Abstract
A cohort study was designed to assess the impact of mass distribution of azithromycin (MDA) for trachoma control on incidence over six months of pediatric diarrhea in eight communities in rural Tanzania. A single dose of azithromycin was offered to all residents in four communities, where trachoma prevalence was ≥ 10%. Four geographically matched communities had trachoma prevalences < 10% and did not receive MDA. All randomly selected children (n = 1036) were followed-up for six months post-MDA with bi-weekly surveillance at home. In the 0–1-month and 1–3-month periods, MDA exposure was associated with a 39% (rate ratio = 0.61, 95% confidence interval = 0.39–0.95) and 24% (rate ratio = 0.76, 95% confidence interval = 0.54–1.07) lower risk of diarrhea, respectively, compared with those unexposed, after adjustment for clustering and covariates. By the 3–6-month period, diarrhea incidence was comparable between groups. Thus, MDA was associated with a short-term reduction in diarrheal morbidity in children.
Introduction
Trachoma, an ocular infection caused by Chlamydia trachomatis, is the leading infectious cause of blindness globally.1 The estimated 3.8 million cases of blindness and 5.3 million cases of low vision caused by trachoma are responsible for significant losses in productivity, which hinders economic development, and increases personal and societal burdens in affected areas.1,2
Results from studies conducted in areas with a high burden of trachoma show that a single dose of azithromycin is effective in treating chlamydial infections and preventing their recurrence.3–5 Mass distribution of azithromycin (MDA) in trachoma-endemic communities has been adopted as a part of World Health Organization (WHO)–endorsed, multi-pronged strategy for reducing the risk of blindness in national trachoma control programs.6,7 In addition to C. trachomatis, evidence from laboratory and experimental studies indicate that azithromycin is effective against infections caused by a broad spectrum of parasites and bacteria, including those pathogens responsible for sexually transmitted infections, acute respiratory infections, possibly malaria and bacterial enteric infections, which are prevalent in developing countries.8–10 Thus, there may be ancillary benefits from mass treatment in these communities.
Diarrheal disease accounts for significant morbidity and mortality among children worldwide, especially in low-income countries.11 In the WHO recent update of the Global Burden of Disease Report, diarrhea is ranked as the third most frequent cause of death in low-income countries, including Tanzania.12 The burden is heaviest on children less than five years of age; it has been estimated that there are 25,000 child deaths caused by diarrhea annually and that it is associated with growth faltering and impaired cognitive development.12–14
Bacteria are the leading cause of severe diarrheal infections in low-income countries where access to clean water and sanitation is limited. The Child Health Epidemiology Research Group estimated that in 2000, more than one-third of the pediatric diarrhea episodes identified in community-based studies were caused by common bacterial pathogens including Salmonella, Shigella, Campylobacter, Vibrio cholerae, and diarrheagenic strains of Escherichia coli.15 Results from several in vitro studies and small randomized controlled trials suggest that azithromycin is efficacious in the treatment of infections caused by Salmonella, Shigella, Campylobacter, and possibly E. coli.16–18 However, the effect of mass prophylactic treatment with azithomycin on incident diarrheal morbidity among young children living in areas where trachoma is prevalent has not been studied. If azithromycin is found to be effective in preventing diarrheal infections and if its effect within a community persists, it may significantly reduce the burden of diarrheal illness in affected communities. Furthermore, it would provide an impetus to increase support for national trachoma control and neglected tropical disease programs. We assessed the impact of MDA for trachoma control and other factors on the risk of diarrhea among children 2–59 months of age living in villages in rural, central Tanzania.
Materials and Methods
Subjects and setting.
The PRET (Partnership for the Rapid Elimination of Trachoma) Plus study was a six-month, population-based, prospective cohort study designed to evaluate the ancillary benefits after MDA for trachoma with respect to the incidence and prevalence of diarrhea, malaria, and acute respiratory illness. For the study of diarrheal disease, random samples of children who were exposed to mass treatment were compared with random samples of children who were not exposed to MDA because their communities were not eligible to receive mass treatment. The PRET Plus study was conducted in the Kongwa District located in rural central Tanzania. The population of Kongwa is mostly herders or subsistence farmers. The regional climate is that of a highland plateau with semi-arid conditions where access to water is poor. For study purposes, the communities represented the smallest population unit for which health services are organized and trachoma control programs are implemented. Tanzanian communities are geographically distinct, averaging 1,500 persons, including an estimated 200–380 persons less than five years of age.
