Abstract
The epidemiology of soil-transmitted helminth infections (hookworm, Trichuris trichiura, Ascaris lumbricoides, and Strongyloides stercoralis) in the United States is poorly understood. To gain understanding of the status of disease, a systematic review was performed to assess the prevalence of soil-transmitted helminth infections in the United States. Of all studies reviewed, 14 were designated as high-quality. High-quality studies were published from 1942 to 1982 and showed that infection was prevalent throughout the southern United States and Appalachia as recently as 1982, finding that hookworm (19.6%), T. trichiura (55.2%), A. lumbricoides (49.4%), and S. stercoralis (3.8%) affected significant percentages of the population. However, because the most recent high-quality studies were published over 25 years ago, the literature does not provide sufficient data to assess current endemic transmission. Because the status of disease remains unclear, there is a need for additional studies to determine if soil-transmitted helminths remain endemic in the United States.
Introduction
Up to one-third of the world's population, many of them children, may be infected with intestinal helminths, principal among them hookworm, Trichuris trichiura, Ascaris lumbricoides, and Strongyloides stercoralis. These intestinal infections may result in malnutrition, iron-deficiency anemia, and malabsorption, and they can be complicated by intestinal obstruction and in the case of S. stercoralis, hyperinfection and disseminated disease that may result in death.1 Additionally, stunting of growth and delays in cognitive development are a frequent consequence of infection.2 Soil-transmitted helminth infections are neglected infections of poverty. This group of parasitic infections that also includes Chagas disease, cysticercosis, toxocariasis, toxoplasmosis, and trichomoniasis are parasitic diseases that cause significant morbidity in those people infected, disproportionately impact impoverished persons, and are poorly characterized in the United States.3
There is a common belief among clinicians and those people in the public health community that soil-transmitted helminths continue to be transmitted within areas of the United States; however, this conclusion is based on unclear or historical evidence.3–5 The purpose of this review is to assess the status of soil-transmitted helminth infection in the United States through a systematic evaluation of the published literature. Summarizing the status of soil-transmitted helminth infections in the United States is the first step to determining current knowledge and the need for future evaluations and interventions.
Methods
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) criteria were applied for this review.6 A comprehensive literature search in MEDLINE/PUBMED (1960 to March 2011) with the medical subject headings (MeSH) intestinal diseases, parasitic/epidemiology, ascariasis, hookworm infections, trichuriasis, and strongyloidiasis was performed. Analysis was restricted to human studies occurring within the United States with no language restrictions. To include literature describing imported infection, the MeSH terms refugee/statistics and health status were also used. Studies were included if they met all of the following criteria: (1) reported data were systematically collected in a defined population, (2) new data were presented, and (3) prevalence of at least one of the queried intestinal parasites was reported. Studies were initially selected based on their titles and abstracts, and then if appropriate, full text was obtained. To identify additional studies, reference lists of selected articles as well as literature reviews were hand-searched, and all articles cited by included studies were reviewed. No study was included if the date of publication was before 1940 to improve likelihood of full text access. If an article reported more than one unique study, each study was considered separately. Authors were contacted whenever possible for clarification if data were incomplete or unclear.
The following data were abstracted as available: first author, year of publication, sample size, population age and ethnicity/race, type of parasitological testing performed, and number of positive samples for each queried parasite. Data were double entered to assure consistency of reporting, and prevalence was calculated for each study.
To provide a summary of the best-quality information available on endemic soil-transmitted helminths transmission within the United States, a methodological quality assessment tool was developed. Because no gold standard assessment tool exists, one was created using the strengthening the reporting of observational studies in epidemiology (STROBE) checklist as well as existing checklists for the assessment of observational studies and review of the literature.7–9 Five criteria were selected, and each study was awarded zero to five points based on study participant selection, sample size, data sources and measurement technique, parasitological analysis technique, and outcome measures (Table 1). Studies with all five points were designated as high quality.
Table 1.
Modified STROBE checklist used for selection of high-quality studies of soil-transmitted infection in the United States
| Criteria | Description |
|---|---|
| Participants | Study clearly gives the eligibility criteria as well as the sources and methods of selection of study participants. Number of participants and number of persons or samples are apparent. These study participants are noted to represent the population under study. Participants selected represented likely endemic transmission of soil-transmitted helminth infection in the United States. |
| Data sources and measurements | Techniques for data collection are clearly detailed. |
| Study size | Study includes more than 350 participants. |
| Quantitative variables | Parasitologic analysis performed is clearly described, and it is sensitive enough to identify parasites using the technological capabilities of the time. |
| Outcome data | Percentages or number of parasite-positive stool samples are given. |
Results
Availability of data.
