Abstract
Post kala-azar dermal leishmaniasis (PKDL) and mucosal leishmaniasis (ML) are serious clinical forms of leishmaniasis caused by Leishmania donovani parasites in Sudan. Although pentavalent antimonys are used as the first line of treatment of all clinical forms of leishmaniasis, persistent PKDL and ML patients are treated with liposomal amphotericin B (Ambisome) as a second-line drug. In this work, we report the development of allergic reactions by a PKDL and a ML Sudanese patient to Ambisome. The findings warrant future close supervision of patients to be treated with the drug.
Introduction
Leishmania donovani in Sudan causes different clinical forms ranging from cutaneous ulcer (cutaneous leishmaniasis [CL]), mucosal leishmaniasis (ML), to visceral leishmaniasis (VL). Visceral leishmaniasis can be complicated by development of a skin rash (post kala-azar dermal leishmaniasis [PKDL]) during or following treatment of VL patients with pentavalent antimonys.1,2
According to the guidelines of the Federal Ministry of Health, Sudan, VL patients are treated with pentavalent antimonys as the first line of treatment, given at a dose of 20 mg/kg for 28 days. The course of treatment is extended for treatment of ML and PKDL patients. Patients, who do not respond to Antimony treatment, are treated with 10 doses of Liposomal Amphotericin B (Ambisome) given at a dose of 2–3 mg/kg every other day.
Ambisome has been shown to be effective against different clinical forms of leishmaniasis and it is strictly used as a second line of treatment according to the guidelines of the Federal Ministry of Health because of its unaffordable high cost. In addition to its effectiveness, Ambisome has a wide range of safety with minimal side effects.3 Nevertheless, infusion reactions to liposomal amphotericin B were previously reported.4,5 In this work, we report the development of allergic reactions by a PKDL and a ML Sudanese patient treated with Ambisome.
PKDL patient.
A young Sudanese male 20 years of age and living in a VL-endemic village in Gadaref eastern Sudan, had a history of VL 8 years ago and was treated with 20 mg/kg Sodium Stibogluconate (SSG; GL Pharmaceuticals, Calcutta, India) for 28 days. Following VL treatment he developed PKDL and received 103 injections of 20 mg/kg/D IV SSG with no remarkable improvement. Because of the persistence of PKDL lesions, he was retreated 2 years later with 48 injections of SSG given at 20 mg/kg with no obvious response. The drug was stopped because of development of an allergic reaction.
The patient was referred to our laboratory in Khartoum for further research and treatment. Skin snips were taken from the PKDL lesions for parasite culture in Novy-MacNeal-Nicolle (NNN) media and parasite growth was positive after 7 days of incubation. The patient was referred for Liposomal Amphotericin B (Ambisome; Geliad Sciences, Inc., Foster City, CA) at Umdurman Tropical Diseases Hospital in Khartoum. The patient was a known asthmatic since 7 years of age, and he was not on regular medication for asthma.
At presentation to the hospital, he had no constitutional symptoms apart from the facial rash. He had an extensive maculo—a popular rash confined to the face, mainly on the forehead and around the lips (Figure 1). On examination all other systems were normal, his weight was 65 kg. He was scheduled for Ambisome treatment at a dose of 2.5 mg/kg in 5% dextrose on alternate days for 20 days.
Figure 1.
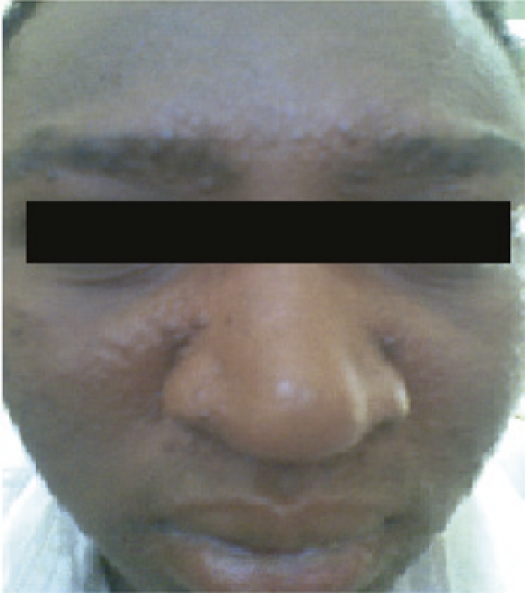
Post kala-azar dermal leishmaniasis Sudanese patient presented with macula-papular rash on the face, and later developed an allergic reaction to Ambisome.