Tanzanian government policy stipulates that all communities in which trachoma prevalence is ≥ 10% receive annual MDA for trachoma control. Rapid assessment of trachoma in each village in Kongwa District in 2008 determined which villages in the district would receive MDA and which villages would not receive MDA. Of the 66 communities in Kongwa, 34 had received MDA within the past year and were excluded. Among the 36 remaining villages, 4 did not meet the government criteria for MDA and would not be receiving MDA in the future. The children randomly selected from these four communities were thus unexposed. The exposed children resided in four communities slated for MDA, which were selected on the basis of geographic proximity to the unexposed villages.
PRET Plus: enrollment and study procedures.
The PRET Plus study was conducted from January 12 through July 21, 2009, during an uncharacteristically dry period in the region where the rainfall total for the year (331.2 mm) was 60% of expected. Prior to study initiation, community leadership provided consent to the overall community participation in the study. The cohorts of children were randomly selected from these eight communities on the basis of complete household census lists. From the census, we identified eligible households (at least one child less than five years of age) and selected at random 130 of the eligible households. Within the selected households, one eligible child was selected at random.
To be eligible to participate in the study, a child had to meet the following criteria: 1) age 2–59 months, 2) resided in an eligible community (defined as either living in the community since birth, or moved in with parents or guardians), and 3) had an identifiable guardian capable of providing consent to participate. Diarrhea prevalence was assessed at the baseline survey and incidence was measured through on-going, bi-weekly surveillance at the household over the study period in all communities (Figure 1).
Figure 1.
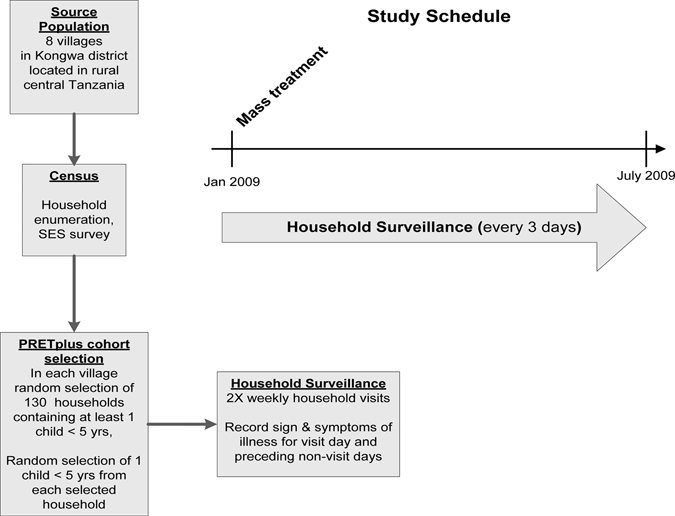
Study overview, Kongwa, Tanzania.
Ethical review.
The PRET Plus study protocol was approved by the Tanzanian National Institute for Medical Research and The Committee for Human Research of the Johns Hopkins School of Medicine, (Baltimore, MD). In Kongwa, community leadership provided consent for overall community participation in the study, and children were included in the studies on the basis of written informed consent obtained from parents or guardians.
Data collection.
Prior to study initiation, team members conducted a full household census in all study communities. Information collected included the age and sex of all household residents; a household resident is defined as a person who has slept in the household for at least the past three months or who intends to reside with the family for the next six months. Additional household characteristics collected included the head of household's years of schooling or formal education(defined as completing a grade in school as opposed to adult education classes), distance to household water source in dry season (measured as time to walk one way), and presence of a latrine (as observed by the interviewer).
On the basis of the census, a random selection of 1,040 children across the eight communities was made. An intensive surveillance was initiated of all randomly selected households. A baseline survey was conducted in January (prior to mass treatment of the four villages). During the baseline survey, children were evaluated for symptoms of diarrhea and acute lower respiratory infection (ALRI). A study team member asked general health questions about the children and evaluated them for symptoms of illness, including diarrhea and ALRI. Symptoms were recorded on a child survey form. Children were treated on site if their symptoms met the presumptive treatment criteria.
After MDA in communities and at a comparable time point in communities that did not receive MDA, surveillance teams visited children's homes to ascertain symptoms (episodes) of diarrhea or ALRI in the past three days; episodes were defined as any new report of diarrheal/ALRI symptoms lasting until a report of absence of symptoms. Symptoms were recorded on a child surveillance form and in a monthly morbidity book. Surveillance continued bi-weekly (every 3–4 days) for the entire study duration for all children.
With respect to diarrhea, guardians were asked if their child had diarrhea in the past three days. A negative response prompted the interviewer to ask about the presence/absence of blood or mucus in feces for the same reporting period. If the guardian indicated that the child had diarrhea in the reporting period, the interviewer continued with follow-up questions regarding the number of loose, watery stools during the reporting period and the presence/absence of blood or mucus in feces. All guardian responses were noted in the monthly morbidity book.
Definition and measurement of diarrhea.