Initial searches identified 902 articles, and their titles and abstracts were reviewed for relevance. Articles were excluded for irrelevance (765), incomplete primary data (12), missing data (4), commentary style (4), unavailable full text (3), duplicated data (3), and non-United States-based data (1) (Figure 1). Therefore, 110 articles consisting of 132 unique studies met inclusion criteria and were included in this review. Of these studies, 44 focused on soil-transmitted helminth infections likely acquired within the United States, whereas 64 focused on imported infections among immigrants, migrant workers, and refugees (Figure 2). In 24 studies, the population studied was not able to be determined from information provided in the article. A full list of articles included in this review, but not cited is available on request.
Figure 1.
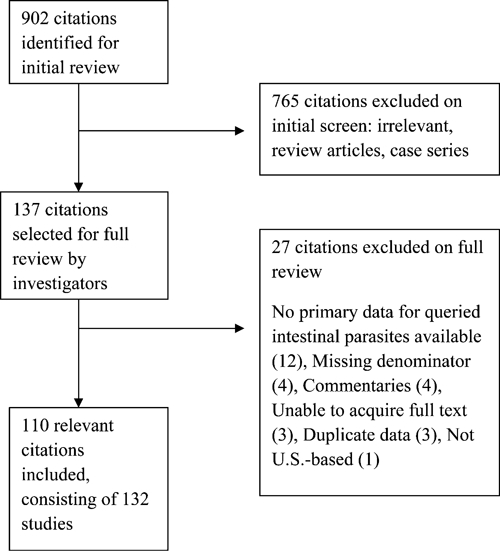
Schematic representation of the study selection process.
Figure 2.
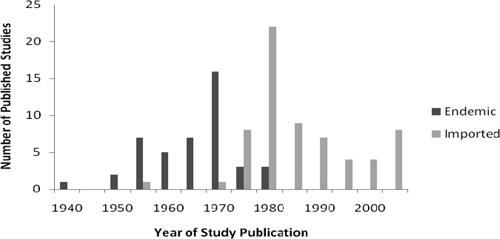
Number of published studies reporting domestic and imported soil-transmitted helminth infection prevalence by year of publication (1940–2010).
Identification of high-quality studies representing infection likely acquired within the United States.
Fourteen high-quality studies of soil-transmitted helminths in the United States with likely endemic transmission were identified using a modified STROBE scale (Table 2).10–22 The oldest included study was performed in 1942, and the most recent study was in 1982. All high-quality studies reported prevalence in the southern United States, with six from Kentucky, three from Louisiana, two from Georgia, and one each from Tennessee, North Carolina, and South Carolina. Studies were all cross-sectional, including nine school-based, four community-based, and one survey of attendees at a community health center. Most studies sampled children, with eight using an elementary school population and one being college-based, whereas the remaining five sampled entire communities. The number of subjects in each of the studies varied widely, with the smallest study consisting of 366 subjects and the largest having nearly 4,000 subjects. All but one of these studies used a single screening stool specimen for parasitologic analysis; one study collected three stool specimens from subjects. Laboratory methods used for soil-transmitted helminth identification varied in each of the 14 high-quality studies; concentration was performed in seven studies, direct smear was performed in eight studies, flotation was performed in six studies, and Kato–Katz technique was used in two studies.
Table 2.
