children were recruited, Haemophilus influenzae b or pneumococcal vaccines were not available in Mozambique.
Laboratory methods.
Packed cell volume was measured by using a microcentrifuge and a hematocrit reader (Hawksley and Sons Ltd., Lancing, United Kingdom). Thick and thin blood films were processed and examined according to standard methods.19 Glycemia was determined by using Accu-Chek® (Roche Inc., Mannheim, Germany) and blood lactate levels were determined by using Lactate Pro® (FaCT Canada, Quesnel, British Columbia, Canada) at the bedside. Full blood counts were determined and biochemical test were conducted by using the CISM hematology analyzer (Kx21; Sysmex, Denver, CO), and biochemistry analyzer (Vitros DT60; Ortho Clinical Diagnostics, Raritan, NJ). Blood cultures were processed as previously described,27 and bacterial isolates identified according to standard microbiologic procedures.28,29 The presence of different respiratory viruses in nasopharyngeal aspirates was investigated by means of different polymerase chain reaction methods.30
Radiograph interpretation.
Radiographs were obtained by using a Siemens (Erlangen, Germany) machine and were interpreted blindly by two independent readers (QB, a pediatrician and JLR, a pediatric radiologist) according to a WHO-designed radiographic interpretation protocol.31,32 Episodes with evidence of consolidation and/or pleural effusion were defined as radiologically confirmed pneumonia (endpoint pneumonia). Other radiologic endpoints included interstitial infiltrates or normal radiographs. Images with discordant results from the two primary readers were interpreted by a third reader, and the result was only accepted as final if concordant with any of the other previous readings.
The study was approved by the Mozambican National Bioethics Committee, the Institutional Review Board of the Hospital Clinic (Barcelona, Spain), and the World Health Organization review board.
Data management and statistical analysis.
All study questionnaires were double-entered by using a program written in FoxPro version 5.0 (Microsoft Corp., Redmond, WA). Statistical analyses were conducted by using Stata 11 (Stata Corp., College Station, TX).
Categorical variables were compared by using a chi-square test or Fisher's exact test. Means of normally distributed variables were compared by using the Student's t-test or analysis of variance. For non-normally distributed variables, medians and interquartile ranges are presented, and the Wilcoxon rank-sum test was used to assess differences.
Crude and multivariate logistic regression analyses were performed to assess whether the different clinical and laboratorial variables were associated with malaria or severe pneumonia according to the gold-standard definitions. For the multivariate analysis, automated backward stepwise estimations were performed by using exclusively those patients with a final diagnosis of malaria or severe pneumonia, and all variables associated with either outcome at a significance level of P < 0.10 in the univariate analysis were included in the model. Variables with more than 20% missing values or not evident on admission were excluded. Odds ratios (ORs) and 95% confidence intervals (CIs) are presented.
Results
General overview.
During the study period, 2,943 children were admitted to the hospital, from which 2,634 (89.5%) fulfilled the IMCI definition of malaria and 780 (26.5%) fulfilled the IMCI definition of severe pneumonia. Only 695 (24%) fulfilled simultaneously the IMCI criteria for malaria and severe pneumonia (Figure 1). A total of 646 (93%) of these patients provided consent, for whom complete collection of data were included in this analysis. Mean age of recruited children was 14.3 months, and 59% (380 of 646) were males.
Figure 1.
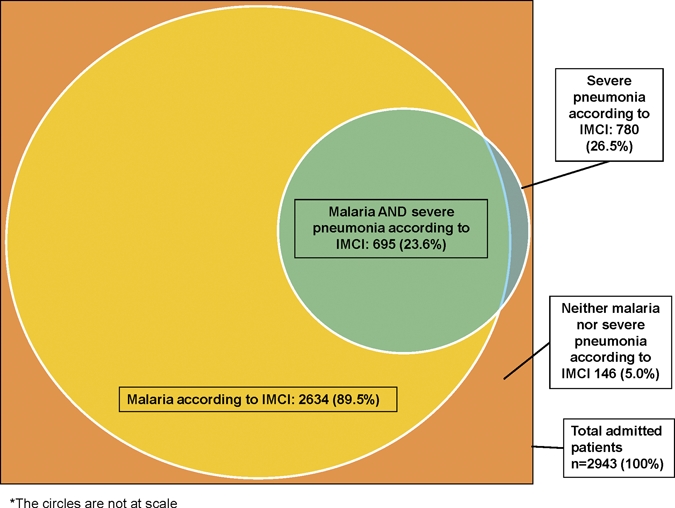
Proportion of patients fulfilling the Integrated Management of Childhood Illness non-exclusive criteria for malaria and severe pneumonia among 2,943 hospitalized patients during the study period, Mozambique.
A total of 1% (5 of 646) of the children did not have results for malaria parasites, and 14% (90 of 641) of the patients had malaria parasites in their blood. A total of 3% (21 of 646) of the children did not have radiographic results. Fifty percent (315 of 625) of the chest radiographs were interpreted were normal, 41% (254 of 625) showed evidence of radiologic pneumonia, and 9% (56 of 625) showed interstitial infiltrates. A total of 99% (637 of 646) of recruited children had blood cultures with a contamination rate of 11% (67 of 637), and a positivity rate of 14% (78 of 566). Streptococcus pneumoniae was the most frequently isolated pathogen (31 of 78, 40%), followed by H. influenzae b (20 of 78, 26%). Non-typhi Salmonella were detected only in three patients.
Nasopharyngeal aspirates were obtained from 97% (626 of 646) of the patients. At least one respiratory virus was detected in 47% (297 of 626) of the children, and co-infections occurred in 9% (59 of 626) of the patients. Rhinovirus (143 of 360, 40%), adenovirus (75 of 360, 21%), and respiratory syncytial virus (41 of 360, 11%) were the most common isolates.
Testing for HIV, which was offered only to study area residents, was performed for 65% (420 of 646) of the patients, 25% (105 of 420) of whom were infected with HIV. A total of 10% (67 of 646) of the recruited patients died during the study. An additional 12 deaths occurring at home within 21 days after discharge were confirmed among patients from the study area. The HIV prevalence among in-hospital deaths with available HIV results was 68% (15 of 22).
