Abstract
Chagas disease is caused by the parasite Trypanosoma cruzi, which is transmitted to humans by blood-sucking triatomine insects. This disease is endemic throughout Mexico and Central and South America, but only a few autochthonous cases have been reported in the United States, despite the fact that infected insects readily invade houses and feed on humans. Competent vectors defecate during or shortly after feeding so that infective feces contact the host. We thus studied the feeding and defecation behaviors of the prevalent species in southern Arizona, Triatoma rubida. We found that whereas defecation during feeding was frequent in females (93%), it was very rare in immature stages (3%), and absent in males. Furthermore, more than half of the immature insects that exhibited multiple feeding bouts (62%) defecated during interruptions of feeding, i.e., while likely on or near the host. These results indicate that T. rubida potentially could transmit T. cruzi to humans.
Introduction
Chagas disease is endemic throughout Mexico and Central and South America, with an estimation of 7.7 million people infected, 108 million people considered at risk, at least 1.7 million symptomatic cases, an annual incidence of 41,200 (through vectorial transmission), and an estimated 10,000 deaths each year.1,2 This disease is caused by the protozoan parasite Trypanosoma cruzi, which is transmitted to humans by blood-sucking insects in the family Reduviidae (Triatominae). Trypanosoma cruzi is transmitted to humans when feces and/or urine from infected bugs come into contact with damaged skin or oral and/or eye mucous membranes, or when the victim rubs the itching wound site. In the United States both infected triatomines and wild mammalian reservoirs are plentiful (e.g., packrats, mice, armadillos, raccoons, and opossums).3–7 Recent studies reported infection rates of 41.5% and 51% in triatomines from Arizona and Texas.8,9 Prevalence in mammalian reservoirs greatly varies with geographical area and host species (e.g., 9–68% in raccoons, depending on the state).10 In spite of this, only seven autochthonous cases of this disease have been reported to date, all in the southern half of the country.11–15
Several reasons might explain why vector transmission of Chagas disease is so rare in the United States, including the lack of suitable domestic dwellings for insect colonies, a low number of insects inside houses, the possible misdiagnosis of the infection, and more importantly, the apparently delayed defecation times of the native species of triatomines.16 Competent vectors defecate during or immediately after feeding so that infective feces contact the host,16–18 and it is known that because of differences in this behavior different species of triatomines differ in their infective capacities.16,19,20 Competent vectors also need to have a high reproductive rate and be highly anthropophilic.21 Other important factors that determine transmission of T. cruzi to humans include the frequency of contacts between infected triatomines and humans, the rate of infection of triatomines with T. cruzi, and the frequency of infective parasites in feces.22,23
In the United States, and particularly in Arizona, Chagas disease could become a health problem because human populations are rapidly expanding into habitats where infected triatomines and wild mammalian reservoirs are plentiful.4,6,24 Arizona is noteworthy as the state with the highest number of triatomine-human contacts reported in the United States (sources: American Association of Poison Control Centers, Arizona Poison and Drug Information Center, and University of Arizona Health Sciences Center). Studies of the hosts of the most abundant triatomine species in southern Arizona, Triatoma rubida, indicate that this species feeds on humans, in addition to canines, felines, swine, and other mammals (Klotz SA and Schmidt JO, personal communication). Moreover, we recently reported that 41.5% of 164 tested T. rubida were infected with T. cruzi.9 Despite that all insects in that study were collected inside or around human houses and therefore were possibly in contact with humans, cases of Chagas disease have not been reported in Arizona. A possible explanation for this could be that T. rubida does not defecate during or shortly after feeding. Wood19 reported that T. rubida collected in Arizona tended to defecate immediately after a blood meal, but the number of insects examined was very low (N = 5 adults). These results contrast with those from a more recent study, which found that most defecation by adult T. rubida (also collected in Arizona) occurred after the insects had left the vicinity of the host.25 Another study, conducted with T. rubida sonoriana from Sonora, Mexico, found that a variable fraction of the insects, depending on their sex and stage, defecate during feeding or soon thereafter.26
The purpose of this study was to clarify contradictory results and to investigate comprehensively the feeding and defecation behaviors of adults and larval stages of T. rubida under laboratory conditions to establish the potential role of this species as a vector of Chagas disease in southern Arizona.
Materials and Methods
Insects.
Experiments with second-, third-, and fourth-instar T. rubida was conducted with insects reared in the laboratory at 28°C under 12 hours light: 12 hours dark photoperiod regimen. These immature stages were the progeny of adult insects collected in suburban Tucson, Arizona. Insects were fed weekly on bovine blood, containing 2% sodium citrate as an anticoagulant and supplemented with 100 mM adenosine 5′-tryphosphate disodium salt (Calbiochem, La Jolla, CA), using an artificial feeder.27 Experiments with fifth-instar (last larval stage) and adult T. rubida were conducted with insects caught around Tucson, Arizona.21 To avoid cross-contamination with T. cruzi, these insects were kept apart from laboratory-reared animals. All material that had been in contact with field-caught insects was disinfected with ethanol 100% or autoclaved if possible. To ensure that our experimental conditions were adequate to study the feeding and defecation behavior of T. rubida, we conducted a positive control experiment using laboratory-reared third-instar Rhodnius prolixus, a triatomine species known to be an efficient vector of Chagas disease in South America and that readily defecates while feeding.18 Those insects were reared as described previously and were provided, free of infection with T. cruzi, by Dr. E. Dotson (Centers for Disease Control and Prevention). All experiments were conducted in a laboratory complying with Biosafety Level 2 regulations.
Procedure.