Summary of published high-quality studies quantifying prevalence of soil-transmitted helminth infection in the United States from 1942 to 1982
| Study and year | Study location | Study design | Population description | N | Hookworm (%) | T. trichiura (%) | A. lumbricoides (%) | S. stercoralis (%) |
|---|---|---|---|---|---|---|---|---|
| Headlee and Cable, 194210 | KY | College-based | College students, 70% Appalachia | 2,393 | 14.6 | 7.9 | 5.1 | 3.8 |
| Young, 195511 | TN | School-based | Rural poor, 5- to 16-year-old children | 2,908 | 19.6 | 1.4 | 6.1 | 0.1 |
| Atchley and others, 195612 | KY | Community-based random sample | January to March 1955 | 1,800 | 0.5 | 14.6 | 21.3 | 2.6 |
| Atchley and others, 195612 | KY | Community-based random sample | April to July 1955 | 843 | 0 | 24.2 | 26.8 | 1.2 |
| Fulmer and Huemphner, 196513 | KY | School-based | Native-born 6–12 years old | 366 | 3.6 | 55.2 | 48.6 | 3.8 |
| Healy and others, 196914 | NC | School-based | Cherokee Native Americans 6–16 years | 631 | 3.0 | 38.0 | 49.4 | –* |
| Gloor and others, 197015 | KY | School-based | 10–14 years old | 439 | 14.8 | 4.8 | 7.7 | 0 |
| Martin, 197216 | GA | School-based | Caucasian children 5–15 years | 3,729 | 4.6 | 0.5 | 1.3 | – |
| Martin, 197217 | GA | Community-based random sample | 550 Caucasians, 199 African-Americans | 749 | 13.6 | 0.5 | 4.3 | – |
| Morgan and others, 197218 | LA | Community-based random sample | Lowest 25% socioeconomic strata | 1,651 | 0.4 | 12.3 | 5.3 | 0.3 |
| Sargent and others, 197219 | SC | School-based | 32% African-American, 5–15 year olds | 2,932 | 1.8 | 1.1 | 21.5 | – |
| Hubbard and others, 197420 | LA | School-based | Mostly African-Americans, 5 years old | 887 | 0.1 | 5.3 | 2.3 | – |
| Farhadian and Schneider, 197521 | LA | Community referral to health center | 10 months to 7 years old | 1,078 | 0.1 | 14.5 | 3.9 | – |
| Walzer and others, 198222 | KY | School-based survey | Native-born 3–7 years old | 561 | 0.2 | 12.6 | 14.4 | 3.0 |
Denotes that S. stercoralis testing was not performed as part of this survey.
Prevalence of soil-transmitted helminths varied widely, although the presence of A. lumbricoides and T. trichiura was noted in each of the 14 studies. The reported prevalence of hookworm infection ranged from 0% to 19.6%, T. trichiura ranged from 0.5% to 55.2%, A. lumbricoides ranged from 2.3% to 49.4%, and S. stercoralis ranged from 0% to 3.8%. The highest hookworm prevalence (19.6%) was reported among school children in Tennessee in 1955; however, hookworm was also as high as 13.6% in a 1972 study in Georgia. T. trichiura infection was present in over one-half of school children in Kentucky surveyed by Fulmer and Huemphner13 in 1965, and it continued to be found among rural schoolchildren in Kentucky (12.6%) as recently as 1982. A. lumbricoides prevalence was highest in a study of Cherokee Native American schoolchildren, but it was also found in 14.4% of schoolchildren in Kentucky in the early 1980s. S. stercoralis was described at low rates throughout these studies, with the most recent high-quality study showing 3.0% of schoolchildren infected. Data on frequency of coinfection among study participants were available in five of the high-quality studies, with none of these studies showing clustering of infection among a small group of subjects.13,14,16,20,21
Discussion
Historically, soil-transmitted helminth infections were endemic throughout Appalachia and the southern United States. The Rockefeller Sanitary Commission invested upwards of $1 million in a campaign designed to eradicate these infections, decreasing hookworm infection from 41.7% among persons in the South in 1914 to 19.3% by 1930.23 Despite these interventions, soil-transmitted helminth infection remained significant in areas of the United States, with some estimating that one in five Kentucky residents or 600,000 persons harbored these parasites in 1945.24 Although transmission of these infections within the United States has likely declined significantly in the intervening years because of improvements in sanitation practices, the current status of these infections is unknown.
Published studies of soil-transmitted helminth infection in the United States show that, as recently as 1982, some regions of Appalachia continued to sustain a significant burden of both T. trichiura and A. lumbricoides, likely from endemic sources. The high-quality studies summarized in this review illustrate that helminth infection was highly prevalent throughout the southern United States as well as Appalachia as recently as the early 1980s. Even less is known about soil-transmitted helminth infections in other parts of the United States outside the southern United States and Appalachia; however, those areas are generally thought to carry lower burdens of infection.
Despite the historically high burden of these infections seen in the United States, the published literature does not provide enough information to determine if soil-transmitted infections remain endemic in the United States, with the most recent high-quality study examining endemic transmission of infection occurring in 1982. Recent publications are case-based, study exclusively immigrant populations, or use a laboratory-based approach that makes determination of endemic transmission impossible.