On the fifth treatment dose the patient developed facial flushing, breathlessness, and generalized expiratory wheezes. The asthmatic attack was controlled and he was placed on a regular Salbutamol inhaler thereafter. One week later, Ambisome (Gilead Sciences Ltd., Cambridge, UK) was rechallenged under close supervision. After 10 cc of the infusion, he felt chest tightness but there were no wheezes and facial flushing. The treatment was immediately stopped and the patient was considered allergic to the drug.
Mucosal leishmaniasis patient.
A 54-year-old Sudanese male had a history of living in a VL-endemic area in Eastern Sudan. The patient developed a chronic ulcer on the lower lip that persisted for more than 6 months (Figure 2). The patient was suspected of having ML. A tissue biopsy was surgically taken for parasite culture and smear preparation. The biopsy was cultured in NNN media and incubated at 24 °C. Mucosal leishmaniasis was confirmed by successful parasite growth from the biopsy. Because of the severity of the lesion and the age of the patient, he was scheduled for Ambisome treatment at a dose of 2.5 mg/kg for 4 weeks and given every other day.
Figure 2.
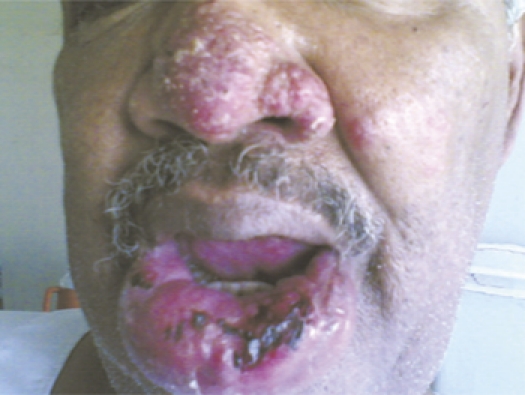
Mucosal leishmaniasis Sudanese patient presented with chronic ulcer on the lower lip that persisted for more than 6 months, and later developed an allergic reaction to Ambisome.
During the ninth dose, the patient developed generalized rash and vesicles, generalized wheezes, and gasped for oxygen that necessitated nublized solbutamol. The patient felt chilly with rigors and complained of chest tightness. His total white blood cell count was 7,200 with 9% eosinophilia. Ambisome was immediately stopped and the patient was given 10 mg antihistamine and 100 mg hydrocortisone intravenously. The patient was observed for 3 days, no further event occurred and the patient was discharged in good condition.
Discussion
Post kala-azar dermal leishmaniasis and ML are two serious clinical forms caused by Leishmania donovani in Sudan.1,2 Because of the persistent lesions of PKDL and ML that require prolonged treatment courses with pentavalent antimony, the two patients were treated with liposomal amphotericin B (Ambisome). The first patients developed PKDL lesions following VL treatment with SSG. Zijlstra and Elhassan2 reported an exceptionally high incidence of PKDL among VL Sudanese patients during or following treatment of VL with SSG. The patient reported in this work received 160 doses of SSG for PKDL treatment in the peripheral hospital where no alternative treatment was available. On the basis of his past repeated SSG treatment, he was recommended for Ambisome treatment. The patient tolerated 4 doses of Ambisome, and unexpectedly, developed symptoms of allergic reaction during the fifth dose. His allergic symptoms included facial flushing, breathlessness, and generalized expiratory wheezes that prompted stopping the Ambisome treatment and use of anti-allergic drugs. The fact that the patient had a history of asthma and allergic reaction to SSG might have increased the risk for his allergic reaction to Ambisome. Unfortunately, no further treatment was provided for the patient who was discharged showing reasonable clinical improvement.