Characteristics of patients according to specific diagnostic groups.
Classification of patients according to our set of highly specific definitions is shown in Figure 2. Diagnoses were distributed as follows: 9% (61 of 646) only malaria, 37% (242 of 646) only severe pneumonia, < 1% (9 of 646) malaria and severe pneumonia, and 52% (334 of 646) other diagnoses.
Figure 2.
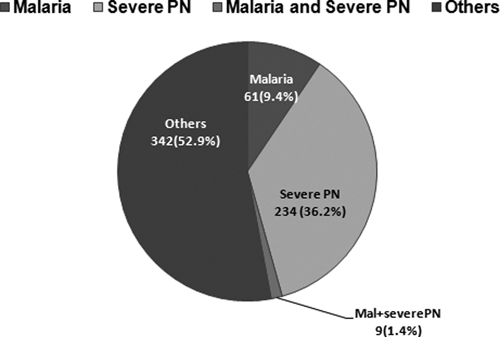
Classification of 646 recruited children according to mutually-exclusive specific definitions, Mozambique.
The prevalence and association of different signs, symptoms, and other characteristics to the specific malaria or severe pneumonia gold-standard diagnoses, by univariate analysis, is shown in Table 2. The nine cases of concomitant malaria and severe pneumonia have been excluded from the analysis.
Table 2.
Features on admission, signs, symptoms, and laboratory results according to mutually exclusive diagnostic group (severe pneumonia or malaria), Mozambique, by univariate analysis*
| Features on admission (signs, symptoms, and laboratory results) | Exclusive group | No. | P‡ | OR (95% CI)§ | ||
|---|---|---|---|---|---|---|
| Severe pneumonia† | No. | Malaria† | ||||
| Female sex | 103 (42.6) | 242 | 29 (47.5) | 61 | 0.483 | 1.22 (0.70–2.15) |
| Age in months, mean (SD) | 14.5 (12.5) | 242 | 19.4 (16.2) | 61 | 0.011 | 1.02 (1.01–1.05) PUI* |
| Admission during rainy season | 136 (56.2) | 242 | 44 (72.1) | 61 | 0.025 | 2.02 (1.09–3.73) |
| Clinical history on arrival | ||||||
| History of convulsions | 9 (3.7) | 242 | 12 (19.7) | 61 | < 0.001 | 6.34 (2.53–15.87) |
| History of any previous treatment | 80 (30.1) | 242 | 10 (16.4) | 61 | 0.011 | 0.40 (0.19–0.82) |
| Previous neurologic abnormalities | 4 (1.7) | 242 | 5 (8.2) | 61 | 0.007 | 5.31 (1.38–20.42) |
| Previous severe malaria | 12 (5.0) | 242 | 8 (13.1) | 61 | 0.022 | 2.89 (1.13–7.43) |
| Clinical examination result on arrival | ||||||
| Temperature (°C), mean (SD) | 38.1 (1.2) | 242 | 38.5 (1.0) | 61 | 0.007 | 1.40 (1.09–1.80) PUI* |
| Hyperpyrexia (axillary temperature ≥ 39°C) | 65 (26.9) | 242 | 25 (41.0) | 61 | 0.031 | 1.89 (1.05–3.39) |
| Oxygen saturation (%), mean (SD) | 88.5 (9.8) | 240 | 92.5 (7.8) | 61 | 0.003 | 0.94 (0.90–0.98) PUD** |
| Low oxygen saturation (< 90%) | 153 (63.8) | 240 | 23 (37.7) | 61 | < 0.001 | 0.34 (0.19–0.62) |
| Respiratory rate, mean (SD) | 65.6 (15.0) | 242 | 60.4 (15.9) | 61 | 0.018 | 0.98 (0.96–0.99) PUI* |
| Nasal flaring | 152 (62.8) | 242 | 26 (42.6) | 61 | 0.004 | 0.44 (0.25–0.78) |
| Deep breathing | 13 (5.4) | 242 | 14 (23.0) | 61 | < 0.001 | 5.25 (2.32–11.89) |
| Unilateral chest signs | 70 (28.9) | 242 | 3 (4.9) | 61 | < 0.001 | 0.13 (0.04–0.42) |
| Crackles | 194 (80.2) | 242 | 17 (27.9) | 61 | < 0.001 | 0.10 (0.05–0.18) |
| Wheezing | 33 (13.6) | 242 | 6 (9.8) | 61 | 0.428 | 0.69 (0.27–1.73) |
| Oral candidiasis | 28 (11.6) | 242 | 2 (3.3) | 61 | 0.053 | 0.26 (0.06–1.12) |
| Splenomegaly | 34 (14.1) | 242 | 33 (54.1) | 61 | < 0.001 | 7.21 (3.88–13.41) |
| Any dehydration | 50 (20.7) | 242 | 21 (34.4) | 61 | 0.023 | 2.02 (1.09–3.72) |
| Deep coma (BCS < 2) | 2 (0.8) | 242 | 4 (6.6) | 61 | 0.004 | 8.42 (1.51–47.11) |
| Prostration | 46 (19.0) | 242 | 24 (39.3) | 61 | 0.001 | 2.76 (1.51–5.07) |
| Heart gallop | 7 (2.9) | 242 | 16 (26.2) | 61 | < 0.001 | 11.94 (4.65–30.67) |
| Pallor | 17 (7.0) | 242 | 30 (49.2) | 61 | < 0.001 | 12.81 (6.34–25.89) |
| Nutritional status | ||||||
| Clinically malnourished | 49 (20.3) | 242 | 5 (8.2) | 61 | 0.028 | 0.35 (0.13–0.92) |
| WAZ > −1 SD | 62 (25.8) | 240 | 23 (33.7) | 61 | 0.008 | ND |
| WAZ −1 SD to −3 SD | 99 (41.3) | 240 | 30 (49.2) | 61 | ||
| WAZ < −3 SD | 79 (32.9) | 240 | 8 (13.1) | 61 | ||
| Waz: Mean (SD) | −2.29 (1.9) | 240 | −1.57 (1.3) | 61 | 0.006 | 0.78 (0.66–0.93) PUD** |
| Anemia status on admission | ||||||