Soil-transmitted helminth infections result in significant disease in those people infected; they are a cause of cognitive and physical growth impairment and anemia in children. Additionally, S. stercoralis infection may result in potentially severe and fatal hyperinfection in immunocompromised hosts.25 All soil-transmitted helminth infections may lead to subtle long-term consequences such as poor school attendance and academic achievement as well as delays in growth and development from chronic malnutrition.1
Although the literature does not provide enough evidence to determine the status of soil-transmitted helminths in the United States, socioeconomic and environmental conditions may remain conducive to ongoing infection. The 1990 poverty rate for Appalachia was 15.2%, higher than the national rate of 13.1%, and in nonmetropolitan areas of central Appalachia, over 27% of the population lives below the poverty level, a rate over two times the national average.26 Substandard housing units are common, facilitating conditions that may encourage transmission of these infections. In Central Appalachia, an area encompassing 179 counties and cities in Kentucky, West Virginia, Tennessee, and Virginia, 57% of counties had substandard housing rates higher than the US rate (5.3%) in 1990.27 Historically, access to indoor plumbing and running water in Appalachia has lagged behind the rest of the United States, with one-half of homes in the region lacking access in 1964. Although sanitation has improved since the last high-quality studies were performed, an estimated 169,000 housing units remained without indoor plumbing as recently as 2000. Poor sanitation continues to persist in some areas of Appalachia, with 3% of the region lacking plumbing, and in some areas, plumbing is not complete in over 25% of housing units.28
This literature review had several limitations. First, search strategies relied on appropriate use of MeSH terminology and may have lead to incomplete identification of existing studies. Additionally, by relying solely on the published literature, this review was subject to publication bias, because studies with minimal or negative results may not have been included. Second, because only a point estimate of prevalence was reported in each study, it was not possible to ascertain the certainty of these estimates of prevalence. Third, most of the studies relied on a single stool screening examination and suboptimal diagnostic methodology. The sensitivity of a single stool examination to detect infection with soil-transmitted helminths may be very low because of variation in egg output and the small amount of stool examined, with reported diagnostic sensitivity of a single stool examination for S. stercoralis ranging from 30% to 50%.29,30 These limitations each contribute to possible underreporting of the current distribution and endemic transmission of soil-transmitted helminths in the United States.
Soil-transmitted helminths were historically considered endemic in several areas of the United States. Although the prevalence of these infections has decreased from levels observed in the early part of the 1900s, it is unclear if these infections continue to be transmitted among those people living in Appalachia or throughout the southern United States. Changes to the US population as a result of immigration have led an increase in attention focused on imported soil-transmitted helminth infections, which are unlikely to contribute to local transmission given the medical care and standards of sanitation in most areas of the United States. However, little attention has been given to possible continued transmission of soil-transmitted helminths in historically endemic areas, where they may cause an underappreciated burden of disease. Because conditions in areas of the United States may still support endemic transmission and these diseases cause significant morbidity among those people infected, determining the status of disease is essential. Additional research, particularly assessment of possible transmission through community level investigations similar to those searches included in this review, should be performed to determine if soil-transmitted helminth infections continue to be endemic in the certain areas of the United States.
Supplementary Material
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Note: Supplemental appendices are available at www.ajtmh.org.
Footnotes
Financial support: This work was made possible through the Centers for Disease Control and Prevention (CDC) Experience Applied Epidemiology Fellowship, a public/private partnership supported by a grant to the CDC Foundation from External Medical Affairs, Pfizer, Inc.
Authors' addresses: Michelle C. Starr and Susan P. Montgomery, Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA, E-mails: kbz4@cdc.gov and zqu6@cdc.gov.
References
- 1.Blumenthal DS, Schultz MG. Incidence of intestinal obstruction in children infected with Ascaris lumbricoides. Am J Trop Med Hyg. 1975;24:801–805. doi: 10.4269/ajtmh.1975.24.801. [DOI] [PubMed] [Google Scholar]