An episode of diarrhea was defined as when a mother reported her child passing ≥ 3 liquid or semi-liquid stools in a 24-hour period. An episode ended when the child experienced three consecutive diarrhea-free days.
Treatment.
Children with signs and symptoms consistent with diarrhea and ALRI were treated presumptively according to the WHO Integrated Management of Childhood Illness guidelines for Tanzania.19 Children diagnosed with diarrhea were provided with oral rehydration solution sachets, and those with evidence of a cough plus a rapid respiratory rate during household visit were treated with amoxicillin for ALRI. Children found to be symptomatic at the baseline survey visit were treated by survey supervisors the same day. Symptomatic children identified during the surveillance period were treated by the surveillance team supervisor the day after symptom recording in the morbidity book. Children found to be symptomatic with diarrhea for more than two weeks after presumptive treatment with oral rehydration solution and without evidence of improvement were referred to Kongwa District Hospital for further evaluation. Children with symptoms consistent with severe illness were transported to the local hospital for treatment.
Data management and statistical analysis.
Detailed protocols to monitor data quality were established and quality control procedures were strictly enforced. Definitions, data collections methods (including household interviews), and data management were standardized. Key data fields were double-entered and data completeness was reconciled by the project manager prior to transmission to the study site's data center. Data were analyzed with Stata 11 software (Stata Corp., College Station, TX). The t test for continuous variables and the two-tailed chi-square analysis were used, as appropriate, to assess differences between the baseline characteristics of all children enrolled. For the bivariate and multivariate analyses, generalized estimating equations with the log link function and a Poisson random component were used to evaluate associations between potential risk factors, adjusted for clustering at the balozi (neighborhood) level with robust standard errors. Risk ratios and 95% confidence intervals (CIs) were used to estimate the association between potential risk factors and diarrhea during the three follow-up periods: 0–1 months, 1–3 months, and 3–6 months. In the multivariate analysis, the estimate of effect of MDA exposure on risk of diarrhea was adjusted for the influence of household characteristics and clustering, incorporating variables statistically significant at the level of P < 0.10 in bivariate analysis.
Results
Characteristics of the study population.
A total of 1,036 children less than five years of age were enrolled in the cohort study. There were four refusals. Of those enrolled, the proportion of children that completed the 1-month, 3-month, and 6-month surveys was 98.1% (1,016 of 1,036), 97.3% (1,008 of 1,036), and 95.8% (992 of 1,036). Males and females were equally represented and approximately one-third of the children were less than two years of age at the time of enrollment (Table 1). Approximately 45% (467 of 1,035) of the participants came from homes where the head of household had no formal schooling. The mean (SD) number of children less than five years of age per household was 1.53 (0.66). The proportion of persons with ≥ 1 less than five years of age living in the same household was 46% (477 of 1,036). Although more than 70% (726 of 1,025) of the children lived in a house with a latrine, nearly 75% had to travel at least a half hour for water during the dry season. The prevalence of diarrheal symptoms reported among children at the baseline survey was 18% (177 of 970). In addition, nearly one-third of parents said that their child had used a drug to treat an infection in the past 30 days.
Table 1.
Baseline characteristics of 1,036 study children living in Kongwa, Tanzania
| Characteristic | No. positive/total | % |
|---|---|---|
| Male | 503/1,036 | 48.6 |
| Age < 2 years | 368/1,036 | 35.5 |
| 1 or more siblings < 5 years of age | 477/1,036 | 46.0 |
| Lives in azithromycin mass treatment village | 519/1,036 | 50.1 |
| Head of household has < 1 year of education | 467/1,035 | 45.1 |
| Household has latrine | 726/1,025 | 70.8 |
| Fresh water source ≥ 30 minutes away in dry season | 752/1,033 | 72.8 |
| Diarrhea present at baseline survey | 177/970 | 18.3 |
| Used a drug to treat suspected infection in past 30 days | 294/969 | 30.3 |
Half of the children lived in MDA villages (519 of 1,036), and azithromycin was administered to 90% (467 of 519) of these children, as assessed in individual family treatment logs Children living the MDA communities were more likely to live in households farther from water and where the head of the household had no formal education than those in communities not receiving MDA (Table 2).
Table 2.
Comparison of baseline characteristics of 1,036 study children living in Kongwa, Tanzania, by exposure status*
| Characteristic | Non-MDA | MDA | ||
|---|---|---|---|---|
| No. positive/total | % | No. positive/total | % | |
| Male | 244/517 | 47.2 | 259/519 | 49.9 |
| Age < 2 years | 180/517 | 34.8 | 188 | 36.2 |
| 1 or more siblings < 5 years of age† | 183/517 | 35.4 | 294/519 | 56.7 |
| Head of household has < 1 year education† | 191/516 | 37.0 | 276/519 | 53.2 |
| Household has latrine | 370/515 | 71.8 | 356/510 | 69.8 |
| Fresh water source ≥ 30 minutes away in dry season† | 299/517 | 57.8 | 453/516 | 87.8 |
| Prevalence of diarrhea at baseline survey | 95/479 | 19.8 | 114/491 | 23.2 |
| Used a drug to treat infection in past 30 days† | 166/479 | 34.7 | 128/490 | 26.1 |
MDA = mass distribution of azithromycin.