| No anemia | 89 (36.8) | 242 | 9 (14.8) | 61 | < 0.001 | 1 |
| Mild anemia (> 25–< 33%) | 120 (49.6) | 242 | 18 (29.5) | 61 | 1.48 (0.64–3.46) | |
| Moderate anemia (> 15–< 25%) | 31 (12.8) | 242 | 30 (49.2) | 61 | 9.57 (4.09–22.38) | |
| Severe anemia (< 15%) | 2 (0.8) | 242 | 4 (6.6) | 61 | 19.78 (3.17–123.36) | |
| Packed cell volume (%), mean (SD) | 30.6 (6.2) | 242 | 23.7 (7.0) | 61 | < 0.001 | 1.21 (1.14–1.29) PUD** |
| Micro-biology and other laboratory results on admission | ||||||
| HIV status positive‖ | 60 (38.2) | 157 | 8 (18.6) | 43 | 0.016 | 0.37 (0.16–0.85) |
| Positive blood culture | 46 (21) | 219 | 0 (0) | 57 | < 0.001 | CBC |
| Resp. virus detected in NPA | 108 (45.6) | 237 | 36 (59.0) | 61 | 0.061 | 1.72 (0.97–3.04) |
| Respiratory syncitial virus in NPA n(%) | 18 (7.6) | 237 | 1 (1.6) | 61 | 0.090 | 0.20 (0.02–1.55) |
| WBC count (109/L), mean (SD) | 18.1 (10.3) | 230 | 13.5 (10.3) | 57 | 0.003 | 0.95 (0.91–0.98) PUI* |
| Leukocytosis (> 20.109/L) | 84 (36.5) | 230 | 10 (17.5) | 57 | 0.006 | 0.37 (0.18–0.77) |
| Platelets (1012/L), mean (SD) | 385 (208) | 236 | 138 (108) | 59 | < 0.001 | 1.01 (1.00–1.01) PUD** |
| Thrombocytopenia (< 100.1012/L) n(%) | 21 (8.9) | 236 | 23 (39.0) | 59 | < 0.001 | 6.54 (3.28–13.03) |
| Lactate: geometric, mean (95% CI) | 2.17 (2.06–2.29) | 236 | 3.81 (3.23–4.50) | 59 | < 0.001 | 7.40 (3.99–13.74) PUI* |
| Lactate > 5 mmol/L on admission | 10 (4.2) | 236 | 19 (32.2) | 59 | < 0.001 | 10.73 (4.65–24.77) |
| Glycemia (mmol/L), mean (SD) | 6.48 (1.82) | 238 | 6.13 (2.08) | 60 | 0.200 | 1.11 (0.95–1.30) PUD** |
| Creatinine (U/L), mean (SD) | 35.1 (12.9) | 237 | 38.0 (9.4) | 61 | 0.100 | 0.98 (0.96–1.01) PUI* |
| Urea (mmol/L) | 2.9 (2.7) | 235 | 4.2 (3.3) | 61 | 0.035 | 1.13 (1.03–1.23) PUI* |
| Bilirubin (micromol/L), mean (SD) | 12.5 (16.4) | 235 | 27.0 (20.1) | 61 | < 0.001 | 1.04 (1.02–1.06) PUI* |
| Abnormal bilirrubin (> 34 micromol/L) n(%) | 9 (3.8) | 235 | 13 (21.3) | 61 | < 0.001 | 6.80 (2.75–16.81) |
| ALT (U/L), mean (SD) | 32.6 (50.9) | 236 | 45.8 (68.6) | 61 | 0.090 | 1.00 (0.99–1.00) PUI* |
| LDH (10–2 U/L), mean (SD) | 11.5 (6.1) | 223 | 14.1 (6.9) | 58 | 0.005 | 1.06 (1.01–1.11) PUI* |
| C-reactive protein (g/L), mean (SD) | 15.8 (11.8) | 83 | 11.8 (7.2) | 38 | 0.049 | 0.96 (0.93–1.00) PUI* |
| Procalcitonine (ng/mL), mean (SD) | 29.8 (82) | 120 | 28.3 (39.5) | 37 | 0.910 | 0.99 (0.99–1.00) PUI* |
| Evolution during admission | ||||||
| Lumbar puncture performed | 32 (13.2) | 242 | 17 (27.9) | 61 | 0.005 | 2.54 (1.29–4.97) |
| Received transfusion | 12 (5.0) | 242 | 24 (39.3) | 61 | < 0.001 | 12.43 (5.73–26.99) |
| Duration of admission (days): Median (IQR) | 5.66 (3.69–8.77) | 242 | 3.87 (2.85–4.96) | 61 | < 0.001 | 0.92 (0.85–0.98) PUI* |
| Case Fatality Rate (in hospital deaths) | 24 (9.9) | 242 | 1 (1.6) | 61 | 0.036 | 0.15 (0.02–1.14) |
OR = odds ratio; CI = confidence interval; WAZ = weight-for-age Z score; BCS = Blantyre coma score; ND = not determined; CBC = cannot be calculated.
PUD = per unit decrease. Change in the OR per unit of the variable decreased.
Values are no. (%) unless otherwise indicated.
P values obtained comparing the association of different values to either the malaria vs. severe pneumonia groups. For comparison of means, t-test or ANOVA used. For medians, Wilcoxon Rank Sum test.
OR and 95% CI obtained with univariate logistic regression comparing only malaria vs. severe pneumonia groups (this being the baseline).
PUI = per unit increase. Change in the OR per unit of the variable increased.
Data only available for study area participants.
Only three (4.9%) of the children with a diagnosis of malaria showed interstitial infiltrates in their chest radiograph, and no viral co-infections were found in any of these three children. The remaining 95% of the malaria patients had a strictly normal chest radiograph.