- 2.Bethony J. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 3.Hotez P. Neglected infections of poverty in the United States of America. PLoS Negl Trop Dis. 2008;2:e256. doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warren KS. Helminthic diseases endemic in the United States. Am J Trop Med Hyg. 1974;23:723–730. [Google Scholar]
- 5.Milder JE, Walzer PD, Kilgore G, Rutherford I, Klein M. Clinical features of Strongyloides stercoralis infection in an endemic area of the United States. Gastroenterology. 1981;80:1481–1488. [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 7.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654. [DOI] [PubMed] [Google Scholar]
- 8.Mallen C, Peat G, Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. J Clin Epidemiol. 2005;59:765–769. doi: 10.1016/j.jclinepi.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Lohr K, Carey T. Assessing best evidence: issues in grading the quality of studies for systematic reviews. Jt Comm J Qual Improv. 1999;25:470–479. doi: 10.1016/s1070-3241(16)30461-8. [DOI] [PubMed] [Google Scholar]
- 10.Headlee WH, Cable RM. Intestinal parasitism among students of Berea College, Kentucky. Am J Trop Med Hyg. 1942;22:351–360. [Google Scholar]
- 11.Young MM. Report of a survey for intestinal parasites in school children in a rural mountain county in Tennessee. South Med J. 1955;48:46–53. doi: 10.1097/00007611-195501000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Atchley FO, Hemphill EC, Hunt DW. Current status of intestinal parasitism of man in eastern Kentucky. J Parasitol. 1956;42:505–509. [PubMed] [Google Scholar]
- 13.Fulmer HS, Huemphner HR. Intestinal helminths in eastern Kentucky: a survey in three rural counties. Am J Trop Med Hyg. 1965;14:269–275. doi: 10.4269/ajtmh.1965.14.269. [DOI] [PubMed] [Google Scholar]
- 14.Healy GR, Gleason NN, Bokat R, Pond H, Roper M. Prevalence of ascariasis and amebiasis in Cherokee Indian school children. Public Health Rep. 1969;84:907–914. [PMC free article] [PubMed] [Google Scholar]
- 15.Gloor RF, Breyley ER, Martinez IG. Hookworm infection in a rural Kentucky county. Am J Trop Med Hyg. 1970;19:1007–1009. doi: 10.4269/ajtmh.1970.19.1007. [DOI] [PubMed] [Google Scholar]
- 16.Martin LK. Hookworm in Georgia. I. Survey of intestinal helminth infections and anemia in rural school children. Am J Trop Med Hyg. 1972;21:919–929. [PubMed] [Google Scholar]
- 17.Martin LK. Hookworm in Georgia. II. Survey of intestinal helminth infections in members of rural households of southeast Georgia. Am J Trop Med Hyg. 1972;21:930–943. [PubMed] [Google Scholar]
- 18.Morgan PM, Hubbard DW, Willis RA, Unglaub WG, Langham RA, Hedmeg AW, Muldrey JE. Intestinal parasitism and nutritional status in Louisiana. J La State Med Soc. 1972;124:197–203. [PubMed] [Google Scholar]
- 19.Sargent RG, Dudley BW, Fox AS, Lease EJ. Intestinal helminths in children in coastal South Carolina: a problem in southeastern United States. South Med J. 1972;65:294–298. doi: 10.1097/00007611-197203000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Hubbard DW, Morgan PM, Yaeger RG, Unglaub WG, Hood MW, Willis RA. Intestinal parasite survey of kindergarten children in New Orleans. Pediatr Res. 1974;8:652–658. doi: 10.1203/00006450-197406000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Farhadian H, Schneider EA. Trichuriasis in Calcasieu Parish, southwest Louisiana. J La State Med Soc. 1975;127:337–340. [PubMed] [Google Scholar]
- 22.Walzer PD, Milder JE, Banwell JG, Kilgore G, Klein M, Parker R. Epidemiologic features of Strongyloides stercoralis infection in an endemic area of the United States. Am J Trop Med Hyg. 1982;31:313–319. doi: 10.4269/ajtmh.1982.31.313. [DOI] [PubMed] [Google Scholar]
- 23.Farmer HF. Hookworm eradication program of Florida in the early 20th century. JFMA. 1986;73:300–304. [PubMed] [Google Scholar]
- 24.Teague RE. The common intestinal parasites in Kentucky. Frankfort, KY: Department of Public Health of Kentucky. Bulletin of the State Department of Health of Kentucky. 1945:431–439. [Google Scholar]
- 25.Crocker C, Reporter R, Redelings M, Mascola L. Strongylodiasis-related deaths in the United States, 1991–2006. Am J Trop Med Hyg. 2010;83:422–426. doi: 10.4269/ajtmh.2010.09-0750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.US Bureau of the Census Income, Poverty, and Health Care Insurance in the United States. 2003. http://pubdb3.census/gov/macro/032004/pov/new41_100_01.htm Available at. Accessed June 10, 2011.
- 27.US Department of Housing and Urban Development . US Housing Market Conditions, First Quarter 2000. Washington, DC: Government Printing Office; 2000. [Google Scholar]
- 28.Glasmeier AK. An Atlas of Poverty in America: One Nation, Pulling Apart, 1960–2003. Distressed Regions Section. New York, NY: Routledge Taylor & Francis Group; 2006. pp. 51–80. [Google Scholar]
- 29.Mirdha B. Human strongyloidiasis: often brushed under the carpet. Trop Gastroenterol. 2009;30:1–4. [PubMed] [Google Scholar]
- 30.Tarafder MR, Carabin H, Joseph L, Balolong E, Olveda R, McGarvey ST. Estimating the sensitivity and specificity of Kato-Katz stool examination technique for detection of hookworms, Ascaris lumbriocides and Trichuris trichiura infections in humans in the absence of a ‘gold standard.'. Int J Parasitol. 2010;40:399–404. doi: 10.1016/j.ijpara.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