P ≤ 0.05.
Incidence of acute diarrhea.
The mean (SD) incidence of diarrhea among participants during the six-month follow-up period was 1.09 (2.0) episodes per person-year, with little variation by follow-up interval. The incidence was 1.10 (3.7) episodes per person-year during the first month of follow-up, which then decreased slightly to 1.09 (2.8) in the 1–3-month follow-up period. The incidence decreased further to 1.05 (2.5) episodes per person-year during the 3–6-month follow-up period.
Risk factors for acute diarrhea.
Bivariate analyses.
Three demographic or host characteristics were associated with diarrhea during the 0–1-month, 1–3-month, and 3–6-month follow-up periods in the bivariate analyses. In the first month of the follow-up period, children living in MDA villages had a 43% lower risk of diarrhea than those living in the villages not receiving mass treatment (relative risk [RR] = 0.57, 95% confidence interval [CI] = 0.37–0.89) (Table 3). Children less than 24 months of age had a three-fold higher risk of diarrhea than older children (RR = 3.10, 95% CI = 2.07–4.63). Children who had been treated with amoxicillin for an acute respiratory illness were twice as likely to experience diarrhea as children who did not receive antibiotics (RR = 1.81, 95% CI = 1.20–2.74). During the 1–3-month follow-up period, living in an MDA village continued to be a protective factor against diarrhea, although the effect was weaker than that observed in the first month of follow-up and was not significant. Children living in these villages experienced a 21% (RR = 0.79, 95% CI = 0.54–1.13) decrease in the incidence of diarrhea compared with those living in villages not treated.
Table 3.
Crude risk ratios for selected risk factors for diarrhea among 1,036 children less than five years of age living in Kongwa, Tanzania, by follow-up period*
| Risk factor | 0–1 months | 1–3 months | 3–6 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Person days | Episodes/person years | Rate | Crude RR (95% CI) | Person days | Episodes/person years | Rate | Crude RR (95% CI) | Person days | Episodes/person years | Rate | Crude RR (95% CI) | |
| Lives in MDA village | ||||||||||||
| Yes | 13,482 | 30 | 0.81 | 0.57 (0.37–0.89) | 27,874 | 74 | 0.97 | 0.79 (0.54–1.13) | 38,034.5 | 97 | 0.93 | 0.88 (0.61–1.27) |
| No | 15,928.5 | 62 | 1.42 | 1 | 27,646.5 | 94 | 1.24 | 1 | 31,839.5 | 93 | 1.07 | 1 |
| Sex | ||||||||||||
| M | 14,189 | 43 | 1.11 | 0.94 (0.63–1.39) | 27,030.5 | 85 | 1.15 | 1.08 (0.77–1.51) | 33,852 | 97 | 1.04 | 1.09 (0.79–1.50) |
| F | 15,221.5 | 49 | 1.17 | 1 | 2,8490 | 83 | 1.06 | 1 | 33,852 | 93 | 0.94 | 1 |
| Age < 2 years | ||||||||||||
| Yes | 10,440.5 | 58 | 2.02 | 3.10 (2.07–4.63) | 1,9845 | 96 | 1.77 | 2.32 (1.74–3.10) | 25,091.5 | 108 | 1.57 | 2.33 (1.71–3.19) |
| No | 18,970 | 34 | 0.65 | 1 | 35,675.5 | 72 | 0.74 | 1 | 44,782.5 | 82 | 0.68 | 1 |
| Siblings < 5 years of age | ||||||||||||
| ≥ 1 | 13,401.5 | 41 | 1.12 | 0.98(0.66–1.46) | 25,886 | 83 | 1.17 | 1.13 (0.85–1.51) | 32,952.5 | 94 | 1.04 | 1.11 (0.83–1.49) |
| None | 16,009 | 51 | 1.16 | 1 | 29,634.5 | 85 | 1.05 | 1 | 36,921.5 | 96 | 0.95 | 1 |
| Household head has < 1 year of education | ||||||||||||
| Yes | 13,048 | 38 | 1.06 | 0.88 (0.59–1.33) | 25,056.5 | 85 | 1.24 | 1.26 (0.93–1.71) | 32,046 | 92 | 1.04 | 1.10 (0.81–1.49) |
| No | 16,331 | 54 | 1.21 | 1 | 30,408 | 83 | 1.00 | 1 | 37,754.5 | 98 | 0.95 | 1 |
| Household has latrine | ||||||||||||
| Yes | 20,601 | 62 | 1.10 | 0.91(0.57–1.47) | 38,829 | 103 | 0.97 | 0.66 (0.49–0.89) | 48,853 | 139 | 1.04 | 1.10 (0.74–1.63) |
