Abstract
Data relating to acute post-streptococcal glomerulonephritis (APSGN) from the notifiable diseases surveillance system in the Northern Territory of Australia was extracted and analyzed. Isolates of Streptococcus pyogenes from confirmed cases were emm sequence typed. From 1991 to July 2008, there were 415 confirmed cases and 23 probable cases of APSGN notified. Four hundred fifteen (94.7%) of these were Indigenous Australians and 428 (97.7%) were people living in remote or very remote locations. The median age of cases was 7 years (range 0–54). The incidence of confirmed cases was 12.5/100,000 person-years, with an incidence in Indigenous Australian children younger than 15 years of age of 94.3 cases/100,000 person-years. The overall rate ratio of confirmed cases in Indigenous Australians to non-Indigenous Australians was 53.6 (95% confidence interval 32.6–94.8). Outbreaks of disease across multiple communities occurred in 1995 (N = 68), 2000 (N = 55), and 2005 (N = 87 [confirmed cases]). Various emm types of S. pyogenes were isolated from cases of APSGN including some types not previously recognized to be nephritogenic. The widespread outbreak in 2005 was caused by emm55.0 S. pyogenes. Acute post-streptococcal glomerulonephritis continues to occur in remote Indigenous communities in Australia at rates comparable to or higher than those estimated in developing countries. Improvements in preventative and outbreak control strategies are needed.
Introduction
The epidemiology of acute post-streptococcal glomerulonephritis (APSGN) has changed substantially over the past 50 years. Before the 1980s APGSN was relatively common worldwide with multiple large and recurrent epidemics reported, especially in the Native Americans in the United States and in Central and South America.1 Many of these epidemics were thought to be related to streptococcal skin rather than throat infection, often associated with preceding scabies.2,3 Over the past 20 years there has been a substantial decline in the reported incidence of APSGN in many industrialized countries.1,3–6
Despite this declining incidence of APSGN in many developed countries, there is still a significant global burden of disease. It has been estimated that there are more than 470,000 cases of APSGN worldwide annually with ~5,000 deaths, with 97% occurring in less developed countries.7 It is likely that APSGN is underreported in many developing countries and these figures are likely to be an underestimate of the true burden of this condition.
As in most developed countries, the southern temperate regions of Australia have sporadic cases of APSGN, mostly related to pharyngitis.8 In contrast, in tropical northern Australia APSGN is far more common and outbreaks have previously been reported in Aboriginal communities.9,10 In the Northern Territory (NT), which comprises a semi-arid central Australian region and a tropical northern region, sporadic cases occur each year but also larger outbreaks in the northern region are documented by public health authorities around every 5–7 years.11 The majority of these cases are related to pyoderma caused by infection with Streptococcus pyogenes (Group A Streptococcus [GAS]), often with underlying scabies.12 The overall incidence and patterns of disease in this population have not been characterized.
It has long been recognized that certain GAS M protein types, as now determined by the emm genotype (emm sequence type), are associated with nephritis and these are mostly different from the emm types that cause acute rheumatic fever.13 Previous studies of the molecular epidemiology of GAS in Aboriginal communities of the NT have shown that there is a wide diversity of emm types present. The GAS carriage is dynamic with high acquisition rates within households.14,15
We reviewed the epidemiology of APSGN within the NT over a 16-year period with the aim of determining the current incidence, patterns of disease, and diversity of emm sequence types of GAS responsible for APSGN in the region. We document that despite declining rates of APSGN in other industrialized countries, rates remain very high for children living in remote Indigenous Australian communities.
Materials and Methods
Setting.
The NT is sparsely populated with a population of ~195,000 in 2000. It occupies 15% of the land mass of Australia but only 1% of the population. The northern third, referred to as the “Top End” has a tropical climate, whereas the southern two-thirds are referred to as “Central Australia” with a much drier climate year round.