In contrast to patients with severe pneumonia, patients with malaria were significantly older, more commonly admitted during the rainy season, or recalled more frequently a history of previous severe malaria episodes, preceding neurologic abnormalities, or convulsions. Patients with malaria had higher axillary temperatures at admission, showed more splenomegaly or heart gallop, and were more frequently dehydrated, prostrated, or comatose. Patients with severe pneumonia were more frequently malnourished, had a higher mean admission respiratory rate, and showed a significantly greater proportion of respiratory signs (nasal flaring, unilateral chest signs, or crackles). They were also more commonly hypoxemic. Patients with malaria were more frequently thrombocytopenic, acidotic, and showed more deep breathing or lower mean PCVs. In contrast, patients with severe pneumonia had higher mean leukocyte counts. For biochemical variables, patients with malaria had significantly higher mean bilirubin, urea, and lactate dehydrogenase levels than patients with severe pneumonia, but mean C-reactive protein levels were higher among patients with severe pneumonia. Infection with HIV was significantly more prevalent among patients with severe pneumonia, who also had longer hospital admissions and higher (although of borderline significance) CFRs.
Risk factors associated with malaria or severe pneumonia by multivariate analysis.
Among children meeting clinical criteria, the risk of having malaria (and therefore not severe pneumonia) was independently associated by multivariate analysis with older ages (OR = 1.09 per month increase, 95% CI = 1.04–1.15) or clinical dehydration (OR = 5.16, 95% CI = 1.31–20.33). Decreasing PCVs were also associated with a higher risk of malaria (OR = 1.29 per PCV unit decrease, 95% CI = 1.14–1.46) and hyperbilirrubinemia (> 34 micromol/L; OR = 15.82, 95% CI = 1.85–135.21) (Table 3). Conversely, the risk of having severe pneumonia (and not malaria) was higher in children with crackles on auscultation (OR = 12.72, 95% CI = 3.92–41.22), nasal flaring (OR = 5.12, 95% CI = 1.59–16.47) or hypoxemia (OR = 5.32, 95% CI = 1.51–18.71). Malnourished children were also at higher risk (OR = 2.23 per unit decrease of weight-for-age Z score, 95% CI = 1.48–3.35), as were children who received any pre-admission treatment (OR = 6.20, 95% CI = 1.40–27.46). Multivariate analysis also suggested that unilateral chest signs (OR = 5.19, 95% CI = 0.95–28.35) and higher leukocyte counts (OR = 1.05 per unit increase, 95% CI = 0.99–1.11) were more associated with severe pneumonia, although at a borderline statistical significance (Table 3).
Table 3.
Independent risk factors for malaria or severe pneumonia diagnoses, Mozambique, by multivariate analysis*
| Factor | Adjusted OR | 95% CI | P | |
|---|---|---|---|---|
| Lower | Upper | |||
| Independent risk factors for malaria | ||||
| Age (per month increase) | 1.09 | 1.04 | 1.15 | 0.001 |
| Clinical dehydration on admission | 5.16 | 1.31 | 20.33 | 0.019 |
| Hematocrit (%) per unit decrease | 1.29 | 1.14 | 1.46 | < 0.001 |
| Hyperbilirubinemia (> 34 micromol/L) | 15.82 | 1.85 | 135.21 | 0.012 |
| Independent risk factors for severe pneumonia | ||||
| Nutritional status (per unit decrease in WAZ) | 2.23 | 1.48 | 3.35 | < 0.001 |
| History of any previous treatment | 6.20 | 1.40 | 27.46 | 0.016 |
| Hypoxemia (oxygen saturation < 94%) | 5.32 | 1.51 | 18.71 | 0.009 |
| Nasal flaring | 5.12 | 1.59 | 16.47 | 0.006 |
| Crackles/crepitations | 12.72 | 3.92 | 41.22 | < 0.001 |
| Unilateral chest signs | 5.19 | 0.95 | 28.35 | 0.058 |
| Leukocyte count (×109/L) per unit increase | 1.05 | 0.99 | 1.11 | 0.062 |
OR = odds ratio, CI = confidence interval; WAZ = weight-for-age Z score.
Impact of HIV.
The prevalence of HIV among study patients (38.2% among severe pneumonia patients, 18.6% among malaria patients) was much higher than the estimated community prevalence in this age group. Univariate analysis confirmed that HIV infection was a risk factor for severe pneumonia. However, due to the large proportion of missing HIV status data (> 20% patients), this variable was not included in the multivariate analysis.
Differences in clinical presentation according to HIV status within the severe pneumonia and malaria groups are shown in Table 4. Within the severe pneumonia group, HIV-positive children had a higher CFR (11.7% versus 3.1%; P = 0.033), and were significantly more malnourished. They also showed more frequently pallor, splenomegaly, and oral candidiasis, and significantly less wheezing. Within the malaria group, the impact of HIV seemed less noticeable because there were no differences in any of the variables when stratified by HIV status, with the sole exception of oral candidiasis, which was significantly more frequent among the HIV-positive patients with malaria.
Table 4.