| No | 8,484 | 27 | 1.16 | 1 | 16,114 | 64 | 1.45 | 1 | 20,202 | 49 | 0.89 | 1 |
| Fresh water source ≥ 30 minutes away in dry season | ||||||||||||
| Yes | 20,909 | 64 | 1.12 | 0.93 (0.57–1.53) | 40,425 | 116 | 1.05 | 0.84 (0.59–1.20) | 51,670.5 | 142 | 1.00 | 0.98 (0.66–1.47) |
| No | 8,414 | 28 | 1.21 | 1 | 14,927.5 | 51 | 1.25 | 1 | 17,969 | 48 | 0.98 | 1 |
| Treated with amoxicillin for ALRI during follow-up period | ||||||||||||
| Yes | 9,950.5 | 44 | 1.61 | 1.81 (1.20–2.74) | 22,942.5 | 101 | 1.61 | 2.08 (1.47–2.94) | 41,051 | 150 | 1.33 | 2.45 (1.76–3.41) |
| No | 19,460 | 48 | 0.90 | 1 | 32,578 | 67 | 0.75 | 1 | 17,969 | 40 | 0.51 | 1 |
Standard errors corrected for clustering at the balozi (neighborhood) level. Values in bold are statistically significant. RR = risk ratios; CI = confidence interval; MDA = mass distribution of azithromycin; ALRI = acute lower respiratory illness.
Children less than 24 months of age were more than twice as likely to experience diarrhea than older children (RR = 2.32, 95% CI = 1.74–3.10). The risk of diarrhea associated with amoxicillin treatment was also observed in this period; treated children had more than twice the risk of diarrhea as untreated children (RR = 2.08, 95% CI = 1.47–2.94). In addition, presence of a latrine in the household was associated with a 34% decreased risk of diarrhea during the period (RR = 0.66, 95% CI = 0.49–0.89). By the 3–6-month follow-up period, MDA was no longer protective against diarrhea (RR = 0.88. 95% CI = 0.61–1.27) and children less than 24 months of age maintained a two-fold increased risk of diarrhea than those more than 24 months of age (RR = 2.32, 95% CI = 1.74–3.10). In addition, the use of amoxicillin remained associated with increased risk of diarrhea (RR = 2.45, 95% CI = 1.76–3.41). Sex, having a sibling less than five years of age, education level of the head of household, and distance to water were not associated with risk of diarrhea during follow-up.
Multivariate analysis.
Multivariate analyses showed that distance to fresh water had no impact on the association between MDA exposure and incidence of diarrhea. After adjusting for the influence of young age, presence of a latrine in the household, treatment of acute respiratory illness, and clustering in the multivariate analysis, living in an MDA village was associated with decreases in the risk of diarrhea of 39% (RR = 0.61, 95% CI = 0.39–0.95), 24% (RR = 0.76, 95% CI = 0.54–1.07), and 15% (RR = 0.85, 95% CI = 0.60–1.20) in the 0–1-months, 1–3-months, and 3–6-months follow-up periods, respectively (Table 4). The effect was significant in the first month of follow-up. There were no significant differences in the incidence of diarrhea between groups in the 1–3-months and 3–6-months periods.
Table 4.
Association between exposure status and risk of diarrhea among 1,036 children less than five years of age living in Kongwa, Tanzania, by follow-up period*
| Risk factor | 0–1 months, rate ratio (95% CI) | 1–3 months, rate ratio (95% CI) | 3–6 months, rate ratio (95% CI) |
|---|---|---|---|
| Lives in MDA village | |||
| Yes | 0.61 (0.39–0.95) | 0.76 (0.54–1.07) | 0.85 (0.60–1.20) |
| No | 1 | 1 | 1 |
Adjusted for effects of child's age < 2 years at enrollment, presence of a toilet in the household, and antibiotic treatment of acute lower respiratory illness during follow-up period. Standard errors corrected for clustering. CI = confidence interval; MDA = mass distribution of azithromycin.