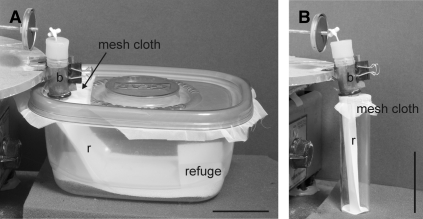
The starvation time of the experimental insects was different for each life stage to ensure similar hunger levels, as different stages have different tolerance to starvation.28 The second, third, and fourth instars were randomly selected from our colony and starved, respectively, for 20–24, 26–36, and 45–50 days since the last molt. In the case of fifth instars and adult insects, the starvation time could not be established precisely because they were caught outdoors and brought to the laboratory for the experiments. All field-caught insects, however, appeared to be starved (i.e., had flattened abdomens) and readily fed when placed on the experimental feeder. Each insect was placed in a clear plastic container (16 × 12 × 7.5 cm, Figure 1A), which was capped with mesh cloth secured with a lid with a hole. Insects could freely extend their mouthparts through the mesh cloth and draw blood from the artificial feeder. Each container had a small piece of filter paper bearing triatomine feces and footprints, which acted as a refuge,29 and a filter paper ramp that allowed the insects to reach the top mesh and feed (Figure 1). Because of their small size, second instars were placed in cylindrical vials (9.5 cm length, 2.5 cm diameter, Figure 1B) capped with mesh cloth secured with a rubber band. The feeding and defecation behavior of each insect was observed continuously by the same experimenter under low-light conditions; up to four individual insects, each in a separate container/vial, were observed simultaneously. To minimize the effect of manipulation, each insect was weighed and placed in the experimental container or vial 12–15 hours before the beginning of an experiment. After that time, the insects were allowed to feed individually on the artificial feeder during 1 hour. Thereafter, the containers with insects were removed carefully from the feeder and observed for an additional hour. Each insect was observed during those 2 hours, and the time at which each behavioral event occurred was recorded. This allowed calculation of parameters such as the number of feeding events, the duration of feeding events, and the number of fecal/urine drops. Insects that did not approach the feeder (16%) and feed were discarded from the analysis. The amount of blood ingested was calculated by weighing each insect after the experiment had ended (2 hours). The relative weight gain of insects was calculated as (weight after feeding – weight before feeding)/(weight before feeding). Because individual fecal/urine drops emitted during the 2-hour experimental period were not weighed, the amount of blood ingested was slightly underestimated (see Discussion).
Figure 1.
Setup used to study the defecation behavior of insects. Insects were fed using an artificial blood feeder. Each insect was individually placed on a clear plastic container lined with filter paper, which was covered on top with a mesh cloth secured with a plastic lid with a hole (A) or rubber bands (B). Insects could freely extend their mouthparts through the mesh cloth and draw blood (b) from the artificial feeder. Each container had a filter paper ramp (r) that allowed the insects to reach the top mesh and feed. (A) Container used for feeding adults, third, fourth, and fifth instars. Each container had a small piece of filter paper impregnated with feces and footprints that acted as a refuge. (B) Container used to feed second instars. Scale bars: 5 cm.
Analysis of infection by T. cruzi.
Adult triatomines, which were collected in the field, were analyzed individually for the presence of T. cruzi using polymerase chain reaction (PCR). The abdomen of each insect was excised with a sterile razor blade and homogenized with a ceramic ball or placed in a 1.5-mL microfuge tube with phosphate-buffered saline (up to 80 μL) and homogenized with a handheld mortar. DNA was extracted following the instructions provided with the QiaAmp DNA Blood Mini Kit (Qiagen 51106, Qiagen, Valencia, CA). The DNA was amplified by PCR following an established T. cruzi sample-processing protocol with two primers (TCZ1/TCZ2 and S35/S36), as previously described.9,30 All PCR experiments included a positive control of known T. cruzi DNA (extracted from cell pellets obtained by sedimenting cultured T. cruzi epimastigotes provided by Dr. Ellen Dotson) and with a negative control in which template DNA was omitted. Results that were positive for both sets of primers (30% of the samples) were considered positive. As we reported previously, in a study in which samples were analyzed under the exact same conditions, no samples were found to be positive for TCZ1/TCZ2 and negative for S35/S36.9 Therefore, samples that were positive with only S35/S36 were considered positive, because we previously found (in 100% of the cases) that such samples were confirmed positive by sequencing.9 Although fifth instars were also collected in the field, we did not analyze them for the presence of T. cruzi, because their defecation behavior was similar to that of the other laboratory-reared (and therefore free of infection) immature stages.
Statistical analysis.
Continuous data (i.e., beginning of feeding, duration of all feeding, weight gain [absolute and relative], and time to first defecation) were analyzed using Kruskal-Wallis ANOVAs, with the different insects stages (second, third, fourth, and fifth instars of T. rubida, females and males of T. rubida, and third instars of R. prolixus) being the categories of the independent variable. Significant results were followed by Dunn's post hoc tests to establish which groups differed from each other. When only two groups (i.e., females versus males) were compared, we used Mann-Whitney tests. Discrete data (e.g., number of feeding events, proportion of insects that defecate while feeding, etc.) from different instars were analyzed using χ2 tests with Yates correction for low numbers.31 For all variables analyzed, statistically significant differences between insect stages are indicated by different lettering in the figures. Correlations between variables (e.g., relative weight gain and number of feeding events) were analyzed using Spearman rank order correlations. In all cases differences were considered statistically significant if P < 0.05.
Results
The feeding and defecation patterns of 141 individual insects were recorded and analyzed. Insects started to feed soon after placement on the feeder (median = 1.2 minutes; range = 0.05–43.7 minutes; N = 141, Figure 2A), and most insects (95%) finished all feeding before the end of the 1-hour feeding period (median = 27.9 minutes; range = 1–60 minutes; N = 141). Although differences in these times initially were statistically significant (Kruskal-Wallis ANOVAs; start of feeding: H = 19.82, degrees of freedom (df) = 6, P < 0.005; end of feeding: H = 11.82, df = 6, P > 0.05), for the most part differences across insect stages, evaluated with post hoc Dunn's tests, were not significant (P > 0.05 for most comparisons; Figure 2A and B). Triatoma rubida, in particular nymphs, stopped feeding multiple times during the feeding period, wandering away from the feeder but returning to resume feeding soon thereafter (Figure 2C). Again, although differences in the number of feeding events were initially statistically significant (Kruskal-Wallis ANOVA, H = 24.77, df = 6, P < 0.001), for the most part differences across life stages were not significant (Figure 2C, post hoc Dunn's tests, P > 0.05). As expected, the number of feeding events positively correlated with the duration of feeding (Spearman rank order correlation; R = 0.544, t(n − 2) = 7.64, N = 141, P < 0.001); that is, the more feeding events, the longer the duration of feeding. The total duration of feeding (i.e., including interruptions) was similar across stages (Figure 2B; Kruskal-Wallis ANOVA: H = 11.56, df = 6, P > 0.05).
Figure 2.