Differences in clinical variables within each of the two distinct diagnostic groups (severe pneumonia and malaria) according to HIV status, Mozambique*
| Signs and symptoms on admission | Severe pneumonia exclusive group* (n=157) | Malaria exclusive group* (n=43) | ||||
|---|---|---|---|---|---|---|
| HIV status | HIV status | |||||
| HIV negative (n = 97) | HIV positive (n = 60) | P† | HIV negative (n = 35) | HIV positive (n = 8) | P† | |
| History of convulsions, no.(%) | 5 (5.2) | 0 (0.0) | 0.07 | 5 (14.3) | 2 (25.0) | 0.60 |
| Temperature (°C), Mean (SD) | 38.0 (1.2) | 38.2 (1.1) | 0.22 | 38.5 (1.0) | 38.3 (1.1) | 0.51 |
| Low oxygen saturation (< 90%), no.(%) | 59 (61.5) | 42 (70.0) | 0.28 | 10 (28.6) | 3 (37.5) | 0.68 |
| Respiratory rate, mean (SD) | 66.3 (15.4) | 66.6 (15.4) | 0.90 | 57.7 (13.6) | 59.1 (14.6) | 0.80 |
| Nasal flaring, no. (%) | 59 (60.8) | 34 (56.7) | 0.61 | 12 (34.3) | 4 (50.0) | 0.44 |
| Deep breathing, no. (%) | 3 (3.1) | 2 (3.3) | 0.93 | 7 (20.0) | 2 (25.0) | 1.00 |
| Unilateral chest signs, no. (%) | 34 (35.1) | 17 (28.3) | 0.38 | 2 (5.7) | 0 (0.0) | 1.00 |
| Crackles, no. (%) | 81 (83.5) | 51 (85.0) | 0.80 | 9 (25.7) | 4 (50.0) | 0.22 |
| Wheezing, no. (%) | 18 (18.6) | 2 (3.3) | 0.005 | 4 (11.4) | 1 (12.5) | 1.00 |
| Oral candidiasis, no. (%) | 4 (4.1) | 12 (20.0) | 0.001 | 0 (0.0) | 2 (25.0) | 0.031 |
| Splenomegaly, no. (%) | 4 (4.1) | 16 (26.7) | < 0.001 | 17 (48.6) | 6 (75.0) | 0.25 |
| Any dehydration, no. (%) | 12 (12.4) | 13 (21.7) | 0.12 | 12 (34.3) | 3 (37.5) | 1.00 |
| Prostration, no. (%) | 14 (14.4) | 10 (16.7) | 0.71 | 13 (37.1) | 3 (37.5) | 1.00 |
| Heart gallop, no. (%) | 1 (1.0) | 6 (10.0) | 0.008 | 9 (25.7) | 2 (25.0) | 1.00 |
| Pallor, no. (%) | 1 (1.0) | 10 (16.7) | < 0.001 | 14 (40.0) | 5 (62.5) | 0.43 |
| Waz: Mean (SD) | -1.6 (1.7) | -3.3 (-2.5) | < 0.001 | -1.5 (1.2) | -2 (1.2) | 0.27 |
| Case fatality rates, no. (%) | 3 (3.1) | 7 (11.7) | 0.033 | 0 (0.0) | 0 (0.0) | NA |
HIV = human immunodeficiency virus.
P values obtained with univariate logistic regression comparing malaria vs. severe pneumonia groups. When necessary, Fisher's exact test applied.
Discussion
The distinction on clinical grounds between malaria and severe pneumonia is not straightforward and has challenged clinicians for a long time.11,12,14,15 This study sought to evaluate clinical signs, symptoms, or other variables that could help clinicians more precisely discriminate at the hospital level between those two diseases. Previous studies addressing this question at the outpatient level have produced disappointing results,10,12,13,33 and hospital-based studies15,34,35 with more complex differentiating techniques, such as microbiology, laboratory biomarkers, pulse oximetry, or radiography, have shown better results at producing models, but still with limited practicality for the medical personnel faced with such complex patients.
Univariate analysis in our study identified significant differences between patients with malaria and patients with severe pneumonia, which likely reflected differential pathophysiologic mechanisms.15 However, multivariate analysis showed that younger age at hospitalization, dehydration, lower PCV counts, or hyperbilirrubinemia were the only independent risk factors associated with malaria. None of these findings can replace an adequate parasitologic diagnosis (either microscopy or rapid diagnostic tests) which remain, despite their limitations,36 the best predictive tools for identifying malaria. These findings justify the recent recommendation of WHO for parasitologic confirmation before antimalarial therapy.37
One could argue that in developing countries, the real diagnostic problem lies not in detection of malaria parasites, but in rapid identification of bacterial disease warranting life-saving antibiotics, especially when considering its much higher associated CFR (6.6 times higher in this study). Clinical signs and symptoms independently associated in this study with severe pneumonia and consequently with the need of antibacterial treatment included a history of preadmission treatment, crackles or unilateral chest signs on auscultation, nasal flaring, hypoxemia, poorer nutritional status, and higher leukocyte counts at admission. However, none of these factors or those associated with malaria seem sufficiently reliable to be used by clinicians to unambiguously discriminate a precise diagnosis because of their low prevalence, poor specificity, or because they are too difficult to ascertain by low-skilled medical personnel or require equipment often unavailable or expensive.
Models constructed with combinations or algorithms including these signs/symptoms did not help to discriminate the groups reliably. Consequently, management of this particular group of patients must include the use of antibiotics, always complemented by antimalarial drugs unless parasitologic diagnosis can rule out malaria infection.
Co-infection with malaria and pneumonia was an infrequent event in this study. Only nine patients (1.4%) were shown to be co-infected with both diseases, a finding that contrasts with that (11.4%) in a similar study with children in Kenya.15 Such disparity could be caused by differences between settings in malaria endemicity, but predominantly result from use of highly specific but less sensitive endpoint definitions, making our case definitions stringent and more difficult to fulfill. Although rigorous case definitions were necessary to accurately differentiate the most likely etiology for our cases, they are also an important limitation of this study because they failed to correctly classify more than half (52.9%) of the patients. Understanding the real etiology behind such a large group of children is the specific object of a different analysis that will be published separately, but the role of other microorganisms, including Pneumocystis jirovecii, Mycobacterium tuberculosis, or other immunosuppression-related infections is likely to be important,38 and bacterial episodes without clear radiologic changes may also have been misclassified.
Our findings were derived from studying a subgroup of severely ill patients admitted to a hospital who fulfilled a pre-defined set of clinical criteria for severe pneumonia and malaria, as defined by IMCI guidelines. Trying to extrapolate such findings to general in-patients or less severe cases might not be valid and necessarily limits the applicability of these conclusions. Because all patients with malaria cases recruited for the study did also fulfill our criteria, they may not be representative of the overall malaria population hospitalized, especially if one takes into account the large proportion of admitted patients with malaria who do not have respiratory symptoms. Furthermore, these results do not contemplate the recent decrease in malaria incidence in many countries in Africa39 or the severe reductions resulting from introduction of conjugate vaccines for H. influenzae b or S. pneumoniae40,41 in countries in Africa. These two factors will also importantly impact the incidence of malaria and severe pneumonia in the future, and thus alter their diagnosis and clinical overlap.