Discussion
In this prospective, longitudinal study, we observed that MDA for trachoma was associated with reduced incidence of diarrheal episodes among young children in rural Tanzania. Although the effect is significant during the time of greatest blood concentrations of the antibiotic, it wanes until by three months to six months no statistically significant further benefit was observed. The results were not confounded by the use of antibiotics for the treatment of acute respiratory illness. These findings are consistent with the results of previous studies evaluating the ancillary effects of MDA in trachoma-prevalent areas. In western Nepal, researchers assessed the effect of azithromycin on morbidity among children 1–10 years of age with surveys at baseline, and again 10 and 180 days post-treatment.20 They reported a 65% decrease from the baseline diarrhea prevalence among children who received treatment compared with a 5% reduction in the control group 10 days after mass treatment. At 180 days, the prevalence rates for both groups were similar to their respective baseline rates. Similarly, Whitty and others compared morbidity rates in children in the Gambia 5 months–14 years of age living in communities randomized to receive three doses of oral azithromycin at weekly intervals or daily treatment with topical tetracycline for 42 days.21 These researchers conducted morbidity surveys at 0, 7, 14, 21, and 28 days after treatment. Their results showed that children in the azithromycin group experienced 40% fewer diarrheal cases than those the tetracycline group at day 14 and the effect was sustained through day 28. Variations in the magnitude and duration of the treatment effect between studies may partially be explained by differences in study design, participant age ranges, and timing of the intervention.
Unlike the current study, the children in both of the aforementioned studies included children more than five years of age and did not evaluate incident diarrhea. We anticipated that the greatest impact would be among the youngest age group in which the risk for diarrhea is highest. Moreover, neither of the previous studies assessed the effect of treatment of longer periods, notably in the period between 29 and 179 days. The studies also differed in the timing of treatment. The magnitude of the treatment effect is likely to be dependent on the seasonal distribution of diarrhea cases caused by susceptible bacterial pathogens. The treatment effect is likely to highest during the season when the incidence of these cases is greatest. This finding has important implications for maximizing the ancillary benefits of mass treatment with azithromycin for trachoma control. Cumulatively, these findings suggest that MDA for treatment and control of trachoma may have short-term, beneficial impact on reducing the burden of enteric infections caused by bacterial pathogens.
With regard to the association between amoxicillin therapy and risk of diarrhea, evidence from previous studies indicates that mild diarrhea is a frequent adverse effect of antibiotic therapy, including amoxicillin with the incidence varying between 5% and 25%. Onset of diarrhea after antibiotic use is frequently linked to disruption of the function of gut microflora or overgrowth of pathogenic bacteria.22–24
One of the potential limitations of this study is that it was an observational study; participants were not randomized to treatment nor were they and the field study team blinded to the study medication. Therefore, information and recall bias or confounding may have accounted for the observed effect. We believe this possibility is unlikely because azithromycin was given for trachoma control, and residents were unlikely to associate it diarrhea episodes, especially months later. Because everyone in the MDA communities was offered azithromycin, it was not biased by evaluating those who chose not to have azithromycin versus those that did. Decisions regarding the selection of villages for MDA were made at the district level prior to the study; we selected eight villages for the cohort study. Children in the MDA villages were evaluated on an intent for treatment basis and to the extent they did not receive treatment would bias the findings towards the null. Because coverage was high, almost all children received azithromycin. Furthermore, our study has findings comparable with other studies, and has extended the findings to determine when the effect might wane. In addition, we adjusted our analyses for demographic and household characteristics, antibiotic treatment of acute respiratory infection, and clustering. However, we cannot rule out that children in trachoma villages were at reduced risk of diarrheal disease, but this seems unlikely because they seemed to live in more disadvantaged households, and we observed that there was no difference in diarrheal incidence by exposure status in the 3–6-month period, well after any effect of azithromycin would be expected. In fact, our findings were similar to those of Fry and others,20 who found no difference in prevalent diarrhea at six months post-MDA.
We also found that previous use of amoxicillin to treat ARI was associated with increased risk of diarrhea. Evidence from previous studies indicates that mild diarrhea is a frequent adverse effect of antibiotic therapy, including amoxicillin with the incidence varying between 5% and 25%. Onset of diarrhea after antibiotic use is frequently linked to disruption of the function of gut microflora or overgrowth of pathogenic bacteria.22–24 The consistency of this finding over the course of the study suggests that this was a real factor.
However, any potential ancillary benefits of MDA must be weighed against its possible adverse effects. There are concerns that MDA over multiple rounds may result in emergence and spread of antimicrobial drug resistance that could undermine the efficacy of first-line therapies used in developing countries to treat serious childhood infections. Additionally, use of antibiotics may lead to colonization with drug-resistant bacteria. Evidence from studies conducted prior to 2001, which were conducted in populations with little exposure to azithromycin, describe temporary increases in the prevalence of antimicrobial drug–resistant Streptococcus pneumoniae carriage in treated children.20,25–27 No drug-resistant isolates were detected six months after dosing. However, exposure to azithromycin and other broad-spectrum antibiotics has increased globally over the past decade, and many trachoma-endemic areas have received several rounds of treatment, which increases distribution of drug-resistant isolates and their duration in communities.28 Given this concern, we are analyzing nasopharyngeal S. pneumoniae specimens and diarrheal and non-diarrheal fecal samples from children in the study to assess the impact of azithromycin on the patterns of drug resistance among pathogenic and non-pathogenic bacteria.