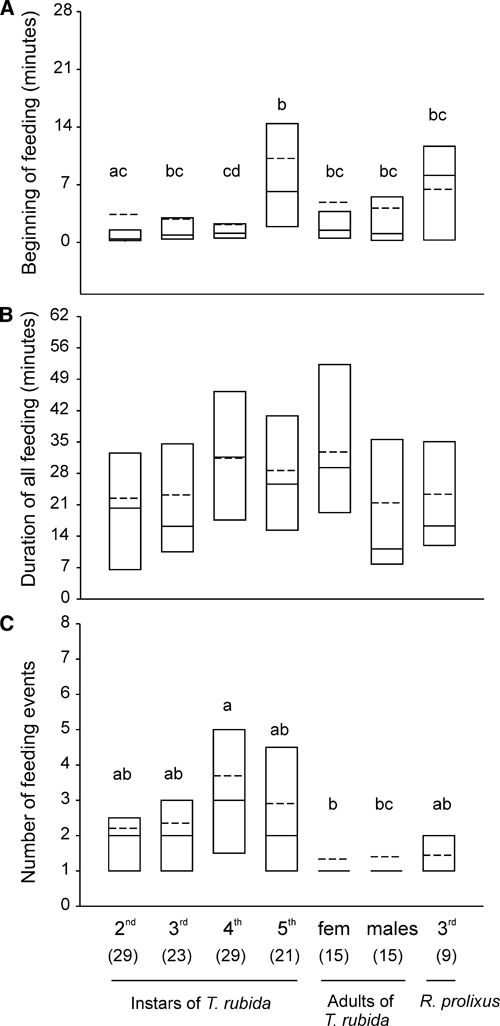
(A) Time at which the different stages (second, third, fourth, and fifth instars and adult females and males) of Triatoma rubida and third-instar Rhodnius prolixus start feeding after being placed in the feeder. (B) Total duration of feeding (i.e., including interruptions). (C) Number of feeding events during the first hour of observation. Shown are the 25th and 75th percentiles (boundaries of the boxes closest and farthest from zero, respectively), the medians (solid line within a box), and the means (dashed line). The number of insects tested in each group is shown in parentheses. Groups that do not share letters differ statistically (P < 0.05; Kruskal-Wallis ANOVAs followed by Dunn's comparisons in panels A and B; Yates corrected χ2 2 × 2 tests in panel C).
Figure 3A and B show the weight gain of triatomines, absolute (in mg) and relative to their initial weight. Because individual fecal/urine drops emitted during the 2-hour feeding/observation period were not weighed, the amount of blood ingested was slightly underestimated (see Discussion). In both cases the amount of blood ingested varied with life stage (Kruskal-Wallis ANOVAs; H = 94.27, df = 6, and P < 0.001 in the case of absolute weight gain; H = 68.23, df = 6, and P < 0.001 in the case of relative weight gain). Juvenile T. rubida fed more and therefore gained more weight as they matured (Figure 3A, post hoc Dunn's comparisons, P < 0.05). Relative to their initial weight, however, second and third instars gained more weight than adults (post hoc Dunn's comparisons, P < 0.05). There was a large variation in the amount of blood each insect ingested, particularly in the case of fifth instars (range: 5.4–391.1 mg; Figure 3A). Relative to their initial weight, third-instar R. prolixus gained more weight than any of the juvenile stages of T. rubida, but the differences were not statistically significant (Dunn's comparisons, P > 0.05, Figure 3B).
Figure 3.
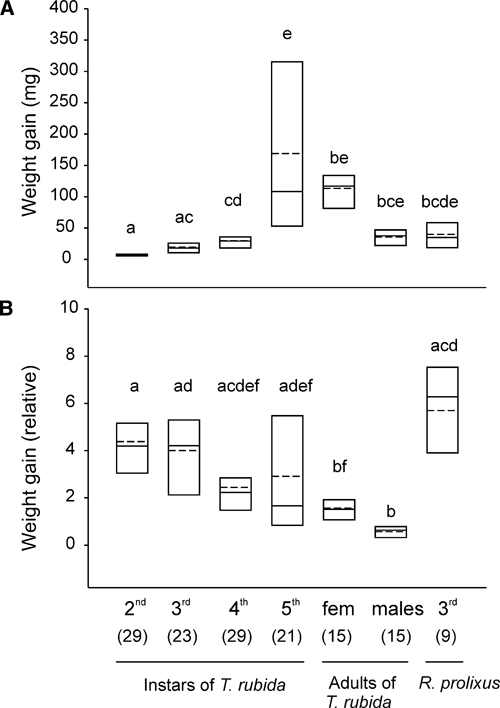
Weight gain in different stages (second, third, fourth, and fifth instars and adult females and males) of Triatoma rubida and third-instar Rhodnius prolixus. (A) Absolute weight gain. (B) Weight gain relative to the initial weight of each insect. Each insect was placed in the feeder for 1 hour. Shown are the 25th and 75th percentiles (boundaries of the boxes closest and farthest from zero, respectively), the medians (solid line within a box), and the means (dashed line). The number of insects tested in each group is shown between parentheses. Groups that do not share letters differ statistically (Kruskal-Wallis tests followed by Dunn's comparisons, P < 0.05).
We next asked if T. rubida defecates while feeding, as the most competent vectors of Chagas disease do.16,18 We found that most adult females (93%, 14 of 15) but none of the adult males (N = 15) defecated while feeding (Figure 4A). Among the immature stages, only 3% and 9.5% of the second- and fifth-instar T. rubida, respectively, defecated while feeding; none of the third- or fourth-instar T. rubida did so (Figure 4A). Of importance, the fact that 45% of third-instar R. prolixus defecated while feeding (Figure 4A, last column) indicates that our experimental design was appropriate for studies of this behavior.
Figure 4.
