Abstract
We demonstrate here the identification and phylogenetic characterization of Babesia microti (B. microti)-like parasite detected from a splenectomized Japanese macaque (Macaca fuscata fuscata) at a facility for laboratory animal science. On Day 133 after splenectomy, intra-erythrocytic parasites were found on light microscopic examination, and the level of parasitemia reached 0.3% on blood smear. Molecular characterization of the parasite using nested-polymerization chain reactions targeting the 18S rRNA, β-tubulin, and subunit 7 (eta) of the chaperonin-containing t-complex polypeptide 1 (CCT7) genes were identified as a B. microti-like parasite, designated the Japanese Macaque Babesia-1 (JM-1).
The genus Babesia belongs to the family Piroplasmida, closely related to Plasmodium and Theileria genera, and comprises over 70 species that parasitize mammals and birds.1 Babesia microti (B. microti) is a rodent-infective Babesia species transmitted by ixodid ticks and is also a major etiological agent of human babesiosis.2–5 Nonhuman primates in Africa and Asia are natural hosts for Entopolypoides macaci, of which is a piroplasm phylogenetically close to the B. microti parasite and similar in morphology.6–9 Babesia microti-like parasites have recently been reported to infect various vertebrate hosts such as the raccoon, domestic dog, fox, squirrel, and rarely humans.10–14 Natural infections with B. microti-like parasites have also been found in some species of nonhuman primates including the African baboon (Papio cynocephalus), cynomolgus macaque (Macaca fascicularis), rhesus macaque (Macaca mulatta), and cercopithecus monkey (Cercopithecus pygerythrus).6,7,9,15,16 Moreover, the susceptibility of the squirrel monkey (Saimiri sciureus) and capuchin monkey (Cebus paella) to B. microti infection has been reported.17,18
The Japanese macaque (Macaca fuscata), as well as rhesus and cynomolgus macaques, is an Old World monkey species native to Japan. It is found only in Japan and classified into two subspecies, Macaca fuscata fuscata, which is widely distributed in three major islands of Japan, and Macaca fuscata yakui, found only in Yaku Islet.19 We demonstrate here the identification and phylogenetic analysis of a B. microti-like parasite detected from M. fuscata fuscata at a primate center for biomedical research.
The monkey (animal no. J79) housed in a cage outdoors, an eight-year-old female, was a second-generation offspring bred in captivity, and was offered by a commercial animal facility after a 28-day quarantine period. No clinical problems or specific pathogens were found during the quarantine period. Monkey J79 was splenectomized for an experiment involving primate malaria infection, and was kept in an individual cage in controlled Biosafety Level II condition at Tsukuba Primate Research Center, given commercial food pellets supplemented with fresh fruits, and maintained in accordance with the Guidelines for the Use of Experimental Animals authorized by the Japanese Association for Laboratory Animal Science. The protocol was approved by the Ethics Committee of Animal Experiments, Dokkyo University of School of Medicine (permit no.: 0536).
Although no marked clinical signs were observed in the monkey during the postoperative period after splenectomy, at 133 days after operation intra-erythrocytic parasites were found on light microscopic examination. Parasitemia reached 0.3% spontaneously, and dot forms (Figure 1A), ovoid forms measuring about 2 μm in diameter (Figure 1B), pyriforms (Figure 1C), and ring-forms (data not shown) were frequently detected on Giemsa-stained thin blood smears. In addition, multiply-infected erythrocytes were often observed (Figure 1D). The parasites were morphologically distinct from primate malarial parasites, but were very similar to B. microti.
Figure 1.
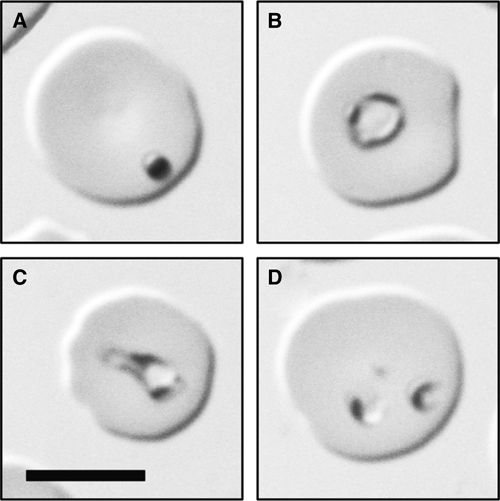
Light micrograph of a Giemsa-stained thin blood smear of peripheral blood showing various forms of intra-erythrocytic parasites. (A) dot form; (B) ovoid form; (C) pyriform; (D) a multiply-infected erythrocyte (bar = 5 μm).