The second patient who presented with a mucosal ulcer developed an allergic reaction to Ambisome treatment, although he had no history of allergy. He tolerated 8 doses of Ambisome, and then developed a serious allergic reaction during the ninth dose accompanied by high eosinophilia suggestive of hypersensitivity to Ambisome. His allergic reaction prompted discontinuation of Ambisome treatment and use of an anti-allergic drug.
Ambisome is known to have a wide margin of safety6; nevertheless, allergic and infusion reactions to the drug were previously reported.4,5,7,8 The documented allergic reaction to Ambisome during treatment of PKDL and ML warrants close supervision of patients, especially since Ambisome is currently considered to be a first-line treatment of leishmaniasis in several endemic regions.
Disclaimer: The study was approved by the institute ethical committee and written consents were obtained from the two patients.
Footnotes
Authors' addresses: Maowia Mukhtar, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan, E-mail: mmukhtar@tropmedicine.org. Mona Aboud, Umdurman Tropical Diseases Hospital, Khartoum, Sudan, E-mail: monaaboud@live.com. Musa Kheir, Department of Medicine, Faculty of Medicine, University of Khartoum, Khartoum, Sudan, E-mail: kheirmusa@hotmail.com. Sahar Bakhiet, Institute of Endemic Diseases, University of Khartoum, Khartoum, Sudan, E-mail: saharbakhite@yahoo.com. Nazik Abdullah, Faculty of Medicine, University of Khartoum, Khartoum, Sudan, E-mail: nazikabdullah@yahoo.com. Ahmed Ali, Faculty of Medicine, Al Nilian University, Khartoum, Sudan, E-mail: drali@gmail.com. Nadia Hassan, Department of Medicine, Faculty of Medicine, Al Nilian University, Khartoum, Sudan, E-mail: amiracr@hotmail.com. Elwaleed Elamin, Faculty of Medical Laboratory Sciences, Al Ziaim Alazhary University, Khartoum, Sudan, E-mail: wmelamin@hotmail.com. Atif Elagib, Tropical Medicine Research Institute, Ministry of Science and Technology, Khartoum, Sudan, E-mail: atifelagib@hotmail.com.
References
- 1.Elhassan AM, Zijlstra EE. Leishmaniasis in Sudan: mucosal leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95((Suppl 1)):S19–S26. doi: 10.1016/s0035-9203(01)90217-2. [DOI] [PubMed] [Google Scholar]
- 2.Zijlstra EE, el-Hassan AM. Leishmaniasis in Sudan. Post kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 2001;95((Suppl 1)):S59–S76. doi: 10.1016/s0035-9203(01)90219-6. [DOI] [PubMed] [Google Scholar]
- 3.Berman J. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis. 2003;16:397–401. doi: 10.1097/00001432-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Malani PN, Depestel DD, Riddell J, Bickley S, Klein LR, Kauffman CA. Experience with community-based amphotericin B infusion therapy. Pharmacotherapy. 2005;25:690–697. doi: 10.1592/phco.25.5.690.63591. [DOI] [PubMed] [Google Scholar]
- 5.Collazos J, Marteniz E, Mayo J, Ibbara S. Pulmonary reactions during treatment with amphotericin B: review of published cases and guidelines for management. Clin Infect Dis. 2001;33:E75–E82. doi: 10.1086/322668. [DOI] [PubMed] [Google Scholar]
- 6.Moen MD, Lyseng-Williamson KA, Scott LJ. Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections. Drugs. 2009;69:361–392. doi: 10.2165/00003495-200969030-00010. [DOI] [PubMed] [Google Scholar]
- 7.Bates CM, Carey PB, Hind CR. Anaphylaxis due to liposomal amphotericin (AmBisome) Genitourin Med. 1995;71:414. doi: 10.1136/sti.71.6.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laing RB, Milne LJ, Leen CL, Malcolm GP, Steers AJ. Anaphylactic reactions to liposomal amphotericin. Lancet. 1994;344:682. doi: 10.1016/s0140-6736(94)92116-4. [DOI] [PubMed] [Google Scholar]