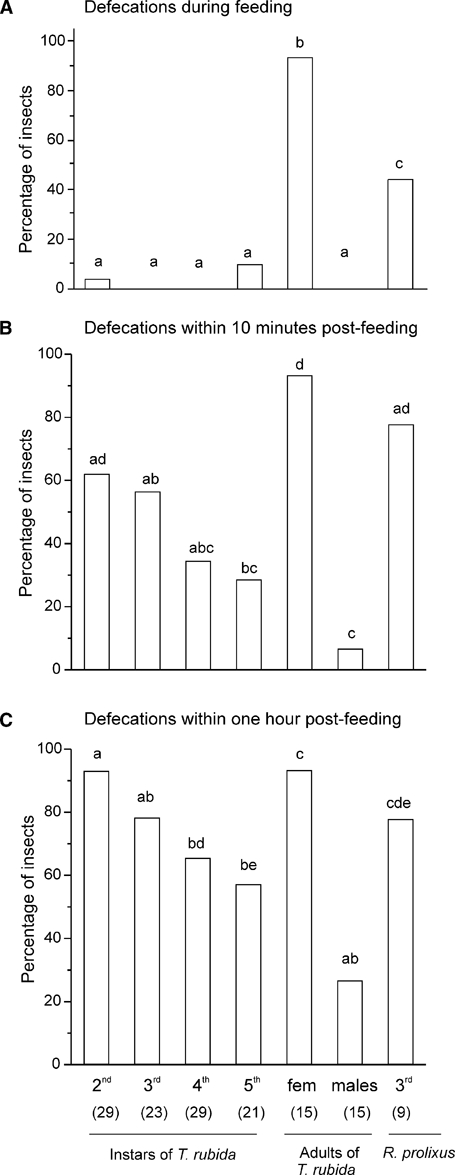
(A) Percentage of insects of different stages (second, third, fourth, and fifth instars and adult females and males of Triatoma rubida and third-instar Rhodnius prolixus) that defecate while feeding. Note that only adult females defecated. We found that 45% of R. prolixus defecated while feeding, which shows that our assay was sufficiently sensitive to study the defecation pattern of T. rubida. (B): Percentage of insects that defecated within 10 minutes after the end of a feeding event. (C) Percentage of insects that defecated within the 1-hour feeding period. In B and C the percentages of insects include those that defecated while feeding. The number of insects tested in each group is shown in parentheses. Groups that do not share letters differ statistically (Yates corrected χ2 2 × 2 tests, P < 0.05).
Could the differences in the amount of blood ingested by the different stages and sexes of T. rubida account for the observed differences in defecation behavior? Females were significantly larger and heavier than males (Mann-Whitney test, T = 299, n1 = 15, n2 = 15, P < 0.01). The median weights of females and males before feeding were 76.7 and 60.7 mg, respectively (ranges = 53.7–123.8 mg and 51.4–92 mg for females and males, respectively). In addition, females ate more than males (Mann-Whitney test, T = 332, n1 = 15, n2 = 15, P < 0.001). Females and males gained respectively 1.52 and 0.63 times (medians) their body weight during a blood meal (Figure 3B).
We also considered the possibility that infection with T. cruzi (because adults were obtained from the field) could explain the differences observed in the defecation behavior of females and males. We found that the proportion of infected adult insects did not differ between sexes (Yates corrected χ2, P > 0.05), with 75% of analyzed females (N = 12) and 60% of males (N = 15), respectively, infected with T. cruzi. Furthermore, females defecated while feeding regardless of whether they were infected or not with T. cruzi (the only female that did not defecate while feeding was positive for T. cruzi). None of the males, whether infected or not with T. cruzi, defecated while feeding.
Although most juvenile T. rubida (98%, N = 102) and all adult males did not defecate while feeding, we considered the possibility that they might defecate shortly after a feeding event, and possibly while on the host. Many T. rubida (more than 50% in the case of second and third instars) defecated within 10 minutes after the end of a feeding event, and most (except for adult males) defecated within the first hour after feeding (Figure 4B and C). In addition, some insects, in particular second instars, defecated many times during the first hour (Kruskal-Wallis ANOVA, H = 41, df = 6, P < 0.001, followed by Dunn's tests, Figure 4C). The percentages of T. rubida that defecated within 10 minutes or 1 hour after the beginning of feeding decreased with life stage, except for females (Figure 4B and C). Moreover, the percentage of nymphs that defecated within 1 hour was similar to that of third-instar R. prolixus (Yates corrected χ2 tests, P > 0.05, Figure 4C). The time to the first defecation within the first hour of feeding varied slightly with stage (Kruskal-Wallis ANOVA, H = 26.77, df = 6, P < 0.001), but most post hoc comparisons were not statistically significant (Dunn's comparisons, Figure 5A). Furthermore, in the case of insects that showed feeding interruptions (62% of immature insects), we found that 28%, 42%, 45%, and 38% of second-, third-, fourth-, and fifth-instar T. rubida, respectively, defecated between feeding events, i.e., while possibly on the host.
Figure 5.
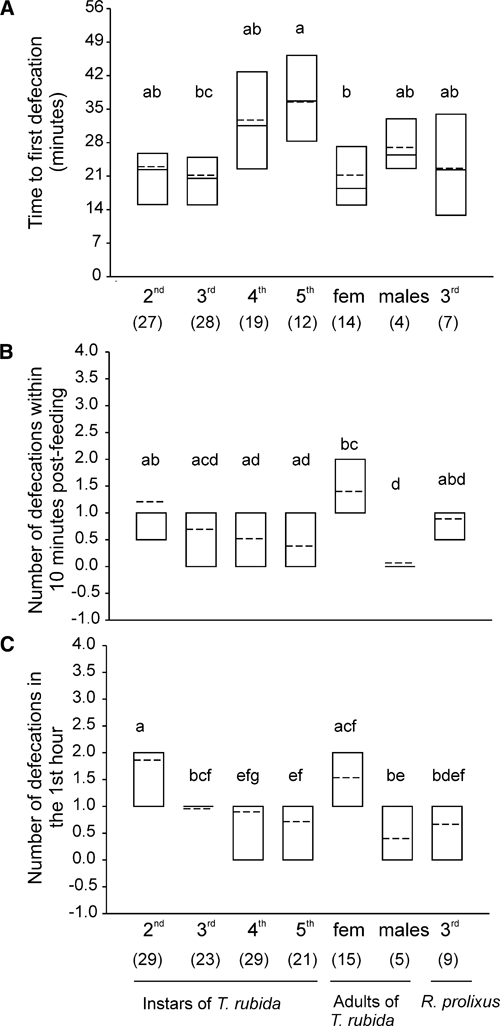
(A) Time to first defecation (calculated for those insects that defecated within the 1-hour feeding period). (B) Number of defecations within 10 minutes after the end of a bout of feeding. (C) Number of defecation events within the 1-hour feeding period. Shown are the 25th and 75th percentiles (boundaries of the boxes closest and farthest from zero, respectively), the medians (solid line within a box), and the means (dashed line). The number of insects in each group is shown in parentheses. Groups that do not share letters differ statistically (Kruskal-Wallis ANOVAs followed by Dunn's comparisons, P < 0.05).