One-fourth of all study patients were infected with HIV and up to 68.2% of all in-hospital deaths occurred among HIV-positive patients. The HIV/acquired immunodeficiency syndrome pandemic has changed the clinical picture in hospitals in Africa and is threatening the advances that have been made on child survival in this area.42 Infection with HIV is known to increase the risk of bacterial infection,43 which likely influences the etiology, distribution, and incidence of severe pneumonia cases,38,44,45 although its relationship to malaria risk is less clear.45 In our study, HIV seemed to differentially impact the two distinct groups, further challenging their discrimination on clinical grounds. Although HIV hardly modified the clinical presentation of malaria cases, it seemed to negatively impact the prognosis and presentation of severe pneumonia episodes, although numbers are small and comparisons may be underpowered. This differential impact might alter the pattern of admission diagnoses, similarly to what has occurred in neighboring South Africa,46 where HIV-related pneumonia cases have become the principal cause of admissions. Implementing specific measures to decrease the burden of HIV-related illness will likely impact morbidity and mortality related to severe pneumonia.47
This study has highlighted that in a setting such as Manhiça, only a small fraction of pediatric admissions with clinical presentation compatible with malaria and severe pneumonia according to IMCI can be attributable to malaria. Although both diseases may have respiratory findings, symptom overlap should not be understood as disease overlap, which is a less frequent finding. Clinical signs or laboratorial determinations remain insufficiently specific to help the clinician ascertain the real etiology in these patients, and should not replace basic routine parasitologic or microbiologic diagnosis. Although these two procedures are increasingly available for malaria, no equivalent gold standard method exists for bacterial pneumonia, a major drawback that needs to be urgently addressed by future research, particularly in areas of high HIV prevalence.
ACKNOWLEDGMENTS
We thank the children and their mothers for participating in the study; Jorge Uqueio for coordinating logistical aspects of the study; Madalena Ripinga for performing clinical work; the clinical officers, medical estagiarios, HIV counselors, data managers, laboratory workers, and laboratory coordinator for obtaining data; Llorenç Quintó and Santi Pérez-Hoyos for providing statistical advice; and Immo Kleinschmidt (London School of Hygiene and Tropical Medicine London, United Kingdom) for providing significant feedback on previous versions of the manuscript.
Footnotes
Financial support: This study was supported by a grant from the World Health Organization (WHO-C6-181-489). The Centro de Investigaçaõ em Saúde da Manhiça received financial support from the Spanish Agency for International Cooperation. Quique Bassat and Anna Roca were supported at the time of the study by the Spanish Ministry of Science and Innovation (FIS: Contrato post Formación Sanitaria Especializada, Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, ref. CM05/00134) and Ramon y Cajal (RYC2008-02777), respectively.
Disclosure: None of the authors has any conflicts of interests.
Authors' addresses: Quique Bassat, Cristina O'Callaghan-Gordo, Núria Díez-Padrisa, Edgar Ayala, Sergi Sanz, Anna Roca, and Pedro L. Alonso, Centre de Recerca en Salut Internacional de Barcelona, Hospital Clínic, Universitat de Barcelona, Rosselló 132, 4art, 08036 Barcelona, Spain, E-mails: quique.bassat@cresib.cat, cristina.ocallaghan@cresib.cat, nuria.diez@cresib.cat, edgar_am12@hotmail.com, ssanz@clinic.ub.es, aroca@mrc.gm, and palonso@clinic.ub.es. Sónia Machevo, Betuel Sigaúque, Luís Morais, Inácio Mandomando, and Tacilta Nhampossa, Centro de Investigaçaõ em Saúde da Manhiça, Rúa 12 Vila da Manhiça, CP1929, Maputo, Mozambique, E-mails: sonia.machevo@manhica.net, betuel.sigauque@manhica.net, musukhamorais@yahoo.com.br, inacio.mandomando@gmail.com, and taciltanha@yahoo.com. Josep L. Ribó, Hospital Universitari Sant Joan de Déu, 08950 Esplugues de Llobregat, Barcelona, Spain, E-mail: jlribo@hsjdbcn.org. Martin Weber, World Health Organization, Indonesia Country Office, Bina Mulia 1 Building, Jalan HR Rasuna Said Kav. 10, Jakarta 12950, Indonesia, E-mail: weberm@who.or.id.
References
- 1.Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, Bassani DG, Jha P, Campbell H, Walker CF, Cibulskis R, Eisele T, Liu L, Mathers C. Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375:1969–1987. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 2.The United Nations Children's Fund (UNICEF)/World Health Organization . Pneumonia: The Forgotten Killer of Children. New York: United Nations; 2006. [Google Scholar]
- 3.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/s1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization The World Malaria Report. 2009. http://www.who.int/malaria/world_malaria_report_2009/en/index.html Available at.