Polymerase chain reaction (PCR) was performed using genomic DNA (gDNA) of the parasite from the peripheral blood. Heparinized blood obtained from J79 was centrifuged at 1,200 × g for 10 min at 4°C. Erythrocytes were washed three times with phosphate-buffered saline (PBS) by centrifugation at 1,200 × g for 10 min at 4°C, and the buffy coat was removed completely. The gDNA was extracted using a QIAamp DNA blood mini kit (Qiagen, Tokyo, Japan) according to the manufacturer's instructions. The gDNA was used as a template for nested PCR, which was carried out targeting the 18S rRNA, β-tubulin, and subunit 7 (eta) of the chaperonin-containing t-complex polypeptide 1 (CCT7) genes of piroplasma belonging to Babesia and Theileria, as described previously with minor modifications.20,21 The 18S rRNA gene was amplified using Piro0F/Piro6R for the first-round PCR and Piro1F/Piro 5.5R for nested PCR (Table 1).12 The β-tubulin gene was amplified using the primers TUBU-ATG5F/Tubu-1538R for the first-round PCR and Tubu-63F/Tubu-3R for nested PCR (Table 1). The CCT7 gene was amplified using the primers TBCCT35F/TBCCTR0 for the first round, and TBCCT70F/TBCCT1519R-3 for nested PCR (Table 1).20 The PCR reaction mixture contained 0.1 μg of template DNA, 5 μL of 10× PCR buffer with 15 mM MgCl2 (TaKaRa Bio Inc., Shiga, Japan), 5 μL of dNTP mix (2 mM of each dNTP) (TaKaRa), 2.5 U of Takara LA Taq DNA polymerase (TaKaRa), and 50 pmol of each primer set for the 18S rRNA, β-tubulin, or CCT7 gene-specific primers for PCR as described previously with minor modifications as reported.21
Table 1.
Polymerase chain reaction (PCR) primers used for amplification of the 18S rRNA, β-tubulin, and CCT7 genes
| Target genes | Primers | Oligonucleotide sequences (5′ to 3′) |
|---|---|---|
| 18S rRNA | Piro 0F | GCCAGTAGTCATATGCTTGTGTTA |
| Piro 6R | CTCCTTCCTYTAAGTGATAAGGTTCAC | |
| Piro 1F | CCATGCATGTCTWAGTAYAARCTTTTA | |
| Piro 5.5R | CCTYTAAGTGATAAGGTTCACAAAACTT | |
| β-tubulin | TUBU-ATG5F | ATGAGAGARATYGTACACATYCAAGC |
| Tubu-1538R | TAYTGYTGGTAYTCGCTRACYA | |
| Tubu-63F | CAAATWGGYGCMAARTTYTGGGA | |
| Tubu-3R | TCGTCCATACCTTCWCCSGTRTACCAGTG | |
| CCT7 | TBCCT35F | TGAAGGARGGNACNGAYACWTCYCARGG |
| TBCCTR0 | GTYTCRTCDATDSWNAGNACHWGGCANGCNGCYTCDGTNGC | |
| TBCCT70F | CAAATYATYAGYAAYATWAAYGCCTGYCA | |
| TBCCT1519R-3 | KTYYTYTTNACMANNBHDGGYTCCCADATRCA |
Nested PCR successfully amplified 18S rRNA, β-tubulin, and CCT7 genes from the gDNA of J79 (data not shown). The PCR products were isolated by 1.0% (w/v) agarose-gel electrophoresis in TAE buffer and purified with a GENECLEAN kit (BIO 101, Inc., Vista, CA). Nucleotide sequences of 18S rRNA, β-tubulin, and CCT7 genes were then determined using a CEQ8000 automated sequencer (Beckman Coulter, Inc., Brea, CA) with the DTCS DNA Sequence kit (Beckman Coulter). The 18S rRNA, β-tubulin, and CCT7 genes were 1,678, 1,300, and 1,720 bp in size, and the sequences were registered in GenBank under accession nos. of AB576641, AB576642, and AB576643, respectively.