To compare the potential infectivity of T. rubida with that of other triatomine species, we calculated a defecation index (DI = % of insects that defecated up to 10 minutes after the end of feeding × average number of defecations up to 10 minutes after the end of feeding/100) as previously reported.18 The only experimental difference is that we used an artificial feeder rather than a live host. Because insects often fed multiple times (Figure 2C), we considered the defecations occurring up to 10 minutes after the end of any feeding event. The DI values for females and males respectively were 1.3 and 0.005 (Figure 6). Among immature stages, we found that second instars had the highest infective capacity, with a DI similar to that observed in third-instar R. prolixus in our experiments (Figure 6).
Figure 6.
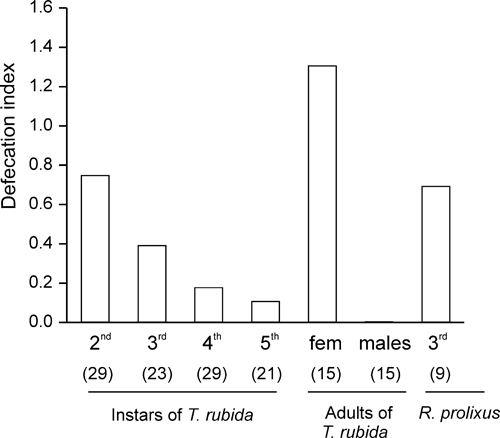
Defecation index for each stage and sex, calculated as (% of insects that defecated up to 10 minutes after the end of feeding × average number of defecations up to 10 minutes after the end of feeding)/100.18 Among nymphs of Triatoma rubida, second instars have the highest infective capacity with a value similar to that of third-instar Rhodnius prolixus.
We also measured how far from the feeder the insects defecated. We found that among juvenile stages, third instars defecated closer to the feeding site (< 3 cm, Figure 7), a distance that probably would enable scratching by the host to introduce feces into the wound site, but the differences across immature stages were not statistically significant (Yates corrected χ2 tests, P > 0.05). Thus, although T. rubida nymphs and adult males did not defecate while feeding, some nymphs defecate soon thereafter, and many did so between feeding events and close to the feeding site, i.e., possibly while on the host or nearby.
Figure 7.
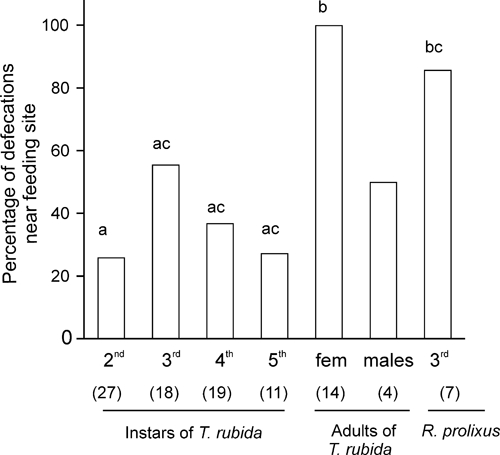
Percentage of insects that defecate near the feeding site (< 3 cm from the feeder) during the first hour of the observation period. Only the first defecation is considered. The number of insects in each group is shown in parentheses. Groups that do not share letters differ statistically (Yates corrected χ2 2 × 2 tests, P < 0.05). Statistical comparisons against males could not be computed because of the low number of males that defecated during the first hour.
Finally, we asked whether any of the parameters analyzed previously (e.g., number of feeding events, time to first defecation from the beginning of feeding, etc.) depended on the amount of blood ingested. That is, do insects that eat larger meals defecate sooner, closer, or more times? To analyze these possibilities we examined correlations between the relative weight gain of T. rubida and the following parameters: number of feeding events, time to the first defecation from the beginning of feeding, time to the first defecation after the end of feeding, number of defecation events up to 10 minutes post feeding, and distance to the first defecation. These correlations were assessed separately for immature stages (i.e., data from all immature insects were pooled), for females and for males. In the case of immature stages, we found that the amount of blood ingested positively correlated with the number of feeding events (Spearman R = −0.3166, t(n − 2) = −3.3381, N = 102, P < 0.005) and with the number of defecation events up to 10 minutes post feeding (Spearman R = 0.2423, t(n − 2) = 2.4983, P < 0.05); none of the other correlations were significant. In the case of females, the amount of blood ingested negatively correlated with the time to the first defecation (Spearman R = 0.6923, t(n − 2) = 3.3235, P < 0.01). In the case of males, none of the correlations were significant (P > 0.05 in all cases).
Discussion
Here, we investigated the feeding and defecation behavior of immature stages and adults of T. rubida to establish their potential role as vectors of Chagas disease in southern Arizona. We found that only adult female T. rubida defecated during feeding, but a relatively large percentage of the insects, depending on their life stage and sex (range: 7–62%), defecated within 10 minutes after feeding—that is, while possibly still in contact with the host.18
It has been reported extensively that the time elapsed between feeding and defecation varies greatly according to the species, stage, and sex. For instance, the percentage of insects that defecated while feeding or soon thereafter ranged between 40% and 63% for R. prolixus, between 10% and 44% for Triatoma infestans, and between 12% and 50% in the case of Rhodnius neglectus,17 and was < 10% in Triatoma rubrofasciata.20 In our experiments we found that the defecation delay post feeding also varies greatly with the developmental stage, sex, and within a group. We found that 93% of adult females, but almost none of the immature stages and none of the adult males, defecated while feeding (Figure 4). Similarly, it was also reported that adult males of Panstrongylus megistus and Triatoma barberi, and nymphs of Triatoma guasayana and Triatoma vitticeps, do not defecate while feeding.17,23,32
Although we found that most juvenile insects and none of the adult males defecated while feeding, transmission of the parasite to humans can occur if the engorged insects remain in close contact with the host after feeding, possibly defecating and urinating to eliminate the extra weight gained during a blood meal.18 Indeed, it has been proposed that triatomines that defecate within 10 minutes after the end of feeding are potentially effective vectors of T. cruzi.18 For example, 63%, 86%, and 90%, respectively, of Triatoma dimidiata, T. infestans, and R. prolixus, all efficient vectors of Chagas disease, defecate during the first 10 minutes after the end of feeding.18 Besides adult female T. rubida, which readily defecated while feeding (Figure 4A), we found that a relatively large percentage of insects defecated within 10 minutes after the end of a feeding episode (range: 29–62% for immature stages; 7% for adult males; Figure 4B). Furthermore, many of the insects, although they did not defecate while feeding, did so close to (within 3 cm) of the feeding site (Figure 7), which implies good vector behavior. By about 1 hour 74% of all immature insects had defecated (Figure 4C), which is lower than the 99% reported for T. infestans and R. prolixus.18
