Abstract
During September 2010, 133 female sand flies were caught inside houses of patients with cutaneous leishmaniasis in the focus for this disease in southeastern Tunisia and subsequently dissected. One specimen was positive for Leishmania protozoa. This sand fly species was identified as Phlebotomus sergenti, and the parasite was identified as L. tropica. This is the first report of P. sergenti involvement in transmission of L. tropica in Tunisia.
Cutaneous leishmaniasis (CL), which is caused by Leishmania tropica, is endemic in southeastern Tunisia. It is found in communities living in villages built on the flanks of the arid mountains of the region of Tataouine.1 Reservoir hosts and vectors of L. tropica in this area are still unknown. This paper reports isolation and species characterization of L. tropica from Phlebotomus sergenti obtained inside houses of persons with CL.
The study was conducted in the village of Ghomrassen, which is located in the Governorate of Tataouine, in southeastern Tunisia. It is a well-known focus of L. tropica in a mountainous area, which has a moderate altitude and an arid climate. During October 2008–September 2009, 5 L. tropica CL cases were identified.1 Houses of persons with CL caused by L. tropica were located in mountains surrounding the village (altitude = 300 meters above sea level).
Sand flies were captured during September 2010 by using Centers for Disease Control light traps. The traps were set up before sunset inside house of persons with CL caused by L. tropica and inspected the next morning. Sand flies were identified according to morphologic characteristics described by Croset and others,2 Leger and others,3 and Boussaa and others.4 Live female sand fly were rinsed briefly in 96% ethanol and dissected in 0.9% sterile saline. The head and genitalia were used for species identification. The gut was examined by using a microscope at 40× magnification. When promastigotes were detected, they were cultured in Novy-Nicolle-MacNeal medium. Cultures were incubated at 26°C and evaluated microscopically after 6 days. Positive cultures were subsequently used for molecular and isoenzyme typing.
DNA extracted by using a Qiamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. LITSR and L5.8S primers were used for amplification of the ribosomal internal transcribed spacer 1 region.1 Positive PCR products were digested with Hae III (Fermentas, Glen Burnie, MD) at 37°C for 1 hour. Restriction profiles were analyzed by electrophoresis on a 3% agarose gel and compared with those obtained in human samples from the same region.1 Positive cultures were also sent to the Pasteur Institute of Algeris for isoenzyme typing by multilocus enzyme electrophoresis.
A total of 68 traps-nights resulted in trapping of 274 sand flies (141 males and 133 female). On the basis of morphologic criteria, 9 species were identified: P. sergenti (n = 84), P. papatasi (n = 84), Sergentomyia fallax (n = 37), S. minuta parroti (n = 29), P. perniciosus (n = 24), P. riouxi and/or P. chabaudi (n = 7), S. antennata (n = 5), and P. alexandri (n = 4).
Thirteen of 41 female P. sergenti had mature eggs in their abdomens (n = 6, 14%) or partially digested blood in their midguts (n = 7, 17%). Results of gut dissections showed that only 1 (2.4%) of 41 P. sergenti female sand flies was infected by Leishmania promastigotes. No eggs were found in this infected female, and the midgut did not contain any blood or blood meal remains. The Leishmania strain isolated from positive cultures was identified as L. tropica by molecular typing and L. tropica MON-8 by isoenzyme typing. A restriction profile obtained for promastigotes from the sand fly was similar to that obtained for amastigotes from human CL samples from the same region (Figure 1).
Figure 1.
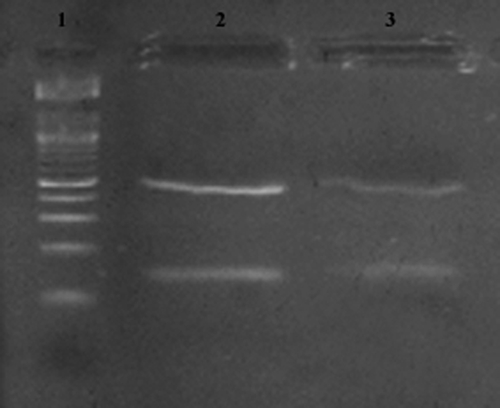
Identification of Leishmania species in Tunisia by using internal transcribed spacer 1 polymerase chain reaction–restriction fragment length polymorphism analysis. Lane 1 = 50-basepair DNA ladder; lane 2 = sand fly isolate from culture promastigotes; lane 3 = cutaneous isolate from dermal scraping (patient with cutaneous leishmaniasis).