- 5.Gove S. Integrated management of childhood illness by outpatient health workers: technical basis and overview. The WHO Working Group on Guidelines for Integrated Management of the Sick Child. Bull World Health Organ. 1997;75((Suppl 1)):7–24. [PMC free article] [PubMed] [Google Scholar]
- 6.Arifeen SE, Hoque DM, Akter T, Rahman M, Hoque ME, Begum K, Chowdhury EK, Khan R, Blum LS, Ahmed S, Hossain MA, Siddik A, Begum N, Sadeq-ur Rahman Q, Haque TM, Billah SM, Islam M, Rumi RA, Law E, Al-Helal ZA, Baqui AH, Schellenberg J, Adam T, Moulton LH, Habicht JP, Scherpbier RW, Victora CG, Bryce J, Black RE. Effect of the Integrated Management of Childhood Illness strategy on childhood mortality and nutrition in a rural area in Bangladesh: a cluster randomised trial. Lancet. 2009;374:393–403. doi: 10.1016/S0140-6736(09)60828-X. [DOI] [PubMed] [Google Scholar]
- 7.English M, Berkley J, Mwangi I, Mohammed S, Ahmed M, Osier F, Muturi N, Ogutu B, Marsh K, Newton CR. Hypothetical performance of syndrome-based management of acute paediatric admissions of children aged more than 60 days in a Kenyan district hospital. Bull World Health Organ. 2003;81:166–173. [PMC free article] [PubMed] [Google Scholar]
- 8.Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, Wangai H, Kimera J, Msuya W, Mtei F, Dekker D, Malahiyo R, Olomi R, Crump JA, Whitty CJ, Reyburn H. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ. 2010;340:c1350. doi: 10.1136/bmj.c1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simoes EA, Peterson S, Gamatie Y, Kisanga FS, Mukasa G, Nsungwa-Sabiiti J, Were MW, Weber MW. Management of severely ill children at first-level health facilities in sub-Saharan Africa when referral is difficult. Bull World Health Organ. 2003;81:522–531. [PMC free article] [PubMed] [Google Scholar]
- 10.Kallander K, Nsungwa-Sabiiti J, Peterson S. Symptom overlap for malaria and pneumonia–policy implications for home management strategies. Acta Trop. 2004;90:211–214. doi: 10.1016/j.actatropica.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 11.La Roche R. Pneumonia: It's Supposed Connection, Pathological and Etiological, with Autumnal Fevers; Including an Inquiry into the Existence and Morbid Agency of Malaria. Philadelphia: Blanchard and Lee; 1854. [Google Scholar]
- 12.O'Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg. 1993;87:662–665. doi: 10.1016/0035-9203(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 13.Redd SC, Bloland PB, Kazembe PN, Patrick E, Tembenu R, Campbell CC. Usefulness of clinical case-definitions in guiding therapy for African children with malaria or pneumonia. Lancet. 1992;340:1140–1143. doi: 10.1016/0140-6736(92)93160-o. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . The Overlap in the Clinical Presentation and Treatment of Malaria and Pneumonia in Children: Report of a Meeting. Geneva: World Health Organization; 1992. WHO/ARI/92.23.WHO/Mal/92.1065. [Google Scholar]
- 15.English M, Punt J, Mwangi I, McHugh K, Marsh K. Clinical overlap between malaria and severe pneumonia in Africa children in hospital. Trans R Soc Trop Med Hyg. 1996;90:658–662. doi: 10.1016/s0035-9203(96)90423-x. [DOI] [PubMed] [Google Scholar]
- 16.Reyburn H, Mwakasungula E, Chonya S, Mtei F, Bygbjerg I, Poulsen A, Olomi R. Clinical assessment and treatment in paediatric wards in the north-east of the United Republic of Tanzania. Bull World Health Organ. 2008;86:132–139. doi: 10.2471/BLT.07.041723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.English M, Esamai F, Wasunna A, Were F, Ogutu B, Wamae A, Snow RW, Peshu N. Assessment of inpatient paediatric care in first referral level hospitals in 13 districts in Kenya. Lancet. 2004;363:1948–1953. doi: 10.1016/S0140-6736(04)16408-8. [DOI] [PubMed] [Google Scholar]
- 18.Alonso PL, Saúte F, Aponte JJ, Gómez-Olivé FX, Nhacolo A, Thomson R, Macete E, Abacassamo F, Ventura PJ, Bosch X, Menéndez C, Dgedge M. Population and Health in Developing Countries. Ottawa, Canada: International Development Research Centre (IDRC) and Manhiça, Mozambique; 2002. pp. 189–195. INDEPTH. [Google Scholar]
- 19.Guinovart C, Bassat Q, Sigauque B, Aide P, Sacarlal J, Nhampossa T, Bardaji A, Nhacolo A, Macete E, Mandomando I, Aponte JJ, Menendez C, Alonso PL. Malaria in rural Mozambique. Part I: children attending the outpatient clinic. Malar J. 2008;7:36. doi: 10.1186/1475-2875-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menendez C, Bardaji A, Sigauque B, Romagosa C, Sanz S, Serra-Casas E, Macete E, Berenguera A, David C, Dobano C, Naniche D, Mayor A, Ordi J, Mandomando I, Aponte JJ, Mabunda S, Alonso PL. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS ONE. 2008;3:e19346. doi: 10.1371/journal.pone.0001934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naniche D, Bardaji A, Lahuerta M, Berenguera A, Mandomando I, Sanz S, Aponte JJ, Sigauque B, Alonso PL, Menendez C. Impact of maternal human immunodeficiency virus infection on birth outcomes and infant survival in rural Mozambique. Am J Trop Med Hyg. 2009;80:870–876. [PubMed] [Google Scholar]
- 22.Bassat Q, Guinovart C, Sigauque B, Aide P, Sacarlal J, Nhampossa T, Bardaji A, Nhacolo A, Macete E, Mandomando I, Aponte JJ, Menendez C, Alonso PL. Malaria in rural Mozambique. Part II: children admitted to hospital. Malar J. 2008;7:37. doi: 10.1186/1475-2875-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sigauque B, Roca A, Bassat Q, Morais L, Quinto L, Berenguera A, Machevo S, Bardaji A, Corachan M, Ribo J, Menendez C, Schuchat A, Flannery B, Soriano-Gabarro M, Alonso PL. Severe pneumonia in Mozambican young children: clinical and radiological characteristics and risk factors. J Trop Pediatr. 2009;55:379–387. doi: 10.1093/tropej/fmp030. [DOI] [PubMed] [Google Scholar]