In addition to the defecation delay, it has been proposed that the number of defecation events occurring shortly after the end of feeding also is important in determining the insect's infective capability.18 This variable, along with the proportion of insects that defecate during that same time period, has been used to calculate a “defecation index” (DI; the higher the DI, the higher the infection capacity) that serves to compare the infective capacity of different species.18 This index greatly varies not only with the species but also with the stage and sex.18,32,33 The only difference is that in our experiments we used an artificial feeder instead of a live host. For instance, this value is highest (3.8) in fourth-instar R. prolixus but 0.5 in adult males of the same species,18 and it is only 0.0004 in adult male T. barberi.32 In our experiments, we found that the DI of adult female T. rubida was 1.3 (but it was only 0.005 in adult males), which is comparable to the DI observed in T. infestans and R. prolixus,18 both very efficient vectors of Chagas disease. Klotz and others25 studied the feeding and defecation behavior of adult T. rubida on immobilized mice and found that the DIs of adult females was only 0.40, but the number of females studied was relatively low (N = 8). Among immature stages of T. rubida, we found that the DI decreased with life stage, ranging from 0.75 for second instars to 0.11 for fifth instars (Figure 6). Although second instars would be the stage with the highest vectorial capability among immature stages, they probably have lower infection rates because they have had fewer blood meals than older stages during their lifetime, and therefore it is less likely that they are infected with T. cruzi. In addition, even if they were infected, they probably would defecate less because they are smaller. A similar pattern of variation of the DI, that is, a higher efficiency of younger instars, was observed in T. barberi.32 In T. dimidiata this pattern was reversed, and in T. infestans the D.I. was similar among immature stages.18
The duration of feeding also contributes to determining the insect's vectorial capacity. According to some authors, species that feed more than 10 minutes could be considered as important potential vectors of T. cruzi.18 We found that T. rubida began feeding soon after being placed on the feeder and fed 23.2 minutes (median; N = 132; Figure 2). Prolonged contact between the vector and the host increases the probability of interrupted feeding and therefore increases the risk of exposure to fecal material.18,32 Indeed, we found that 62% of immature T. rubida (N = 102) interrupted feeding multiple times (Figure 2C), 50% of which defecated during interruptions. A similar pattern of increased defecation during periods of interrupted feeding was also observed in T. barberi and T. dimidiata.18,32 Interrupted feeding might increase the chance that an insect acquires the infection because it increases the possibility that the insect changes hosts to complete a blood meal.32
As others have reported previously for other triatomine species,17,32 we found a striking difference between the defecation behavior of adult male and female T. rubida. We found that 93% of adult females—but none of the males—defecate while feeding (Figure 4A). Because we used dispersing adult insects collected in the field, we could not establish how long they had gone without feeding, but it is likely that both adult males and females were starved, as hunger and low nutritional status are the main drivers of dispersal.33,34 Longer fasting periods could increase vector potential by decreasing defecation delay.16,35 Although adult females were heavier and ate more than males (Figure 3), meal size might not be the only factor affecting defecation behavior. For instance, second and third instars ate (relative to their initial weight) more than adult females, but almost none of them defecated while feeding (Figures 3B and 4A). In our experiments we did not weigh the fecal/urine drops emitted during the 2-hour experimental period. This resulted in a relatively slight underestimation of the amount of blood ingested by each individual insect. On the basis of previously published studies in Triatoma sordida, we calculated that we underestimated the amount of blood ingested by adult females by 5–6%, although the amount of feces deposited during and shortly after feeding greatly varies with the species and insect stage.33,36
Is it possible that a minimum blood weight gain is necessary for defecation to occur during feeding? For instance, fifth-instar T. sordida and T. guasayana need to eat an average minimum of 60–100 and 115 mg of blood, respectively, for defecation to occur during feeding or soon thereafter.23,33 In our experiments, although 52% of fifth-instar T. rubida ingested more than 100 mg of blood, we found that only 18% of those insects defecated while feeding. We also found that most female T. rubida ate 114 ± 9 mg (mean ± SE; median = 117 mg) of blood, which is more than the amount reportedly required for defecation to occur during feeding in female T. sordida and T. guasayana.23,33 Our experimental design, in which insects were allowed to feed ad libitum during the experimental period, did not allow us to establish this minimum value because all but one of the females tested defecated while feeding (Figure 4A). We found, however, that the amount of blood ingested by females correlated negatively with the time to the first defecation, as described in other triatomine species.33,37,38 Because dispersing adults (like those used in our study) have a low nutritional status,33,34 they likely ingest larger amounts of blood and defecate sooner, thus increasing their vectorial capacity. The amount of blood ingested may also affect the vectorial capacity of immature insects, as we found that this variable positively correlated with the number of defecation events up to 10 minutes post feeding.
Infection of T. rubida with T. cruzi did not accelerate defecation, as reported in R. prolixus.35 We found that both adult males and females were equally infected in our study, but only the females defecated while feeding. Moreover, they did so while feeding regardless of whether they were infected with T. cruzi.
In this work, we have used an artificial-membrane feeding system instead of a live host. In nature, movements of host animals naturally occur and might cause feeding interruptions. Furthermore, it has been shown that the rate of engorgement, the probing time, and the number of feeding interruptions are affected by the type of host.39 Although it cannot be assumed that these two different feeding methods (use of an artificial feeder or a live host) produce identical results, our findings are consistent with those observed by others in experiments with T. rubida feeding on live hosts.19,26 Wood19 reported that T. rubida (also collected in southern Arizona) tend to defecate immediately after a blood meal, but the number of insects examined was very low (N = 5 adults). Martínez-Ibarra and others26 found that adult female T. rubida sonoriana (likely a subspecies different from the one we studied) fed on immobilized rabbits tended to defecate before finishing a blood meal or soon (on average, < 1 minute) thereafter, while adult males have a longer defecation delay (average = 55 min). In another study, Klotz and others25 found that none of a small number (8 females and 7 males) of adult T. rubida tested defecated on a restrained host. Furthermore, Takano-Lee and Edman35 tested R. prolixus using both an artificial feeder and restrained guinea pigs and found that the defecation behavior of insects fed on the artificial feeder (and the calculated DIs) was similar to that of insects fed on live hosts. In addition, the fact that in a parallel experiment with third-instar R. prolixus we obtained results comparable to those reported by others using restrained mice, validates our results.