Inside the houses of persons with CL caused by L. tropica, P. sergenti was the most abundant sand fly species (30.6%). A few specimens of other Paraphlebotomus species were also caught. This result confirms those of previous entomologic surveys carried out in the same region.5 It underlines the abundance of this species in suburban habitats of the mountainous area of southeastern Tunisia. The high proportion of P. sergenti females inside houses (sex ratio = 104 males per 100 females) and the high percentage of gorged and gravid specimens indoors suggest the endophily, endophagy, and anthropophagy of P. sergenti. The isolation from one specimen of Leishmania promastigotes characterized as L. tropica and its identity to human isolates provides evidence of P. sergenti as the vector of this Leishmania species in Tataouine region. Interestingly, P. sergenti is the confirmed or suspected vector of L. tropica in many foci of anthroponotic CL in Morocco (northern Africa), the Middle East, and Central Asia.6,7
ACKNOWLEDGMENTS
We thank Dr. Mohamed Raouane and his staff (Regional Directory of Public Health of Tataouine) for contributions to this study and Dr. Zoubir Harrat (Institut Pasteur d'Algérie) for isoenzyme typing.
Footnotes
Financial support: This study was supported by the network of Pasteur Institutes (project ACIP A-04-2007).
Authors' addresses: Ahmed Tabbabi, Nadia Bousslimi, Adel Rhim, Karim Aoun, and Aïda Bouratbine, Laboratoire de Recherche LR 05SP03, Institut Pasteur de Tunis, 1002 Tunis Belvédère, Tunisia, E-mails: tabbabiahmed@gmail.com, nguetari@gmail.com, adel.rhim@pasteur.rns.tn, karim.aoun@pasteur.rns.tn, and aida.bouratbine@pasteur.rns.tn.
Reprint requests: Aïda Bouratbine, Laboratoire de Parasitologie, Institut Pasteur de Tunis, 13 Place Pasteur, BP 74, 1002 Tunis Belvédère, Tunisia, E-mail: aida.bouratbine@pasteur.rns.tn.
References
- 1.Bousslimi N, Aoun K, Ben-Abda I, Ben-Alaya-Bouafif N, Raouane M, Bouratbine A. Epidemiologic and clinical features of cutaneous leishmaniasis in southeastern Tunisia. Am J Trop Med Hyg. 2010;83:1034–1039. doi: 10.4269/ajtmh.2010.10-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croset H, Rioux JA, Maistre M, Bayar N. Les phlébotomes de Tunisie (Diptera, Phlebotomidae) Mise au point systématique, chorologique et éthologique. Ann Parasitol Hum Comp. 1978;53:711–749. [PubMed] [Google Scholar]
- 3.Léger N, Pesson B, Madulo-leblond G, Abonnec E. Sur la différentiation des femelles du sous-genre Larroussius Nitzulescu, 1931 (Diptera-Phlebotomidae) de la région Méditerranéenne. Ann Parasitol Hum Comp. 1983;58:611–623. [PubMed] [Google Scholar]
- 4.Boussaa S, Boumezzougha A, Remyc PE, Glasserc N, Pesson B. Morphological and isoenzymatic differentiation of Phlebotomus perniciosus and Phlebotomus longicuspis (Diptera: Psychodidae) in southern Morocco. Acta Trop. 2008;106:184–189. doi: 10.1016/j.actatropica.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Ghrab J, Rhim A, Bach-Hamba D, Chahed MK, Aoun K, Nouira S, Bouratbine A. Phlebotominae (Diptera: Psychodidae) of human leishmaniasis sites in Tunisia. Parasite. 2006;13:23–33. doi: 10.1051/parasite/2006131023. [DOI] [PubMed] [Google Scholar]
- 6.Guilvard E, Rioux JA, Gallego M, Pratlong F, Mahjour J, Martinezortega E, Dereure J, Saddiki A, Martini A. Leishmania tropica in Morocco. 3. Identification of 89 isolates from the vector Phlebotomus sergenti. Ann Parasitol Hum Comp. 1991;66:96–99. doi: 10.1051/parasite/199166396. [DOI] [PubMed] [Google Scholar]
- 7.Killick-kendrick R. Phlebotomine vectors of the leishmaniasis: a review. Med Vet Entomol. 1990;4:1–24. doi: 10.1111/j.1365-2915.1990.tb00255.x. [DOI] [PubMed] [Google Scholar]