A BLAST search with the 18S rRNA, β-tubulin, and CCT7 sequences detected no identical sequences in the public database. The most closely related sequences, with 98% sequence similarity, were from a B. microti-like parasite, which was isolated from a Hokkaido Squirrel.13 We refer to the cognate sequence as Japanese Macaque Babesia-1 (JM-1).
Phylogenetic relationships among JM-1 and other Babesia and Theileria species were analyzed with the sequences for 18S rRNA, β-tubulin, and CCT7 genes using MacVector software version 8.0 (Genetic Computer Group Inc., Madison, WI). Published sequences of these genes were retrieved from the public database (Figure 2). These sequences were aligned using the CLUSTAL W Alignment program,22 and a phylogenetic tree was constructed by the neighbor-joining (NJ) method from the aligned sequences with the Phylogenetic Analysis in the Mac Vector software package, version 8.0. Support for tree nodes was calculated with 1,000 bootstrap replicates using the bootstrap tree algorithm.21,23
Figure 2.
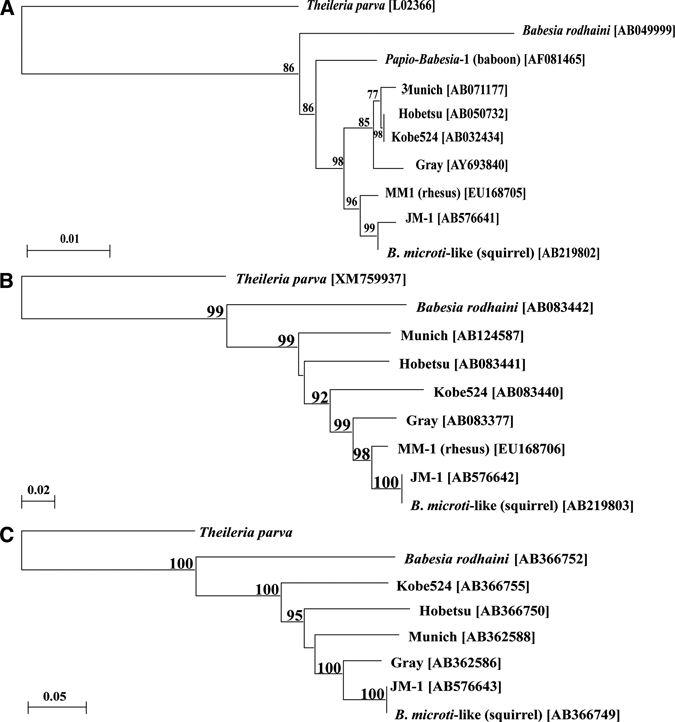
Neighbor-joining phylogenetic trees showing relationships between the (A) 18S rRNA, (B) β-tubulin, and (C) CCT7 gene sequences from the Japanese macaque J79 Babesia microti-like parasite (JM-1) and other Babesia isolates. GenBank accession numbers are shown in the trees for sequences from B. microti Gray strain, B. microti-like parasites (Kobe524, Hobetsu, and Munich strains; squirrel isolate, Japan), and Babesia rodhaini. The corresponding Theileria parva sequence served as the outgroup for each tree. The GenBank accession numbers are shown for 18S rRNA, β-tubulin, and CCT7 genes. The gene sequence was obtained from the Institute for Genomic Research (TIGR) website (http://www.tigr.org). Numbers at the nodes indicate bootstrap support form 1,000 repetitions.
The NJ phylogenetic tree constructed using the 18S rRNA gene sequences separates the B. microti group into two closely related clades, one holding the rodent and human isolates including the Gray strain (B. microti sensu stricto), and one holding the nonhuman primate and squirrel isolates (Figure 2A). The trees constructed from β-tubulin and CCT7 gene sequences, however, both place JM-1 within a single clade holding all corresponding sequences from B. microti sensu stricto and B. microti-like isolates, but more distant from B. microti sensu stricto (Figure 2B and C). JM-1 was most closely related to the B. microti-like parasite from a squirrel by all three analyses (Figure 2).