To our knowledge, this is the first systematic study of one of the most important factors (defecation behavior) determining the vectorial capacity of immature stages and adult T. rubida, the most abundant triatomine species in southern Arizona. Our results, together with our previous findings show that ∼41.5% of analyzed T. rubida were infected with T. cruzi,9 indicating that this species has the potential to transmit the parasite to humans. Its vectorial capacity, however, appears to be lower than that of the most important triatomine vectors, because only adult females defecate while feeding. From an epidemiological standpoint, however, this is relevant for Arizonans, because the insects that invade houses and feed on humans are dispersing, flying adults.9,40 Other factors, such as possibly low pathogenicity of the strain of T. cruzi harbored in local triatomines, likely explain the lack of reported human cases of Chagas disease in Arizona, although this remains to be investigated.
ACKNOWLEDGMENTS
We thank Ellen Dotson for kindly providing specimens of Rhodnius prolixus and T. cruzi DNA; B. Savary, P. Jenkins, R. Smith, C. Olson, and other Tucson residents for providing T. rubida, and K. Peck (supported by an UA/NASA space grant program) for helping with the analysis of T. cruzi. We also acknowledge two anonymous reviewers for their helpful comments and suggestions that greatly improved this manuscript.
Footnotes
Financial support: This work was supported by an Arizona Biomedical Research Commission grant no. 0708 to JGH.
Authors' addresses: Carolina E. Reisenman, Teresa Gregory, and John G. Hildebrand, Department of Neuroscience, College of Science, University of Arizona, Tucson, Arizona, E-mails: Carolina@neurobio.arizona.edu, tgregory@neurobio.arizona.edu, and jhildebr@email.arizona.edu. Pablo G. Guerenstein, CICyTTP-CONICET and UNER, Entre Ríos, Argentina, E-mail: pabloguerenstein@cicyttp.org.ar.
References
- 1.Pan American Health Organization Estimación cuantitativa de la enfermedad de Chagas en las Américas. 2006. http://www.bvsops.org.uy/pdf/chagas19.pdf Available at. Accessed March 7, 2011.
- 2.World Health Organization Chagas Disease (American trypanosomiasis) 2011. http://www.who.int/mediacentre/factsheets/fs340/en/index.html Fact sheet no. 340. Available at. Accessed March 4, 2011. [PMC free article] [PubMed]
- 3.Wood S, Wood F. Observations on vectors of Chagas' disease in the United States. III. New Mexico. Am J Trop Med Hyg. 1961;10:155–165. doi: 10.4269/ajtmh.1961.10.155. [DOI] [PubMed] [Google Scholar]
- 4.Wood S. Additional observations on Trypanosoma cruzi Chagas, from Arizona in insects, rodents and experimentally infected animals. Am J Trop Med Hyg. 1940;29:43–55. doi: 10.4269/ajtmh.1949.s1-29.43. [DOI] [PubMed] [Google Scholar]
- 5.Wood S. Observations on vectors of Chagas' disease in the United States. I. California. Bull South Calif Acad Sci. 1942;41:61–69. [Google Scholar]
- 6.Ryckman R. The vertebrate hosts of the Triatominae of North and Central America and the West Indies (Hemiptera: Reduviidae: Triatominae) Bull Soc Vector Ecol. 1986;11:221–241. [Google Scholar]
- 7.Packchanian A. Reservoirs hosts of Chagas' disease in the state of Texas. Am J Trop Med. 1942;22:623–631. [Google Scholar]
- 8.Kjos SA, Snowden KF, Olson JK. Biogeography and Trypanosoma cruzi infection prevalence of Chagas disease vectors in Texas. USA Vector-Borne Zoonotic Dis. 2009;9:41–50. doi: 10.1089/vbz.2008.0026. [DOI] [PubMed] [Google Scholar]
- 9.Reisenman CE, Lawrence G, Guerenstein PG, Gregory T, Dotson E, Hildebrand JG. Infection of kissing bugs with Trypanosoma cruzi, Tucson, Arizona, USA. Emerg Infect Dis. 2010;16:400–405. doi: 10.3201/eid1603.090648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown EL, Roellig DM, Gompper ME, Monello RJ, Wenning KM, Gabriel MW, Yabsley MJ. Seroprevalence of Trypanosoma cruzi among eleven potential reservoir species from six states across the southern United States. Vector Borne Zoonotic Dis. 2010;10:757–763. doi: 10.1089/vbz.2009.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woody NC, Woody HB. American trypanosomiasis (Chagas' disease): first indigenous case in the United States. J Am Med Assoc. 1955;159:676–677. doi: 10.1001/jama.1955.02960240042010a. [DOI] [PubMed] [Google Scholar]
- 12.Ochs DE, Hnilica VS, Moser DR, Smith JH, Kirchoff LV. Postmortem diagnosis of autochthonous acute chagasic myocarditis by polymerase chain reaction amplification of a species-specific DNA sequence of Trypanosoma cruzi. Am J Trop Med Hyg. 1996;54:526–529. doi: 10.4269/ajtmh.1996.54.526. [DOI] [PubMed] [Google Scholar]
- 13.Herwaldt B, Grijalva M, Newsome A, McGhee C, Powell M, Nemec D, Steurer F, Eberhard ML. Use of polymerase chain reaction to diagnose the fifth reported US case of autochtonous transmission of Trypanosoma cruzi, in Tennessee, 1998. J Infect Dis. 2000;181:395–399. doi: 10.1086/315212. [DOI] [PubMed] [Google Scholar]
- 14.Schiffler RJ, Mansur GP, Navin TR. Indigenous Chagas' disease (American trypanosomiasis) in California. JAMA. 1984;251:2983–2984. [PubMed] [Google Scholar]
- 15.Dorn P, Perniciario L, Yabsley M, Roellig D, Balsamo G, Diaz J, Wesson D. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007;13:605–607. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeledón R, Rabinovich JE. Chagas' disease: an ecological appraisal with special emphasis on its insect vectors. Annu Rev Entomol. 1981;26:101–133. doi: 10.1146/annurev.en.26.010181.000533. [DOI] [PubMed] [Google Scholar]