- 24.Sacarlal J, Nhacolo AQ, Sigauque B, Nhalungo DA, Abacassamo F, Sacoor CN, Aide P, Machevo S, Nhampossa T, Macete EV, Bassat Q, David C, Bardaji A, Letang E, Saute F, Aponte JJ, Thompson R, Alonso PL. A 10 year study of the cause of death in children under 15 years in Manhica, Mozambique. BMC Public Health. 2009;9:67. doi: 10.1186/1471-2458-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization . Pocket Book for Hospital Care of Children: Guidelines for the Management of Common Illness with Limited Resources. Geneva: World Health Organization; 2005. [Google Scholar]
- 26.Bassat Q, Guinovart C, Sigauque B, Mandomando I, Aide P, Sacarlal J, Nhampossa T, Bardaji A, Morais L, Machevo S, Letang E, Macete E, Aponte JJ, Roca A, Menendez C, Alonso PL. Severe malaria and concomitant bacteraemia in children admitted to a rural Mozambican hospital. Trop Med Int Health. 2009;14:1011–1019. doi: 10.1111/j.1365-3156.2009.02326.x. [DOI] [PubMed] [Google Scholar]
- 27.Sigauque B, Roca A, Mandomando I, Morais L, Quinto L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, Bassat Q, Bardaji A, Nhalungo D, Soriano-Gabarro M, Flannery B, Menendez C, Levine MM, Alonso PL. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J. 2009;28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 28.Roca A, Sigauque B, Quinto L, Mandomando I, Valles X, Espasa M, Abacassamo F, Sacarlal J, Macete E, Nhacolo A, Levine M, Alonso P. Invasive pneumococcal disease in children < 5 years of age in rural Mozambique. Trop Med Int Health. 2006;11:1422–1431. doi: 10.1111/j.1365-3156.2006.01697.x. [DOI] [PubMed] [Google Scholar]
- 29.Valles X, Flannery B, Roca A, Mandomando I, Sigauque B, Sanz S, Schuchat A, Levine M, Soriano-Gabarro M, Alonso P. Serotype distribution and antibiotic susceptibility of invasive and nasopharyngeal isolates of Streptococcus pneumoniae among children in rural Mozambique. Trop Med Int Health. 2006;11:358–366. doi: 10.1111/j.1365-3156.2006.01565.x. [DOI] [PubMed] [Google Scholar]
- 30.O'Callaghan-Gordo C, Bassat Q, Morais L, Diez-Padrisa N, Machevo S, Nhampossa T, Nhalungo D, Sanz S, Quinto L, Alonso PL, Roca A. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: a malaria endemic area with high prevalence of human immunodeficiency virus. Pediatr Infect Dis J. 2011;30:39–44. doi: 10.1097/INF.0b013e3181f232fe. [DOI] [PubMed] [Google Scholar]
- 31.Cherian T, Mulholland EK, Carlin JB, Ostensen H, Amin R, de Campo M, Greenberg D, Lagos R, Lucero M, Madhi SA, O'Brien KL, Obaro S, Steinhoff MC. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull World Health Organ. 2005;83:353–359. [PMC free article] [PubMed] [Google Scholar]
- 32.Roca A, Sigauque B, Quinto L, Morais L, Berenguera A, Corachan M, Ribo JL, Naniche D, Bassat E, Sacoor C, Nhalungo D, Macete E, Schuchat A, Soriano-Gabarro M, Flannery B, Alonso PL. Estimating the vaccine-preventable burden of hospitalized pneumonia among young Mozambican children. Vaccine. 2010;28:4851–4857. doi: 10.1016/j.vaccine.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 33.Berkley JA, Maitland K, Mwangi I, Ngetsa C, Mwarumba S, Lowe BS, Newton CR, Marsh K, Scott JA, English M. Use of clinical syndromes to target antibiotic prescribing in seriously ill children in malaria endemic area: observational study. BMJ. 2005;330:995. doi: 10.1136/bmj.38408.471991.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNally LM, Jeena PM, Gajee K, Thula SA, Sturm AW, Cassol S, Tomkins AM, Coovadia HM, Goldblatt D. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet. 2007;369:1440–1451. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 35.Muller B, Harbarth S, Stolz D, Bingisser R, Mueller C, Leuppi J, Nusbaumer C, Tamm M, Christ-Crain M. Diagnostic and prognostic accuracy of clinical and laboratory parameters in community-acquired pneumonia. BMC Infect Dis. 2007;7:10. doi: 10.1186/1471-2334-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT) Am J Trop Med Hyg. 2007;77:119–127. [PubMed] [Google Scholar]
- 37.World Health Organization Guidelines for the Treatment of Malaria, Second Edition. 2010. http://whqlibdoc.who.int/publications/2010/9789241547925.pdf Available at.
- 38.Graham SM, Mankhambo L, Phiri A, Kaunda S, Chikaonda T, Mukaka M, Molyneux EM, Carrol ED, Molyneux ME. Impact of human immunodeficiency virus infection on the etiology and outcome of severe pneumonia in Malawian children. Pediatr Infect Dis J. 2011;30:33–38. doi: 10.1097/INF.0b013e3181fcabe4. [DOI] [PubMed] [Google Scholar]
- 39.O'Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect Dis. 2010;10:545–555. doi: 10.1016/S1473-3099(10)70096-7. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 41.Watt JP, Wolfson LJ, O'Brien KL, Henkle E, Deloria-Knoll M, McCall N, Lee E, Levine OS, Hajjeh R, Mulholland K, Cherian T. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–911. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 42.Zwi K, Pettifor J, Soderlund N, Meyers T. HIV infection and in-hospital mortality at an academic hospital in South Africa. Arch Dis Child. 2000;83:227–230. doi: 10.1136/adc.83.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenwood B. The epidemiology of pneumococcal infection in children in the developing world. Philos Trans R Soc Lond B Biol Sci. 1999;354:777–785. doi: 10.1098/rstb.1999.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MP, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott JA. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med. 2005;352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 45.Laufer MK, van Oosterhout JJ, Thesing PC, Thumba F, Zijlstra EE, Graham SM, Taylor TE, Plowe CV. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 46.Zwi KJ, Pettifor JM, Soderlund N. Paediatric hospital admissions at a South African urban regional hospital: the impact of HIV, 1992–1997. Ann Trop Paediatr. 1999;19:135–142. doi: 10.1080/02724939992455. [DOI] [PubMed] [Google Scholar]
- 47.Enarson PM, Gie RP, Enarson DA, Mwansambo C, Graham SM. Impact of HIV on standard case management for severe pneumonia in children. Expert Rev Respir Med. 2011;4:211–220. doi: 10.1586/ers.10.14. [DOI] [PubMed] [Google Scholar]