According to a previous survey in 1979, infections with Babesia sp. were found in 4 of 93 (4.3%) Japanese macaques (M. fuscata yakui) that had been reared in a monkey park in Japan.19 Although the origin of the JM-1 infection in the Japanese macaque is unclear, it is likely that the infection was from colonies of Japanese macaques at the facility of animal science laboratory or the cage outdoors at the breeding facility. Our case is most likely an example of subclinical or opportunistic infection that manifested in the postoperative period after splenectomy in a previously immunocompetent host.
ACKNOWLEDGMENTS
We thank the late Professor Masayoshi Tsuji for his seminal contributions to this research.
Footnotes
Financial support: This study was supported in part by a Grant-in-Aid for Cooperative Research from Rakuno Gakuen University, Rakuno Gakuen University Dairy Science Institute, 2010-6, a Cooperative Research Grant (1423-joint-1) from the National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, and grants from the Ministry of Health, Labor and Welfare of Japan and Ministry of Education, Culture, Sports, Science and Technology of Japan.
Authors' addresses: Haruyuki Hirata, Mari Maeda, Michio Jinnai, Kohei Fujisawa, and Chiaki Ishihara, School of Veterinary Medicine, Rakuno Gakuen University, Ebetsu, Japan, E-mails: hirata@rakuno.ac.jp, s20603057@stu.rakuno.ac.jp, s20741005@stu.rakuno.ac.jp, s20741003@stu.rakuno.ac.jp, and ishihara@rakuno.ac.jp. Satoru Kawai, Laboratory of Tropical Medicine and Parasitology, Dokkyo Medical University, Tochigi, Japan, E-mail: skawai@dokkyomed.ac.jp. Yuko Katakai, Corporation for Production and Research of Laboratory Primates, Hachimandai, Tsukuba, Japan, E-mail: katakai@primate.or.jp. Kenji Hikosaka and Kazuyuki Tanabe, Laboratory of Malariology, International Research Center of Infectious Diseases, Research Institute for Microbial Diseases, Osaka University, Suita, Osaka, Japan, E-mails: hikosaka@biken.osaka-u.ac.jp and kztanabe@biken.osaka-u.ac.jp. Yasuhiro Yasutomi, Laboratory of Immunoregulation and Vaccine Research, Tsukuba Primate Research Center, National Institute of Biomedical Innovation, Tsukuba, Ibaraki, Japan, E-mail: yasutomi@nibio.go.jp.
References
- 1.Martin-Rabadán P, Emilio B. In: Infectious Diseases. Third edition. Cohen J, Powderly WG, Opal SM, editors. Philadelphia, PA: Mosby; 2010. 1892. (Blood and tissue protozoa). [Google Scholar]
- 2.Goethert HK, Telford SR., 3rd What is Babesia microti? Parasitology. 2003;127:301–309. doi: 10.1017/s0031182003003822. [DOI] [PubMed] [Google Scholar]
- 3.Homer MJ, Aguilar-Delfin I, Telford SR, 3rd, Krause PJ, Persing DH. Babesiosis. Clin Microbiol Rev. 2000;13:451–469. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Telford SR III, Gorenflot A, Brasseur P, Spielman A. In: Parasitic Protozoa. Kreier JP, editor. New York, NY: Academic Press, Inc.; 1993. pp. 1–47. (Babesial infections in humans and wildlife). [Google Scholar]
- 5.Tsuji M, Wei Q, Zamoto A, Morita C, Arai S, Shiota T, Fujimagari M, Itagaki A, Fujita H, Ishihara C. Human babesiosis in Japan: epizootiologic survey of rodent reservoir and isolation of new type of Babesia microti-like parasite. J Clin Microbiol. 2001;39:4316–4322. doi: 10.1128/JCM.39.12.4316-4322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bronsdon MA, Homer MJ, Magera JM, Harrison C, Andrews RG, Bielitzki JT, Emerson CL, Persing DH, Fritsche TR. Detection of enzootic babesiosis in baboons (Papio cynocephalus) and phylogenetic evidence supporting synonymy of the genera Entopolypoides and Babesia. J Clin Microbiol. 1999;37:1548–1553. doi: 10.1128/jcm.37.5.1548-1553.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emerson CL, Tsai CC, Holland CJ, Ralston P, Diluzio ME. Recrudescence of Entopolypoides macaci Mayer, 1933 (Babesiidae) infection secondary to stress in long-tailed macaques (Macaca fascicularis) Lab Anim Sci. 1990;40:169–171. [PubMed] [Google Scholar]