- 17.Dias E. Observations on defecation and contact feeding time of several South American Triatoma. Mem Inst Oswaldo Cruz. 1956;54:115–124. doi: 10.1590/s0074-02761956000100006. [DOI] [PubMed] [Google Scholar]
- 18.Zeledón R, Alvarado R, Jirón LF. Observations on the feeding and defecation patterns of three triatomine species (Hemiptera: Reduviidae) Acta Trop. 1977;34:65–77. [PubMed] [Google Scholar]
- 19.Wood SF. Importance of feeding and defecation times of insect vectors in transmission of Chagas' disease. J Med Entomol. 1951;44:52–54. [Google Scholar]
- 20.Vianna Braga M, Marli ML. Feeding and defecation patterns of nymphs of Triatoma rubrofasciata (De Geer, 1773) (Hemiptera: Reduviidae), and its potential role as vector for Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 1998;94:127–129. doi: 10.1590/s0074-02761999000100025. [DOI] [PubMed] [Google Scholar]
- 21.Lent D, Wygodzinsky PW. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas' disease. Bull Am Mus Nat Hist. 1979;163:123–520. [Google Scholar]
- 22.Rodriguez Coura J, Borges-Pereira J. Chagas disease: 100 years after its discovery. A systemic review. Acta Trop. 2010;115:5–13. doi: 10.1016/j.actatropica.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Loza-Murguía M, Noireau F. Vectorial capacity of Triatoma guasayana (Wygodzinsky & Abalos) (Hemiptera: Reduviidae) compared with two other species of epidemic importance. Neotrop Entomol. 2010;39:799–809. doi: 10.1590/s1519-566x2010000500020. [DOI] [PubMed] [Google Scholar]
- 24.Wood S. Observations on vectors of Chagas' disease in the United States. II. Arizona. Am J Trop Med Hyg. 1943;23:315–320. doi: 10.4269/ajtmh.1961.10.155. [DOI] [PubMed] [Google Scholar]
- 25.Klotz SA, Dorn PL, Klotz JH, Pinnas JL, Weirauch C, Kurtz JR, Schmidt J. Feeding behavior of triatomines from the southwestern United States: an update on potential risk for transmission of Chagas disease. Acta Trop. 2009;111:114–118. doi: 10.1016/j.actatropica.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Ibarra JA, Nogueda-Torres B, Paredes-González E, Alejandre-Aguilar R, Solorio-Cibrián M, Barreto SP, Gómez-Estrada HI, Trujillo-García JC. Development of Triatoma rubida sonoriana, Triatoma barberi, and Meccus mazzottii (Heteroptera, Reduviidae) under laboratory conditions. J Am Mosq Control Assoc. 2005;21:310–315. doi: 10.2987/8756-971X(2005)21[310:DOTRST]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 27.Núñez J, Lazzari CR. Rearing of Triatoma infestans Klug (Hemiptera: Reduviidae) in the absence of a live host. I. Some factors affecting the artificial feeding. J Appl Entomol. 1990;109:87–92. [Google Scholar]
- 28.Guerenstein PG, Núñez J. Feeding response of the hematophagous bugs Rhodnius prolixus and Triatoma infestans to saline solutions: a comparative study. J Insect Physiol. 1994;40:747–752. [Google Scholar]
- 29.Lorenzo MG, Lazzari CR. The spatial pattern of defecation in Triatoma infestans and the role of feces as a chemical mark of the refuge. J Insect Physiol. 1996;42:903–907. [Google Scholar]
- 30.Virreira M, Torrico F, Truyens C, Alonso-Vega C, Solano M, Carlier Y, Svoboda M. Comparison of polymerase chain reaction methods for reliable and easy detection of congenital Trypanosoma cruzi infection. Am J Trop Med Hyg. 2003;68:574–582. doi: 10.4269/ajtmh.2003.68.574. [DOI] [PubMed] [Google Scholar]
- 31.Zar JH. Biostatistical Analysis. Fourth edition. Upper Saddle River, NJ: Prentice-Hall, Inc; 1999. [Google Scholar]
- 32.Zárate LG, Morales López G, Cabrera Ozuna M, García Santiago G, Zárate RJ. The biology and behavior of Triatoma barberi (Hemiptera: Reduviidae) in México. J Med Entomol. 1984;21:548–560. doi: 10.1093/jmedent/21.5.548. [DOI] [PubMed] [Google Scholar]
- 33.Crocco LB, Catalá SS. Feeding and defecation patterns in Triatoma sordida. Mem Inst Oswaldo Cruz. 1996;91:409–413. doi: 10.1590/s0074-02761996000400004. [DOI] [PubMed] [Google Scholar]
- 34.Vázquez-Prokopec GM, Ceballos LA, Marcet PL, Cécere MC, Cardinal MV, Kitron U, Gürtler RE. Seasonal variations in active dispersal of natural populations of Triatoma infestans in rural north-western Argentina. Med Vet Entomol. 2006;20:273–279. doi: 10.1111/j.1365-2915.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takano-Lee M, Edman JD. Lack of manipulation of Rhodnius prolixus (Hemiptera: Reduviidae) vector competence by Trypanosoma cruzi. J Med Entomol. 2002;39:44–51. doi: 10.1603/0022-2585-39.1.44. [DOI] [PubMed] [Google Scholar]
- 36.Piesman J, Sherlock LA. Factors controlling the volume of feces produced by triatomine vectors of Chagas' disease. Acta Trop. 1983;40:351–358. [PubMed] [Google Scholar]
- 37.Trumper EV, Gorla DE. Density-dependent timing of defecation by Triatoma infestans. Trans R Soc Trop Med Hyg. 1991;85:800–802. doi: 10.1016/0035-9203(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 38.Kirk ML, Schofield CJ. Density-dependent timing of defecation by Rhodnius prolixus, and its implications for the transmission of Trypanosoma cruzi. Trans R Soc Trop Med Hyg. 1987;81:348–349. doi: 10.1016/0035-9203(87)90262-8. [DOI] [PubMed] [Google Scholar]
- 39.Guarnieri A, Diotaiuti L, Gontijo N, Gontijo A, Pereira M. Comparison of feeding behaviour of Triatoma infestans, Triatoma brasiliensis and Triatoma pseudomaculata in different hosts by electronic monitoring of the cibarial pump. J Insect Physiol. 2000;46:1121–1127. doi: 10.1016/s0022-1910(99)00222-x. [DOI] [PubMed] [Google Scholar]
- 40.Ekkens D. Nocturnal flights of Triatoma (Hemiptera: Reduviidae) in Sabino Canyon, Arizona. I. Light collections. J Med Entomol. 1981;18:211–227. [Google Scholar]