In summary, our data suggest an ancillary benefit from MDA in reducing episodes of diarrheal disease in children in addition to reducing trachoma prevalence. A protective effect was observed up to three months after treatment. Knowledge of the seasonal distribution of diarrheagenic bacteria in particular regions could be used to optimize the timing of a mass treatment to maximize the impact on bacterial infections. More studies are needed to evaluate the impact of mass treatment on diarrheal mortality and consideration should be given to monitoring the long-term impact of mass treatment on antimicrobial drug resistance.
Footnotes
Financial support: This study was supported by a grant from the Bill and Melinda Gates Foundation (Seattle, WA) (#48027) and an unrestricted grant from Research to Prevent Blindness. Pfizer, Inc. donated the azithromycin for the study. Sheila West is supported by a Senior Scientific Award from Research to Prevent Blindness.
Disclosure: None of the authors have any conflicts of interest.
Authors' addresses: Christian L. Coles and Jessica C. Seidman, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, E-mails: ccoles@jhsph.edu and jseidman@jhsph.edu. Joshua Levens, Beatriz Munoz, and Sheila West, Dana Center for Preventive Ophthalmology, Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, E-mails: joshualevens@gmail.com, bmunoz@jhmi.edu, and shwest@jhmi.edu. Harran Mkoche, Trachoma Project Kongwa, Tanzania c/o Dana Center for Preventive Ophthalmology, Wilmer Eye Institute, Johns Hopkins University, Baltimore, MD, E-mail: harry_mkocha2@yahoo.com.
References
- 1.Mariotti SP, Pascolini D, Rose-Nussbaumer J. Trachoma: global magnitude of a preventable cause of blindness. Br J Ophthalmol. 2009;93:563–568. doi: 10.1136/bjo.2008.148494. [DOI] [PubMed] [Google Scholar]
- 2.Wright HR, Turner A, Taylor HR. Trachoma. Lancet. 2008;371:1945–1954. doi: 10.1016/S0140-6736(08)60836-3. [DOI] [PubMed] [Google Scholar]
- 3.Chidambaram JD, Alemayehu W, Melese M, Lakew T, Yi E, House J, Cevallos V, Zhou Z, Maxey K, Lee DC, Shapiro BL, Srinivasan M, Porco T, Whitcher JP, Gaynor BD, Lietman TM. Effect of a single mass antibiotic distribution on the prevalence of infectious trachoma. JAMA. 2006;295:1142–1146. doi: 10.1001/jama.295.10.1142. [DOI] [PubMed] [Google Scholar]
- 4.West SK, Munoz B, Mkocha H, Holland MJ, Aguirre A, Solomon AW, Foster A, Bailey RL, Mabey DC. Infection with Chlamydia trachomatis after mass treatment of a trachoma hyperendemic community in Tanzania: a longitudinal study. Lancet. 2005;366:1296–1300. doi: 10.1016/S0140-6736(05)67529-0. [DOI] [PubMed] [Google Scholar]
- 5.Solomon AW, Holland MJ, Alexander ND, Massae PA, Aguirre A, Natividad-Sancho A, Molina S, Safari S, Shao JF, Courtright P, Peeling RW, West SK, Bailey RL, Foster A, Mabey DC. Mass treatment with single-dose azithromycin for trachoma. N Engl J Med. 2004;351:1962–1971. doi: 10.1056/NEJMoa040979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariotti SP. New steps toward eliminating blinding trachoma. N Engl J Med. 2004;351:2004–2007. doi: 10.1056/NEJMe048205. [DOI] [PubMed] [Google Scholar]
- 7.Negrel AD, Mariotti SP. WHO alliance for the global elimination of blinding trachoma and the potential use of azithromycin. Int J Antimicrob Agents. 1998;10:259–262. doi: 10.1016/s0924-8579(98)00046-6. [DOI] [PubMed] [Google Scholar]
- 8.Diniz-Santos DR, Silva LR, Silva N. Antibiotics for the empirical treatment of acute infectious diarrhea in children. Braz J Infect Dis. 2006;10:217–227. doi: 10.1590/s1413-86702006000300011. [DOI] [PubMed] [Google Scholar]
- 9.Carbon C, Poole MD. The role of newer macrolides in the treatment of community-acquired respiratory tract infection. A review of experimental and clinical data. J Chemother. 1999;11:107–118. doi: 10.1179/joc.1999.11.2.107. [DOI] [PubMed] [Google Scholar]
- 10.DuPont HL. Azithromycin for the self-treatment of traveler's diarrhea. Clin Infect Dis. 2007;44:347–349. doi: 10.1086/510594. [DOI] [PubMed] [Google Scholar]