- 8.Gleason NN, Wolf RE. Entopolypoides macaci (Babesiidae) in Macaca mulatta. J Parasitol. 1974;60:844–847. [PubMed] [Google Scholar]
- 9.Hawking F. Entopolypoides macaci, a Babesia-like parasite in Cercopithecus monkeys. Parasitology. 1972;65:89–109. doi: 10.1017/s0031182000044267. [DOI] [PubMed] [Google Scholar]
- 10.Birkenheuer AJ, Horney B, Bailey M, Scott M, Sherbert B, Catto V, Marr HS, Camacho AT, Ballman AE. Babesia microti-like infections are prevalent in North American foxes. Vet Parasitol. 2010;172:179–182. doi: 10.1016/j.vetpar.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Clancey N, Horney B, Burton S, Birkenheuer A, McBurney S, Tefft K. Babesia (Theileria) annae in a red fox (Vulpes vulpes) from Prince Edward Island, Canada. J Wildl Dis. 2010;46:615–621. doi: 10.7589/0090-3558-46.2.615. [DOI] [PubMed] [Google Scholar]
- 12.Kawabuchi T, Tsuji M, Sado A, Matoba Y, Asakawa M, Ishihara C. Babesia microti-like parasites detected in feral raccoons (Procyon lotor) captured in Hokkaido, Japan. J Vet Med Sci. 2005;67:825–827. doi: 10.1292/jvms.67.825. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji M, Zamoto A, Kawabuchi T, Kataoka T, Nakajima R, Asakawa M, Ishihara C. Babesia microti-like parasites detected in Eurasian red squirrels (Sciurus vulgaris orientis) in Hokkaido, Japan. J Vet Med Sci. 2006;68:643–646. doi: 10.1292/jvms.68.643. [DOI] [PubMed] [Google Scholar]
- 14.Zahler M, Rinder H, Schein E, Gothe R. Detection of a new pathogenic Babesia microti-like species in dogs. Vet Parasitol. 2000;89:241–248. doi: 10.1016/s0304-4017(00)00202-8. [DOI] [PubMed] [Google Scholar]
- 15.Gleason NN, Healy GR, Western KA, Benson GD, Schultz MG. The “Gray” strain of Babesia microti from a human case established in laboratory animals. J Parasitol. 1970;56:1256–1257. [PubMed] [Google Scholar]
- 16.Voorberg-vd Wel A, Kocken CH, Zeeman AM, Thomas AW. Detection of new Babesia microti-like parasites in a rhesus monkey (Macaca mulatta) with a suppressed Plasmodium cynomolgi infection. Am J Trop Med Hyg. 2008;78:643–645. [PubMed] [Google Scholar]
- 17.Chin W, Campbell CC, Collins WE, Roberts JM. Plasmodium inui and Babesia microti infections in the squirrel monkey, Saimiri sciureus. Am J Trop Med Hyg. 1983;32:691–693. doi: 10.4269/ajtmh.1983.32.691. [DOI] [PubMed] [Google Scholar]
- 18.Moore JA, Kuntz RE. Babesia microti infections in nonhuman primates. J Parasitol. 1981;67:454–456. [PubMed] [Google Scholar]
- 19.Otsuru M, Sekikawa H. Surveys of simian malaria in Japan. Zentralbl Bakteriol. 1979;244:345–350. [Orig A] [PubMed] [Google Scholar]
- 20.Nakajima R, Tsuji M, Oda K, Zamoto-Niikura A, Wei Q, Kawabuchi-Kurata T, Nishida A, Ishihara C. Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTeta gene in 36 isolates. J Vet Med Sci. 2009;71:55–68. doi: 10.1292/jvms.71.55. [DOI] [PubMed] [Google Scholar]
- 21.Zamoto A, Tsuji M, Wei Q, Cho SH, Shin EH, Kim TS, Leonova GN, Hagiwara K, Asakawa M, Kariwa H, Takashima I, Ishihara C. Epizootiologic survey for Babesia microti among small wild mammals in northeastern Eurasia and a geographic diversity in the beta-tubulin gene sequences. J Vet Med Sci. 2004;66:785–792. doi: 10.1292/jvms.66.785. [DOI] [PubMed] [Google Scholar]
- 22.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]