- 11.O'Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005;16:125–136. doi: 10.1053/j.spid.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization . The Global Burden of Disease: 2004 update. 16–27. Geneva: World Health Organization; 2008. pp. 16–27. [Google Scholar]
- 13.Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, Guyatt H, Lima AM, Guerrant RL. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J. 2006;25:513–520. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- 14.Niehaus MD, Moore SR, Patrick PD, Derr LL, Lorntz B, Lima AA, Guerrant RL. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg. 2002;66:590–593. doi: 10.4269/ajtmh.2002.66.590. [DOI] [PubMed] [Google Scholar]
- 15.Lanata CF, Mendoza W. Improving Diarrhea Estimates. Geneva: World Health Organization; 2002. [Google Scholar]
- 16.Yates J. Traveler's diarrhea. Am Fam Physician. 2005;71:2095–2100. [PubMed] [Google Scholar]
- 17.Khan WA, Seas C, Dhar U, Salam MA, Bennish ML. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. A double-blind, randomized, controlled trial. Ann Intern Med. 1997;126:697–703. doi: 10.7326/0003-4819-126-9-199705010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Tribble DR, Sanders JW, Pang LW, Mason C, Pitarangsi C, Baqar S, Armstrong A, Hshieh P, Fox A, Maley EA, Lebron C, Faix DJ, Lawler JV, Nayak G, Lewis M, Bodhidatta L, Scott DA. Traveler's diarrhea in Thailand: randomized, double-blind trial comparing single-dose and 3-day azithromycin-based regimens with a 3-day levofloxacin regimen. Clin Infect Dis. 2007;44:338–346. doi: 10.1086/510589. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization . Integrated Management of Childhood Illness. Geneva: World Health Organization; 2008. [Google Scholar]
- 20.Fry AM, Jha HC, Lietman TM, Chaudhary JS, Bhatta RC, Elliott J, Hyde T, Schuchat A, Gaynor B, Dowell SF. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis. 2002;35:395–402. doi: 10.1086/341414. [DOI] [PubMed] [Google Scholar]
- 21.Whitty CJ, Glasgow KW, Sadiq ST, Mabey DC, Bailey R. Impact of community-based mass treatment for trachoma with oral azithromycin on general morbidity in Gambian children. Pediatr Infect Dis J. 1999;18:955–958. doi: 10.1097/00006454-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Ayyagari A, Agarwal J, Garg A. Antibiotic associated diarrhoea: infectious causes. Indian J Med Microbiol. 2003;21:6–11. [PubMed] [Google Scholar]
- 23.Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med. 2002;346:334–339. doi: 10.1056/NEJMcp011603. [DOI] [PubMed] [Google Scholar]
- 24.Hogenauer C, Hammer HF, Krejs GJ, Reisinger EC. Mechanisms and management of antibiotic-associated diarrhea. Clin Infect Dis. 1998;27:702–710. doi: 10.1086/514958. [DOI] [PubMed] [Google Scholar]
- 25.Batt SL, Charalambous BM, Solomon AW, Knirsch C, Massae PA, Safari S, Sam NE, Everett D, Mabey DC, Gillespie SH. Impact of azithromycin administration for trachoma control on the carriage of antibiotic-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 2003;47:2765–2769. doi: 10.1128/AAC.47.9.2765-2769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaynor BD, Holbrook KA, Whitcher JP, Holm SO, Jha HC, Chaudhary JS, Bhatta RC, Lietman T. Community treatment with azithromycin for trachoma is not associated with antibiotic resistance in Streptococcus pneumoniae at 1 year. Br J Ophthalmol. 2003;87:147–148. doi: 10.1136/bjo.87.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leach AJ, Shelby-James TM, Mayo M, Gratten M, Laming AC, Currie BJ, Mathews JD. A prospective study of the impact of community-based azithromycin treatment of trachoma on carriage and resistance of Streptococcus pneumoniae. Clin Infect Dis. 1997;24:356–362. doi: 10.1093/clinids/24.3.356. [DOI] [PubMed] [Google Scholar]
- 28.Haug S, Lakew T, Habtemariam G, Alemayehu W, Cevallos V, Zhou Z, House J, Ray K, Porco T, Rutar T, Keenan J, Lietman TM, Gaynor BD. The decline of pneumococcal resistance after cessation of mass antibiotic distributions for trachoma. Clin Infect Dis. 2010;51:571–574. doi: 10.1086/655697. [DOI] [PubMed] [Google Scholar]