There are ~65,000 Indigenous Australians in the NT, representing around 30% of the population. In comparison, Indigenous Australians make up only 2.4% of the total Australian population and comprise both Aboriginal and Torres Strait Islanders.16 In the NT, 80% of Indigenous Australians live in remote or very remote locations. Indigenous Australians have poorer health outcomes than non-Indigenous Australians with a life expectancy that is estimated to be almost 20 years less than non-Indigenous Australians.16
The Top End of the NT has two seasons. The “dry” season runs from April to September when the weather is cooler, less humid, and there is little rain. The monsoonal “wet” season runs from October to the end of March and during this period it is hot, humid, and rainy. In Central Australia there is a desert climate with occasional rain throughout the year. During the period of April–September the weather is cooler than the months of October–March.
Epidemiological data.
Epidemiological data was obtained from the Northern Territory Center for Disease Control (NTCDC). Acute post-streptococcal glomerulonephritis is a notifiable disease in the Northern Territory and electronic collection of data were established in 1991. The case definition of a confirmed case is a clinically compatible illness (two of hematuria, hypertension, and facial or peripheral edema) and laboratory evidence including hematuria (> 10 red blood cells/μL), plus evidence of streptococcal infection with either positive streptococcal serology (anti-streptolysin O[ASO] > 250 IU/mL, anti-DNAse B > 200 IU/mL), or positive culture from throat or skin plus a reduced C3 level (normal 0.86–1.84 g/L). Probable cases were defined as subclinical cases with laboratory evidence only.
Patients were defined as living in a remote or very remote location if their place of residence had a score of 5.92 or more using the Accessibility/Remoteness Index of Australia (ARIA).17 Notifications of cases from outside the Northern Territory (often communities close to the borders) were excluded from incidence calculations but included in other analyses.
Source of streptococcal isolates and laboratory methods.
All emm sequence typing of S. pyogenes isolates was performed at the Menzies School of Health Research (MSHR) streptococcal research laboratory in Darwin. The streptococcal isolates used in this study were collected between 1990 and 2008 and included isolates from community screening projects run through MSHR and isolates from patients and contacts cultured at the Royal Darwin Hospital microbiology laboratory or the local private pathology laboratory that services many of the remote communities (Western Diagnostic Pathology). Isolates were included in this study if they were collected from a skin sore swab or throat swab from a patient confirmed to have APSGN using the above diagnostic criteria. Swabs were performed at the discretion of the treating clinician and not all patients had both skin swabs and throat swabs performed. The vast majority of positive cultures were S. pyogenes from skin sore swabs. The NTCDC notification database and hospital patient records were used to obtain epidemiological, clinical, and laboratory data. If a case had not been notified to NTCDC but clinical information and pathology results were available to confirm a diagnosis, that case was included.
In addition to bacterial isolates from confirmed cases, 16 isolates were available from contacts and community screening done in one community during a widespread outbreak in 2005. These were also emm sequence typed to gain further insight into the streptococcal strains responsible for this large outbreak.
The streptococcal isolates underwent emm sequence typing based on methods described by the U.S. Centers for Disease Control and Prevention (CDC) with minor modifications as previously described.14,15 Genetic sequences were compared with the S. pyogenes emm sequence database on the CDC website18 to determine emm types and subtypes.
Analytical methods.
Incidences were expressed as rates/100,000 person-years from years 1992 to 2007. Population data was obtained from Northern Territory Department of Health and Families and Australian Bureau of Statistics.19 Confidence intervals (CI) were calculated assuming the Poisson distribution using Stata 9 (College Station, TX). Proportions were compared using a χ2 test. A P value of < 0.05 was used as the level of significance.
Ethics.
Ethics approval was obtained from the Human Research Ethics Committee of the Northern Territory Department of Health and Families and the Menzies School of Health Research (approval no. 08/34). This included approval from the Aboriginal Ethics Sub-Committee.
Results
Epidemiology.
From 1991 until July 2008 there were 415 confirmed cases, including 13 cases notified in people living in communities outside the NT. There were 23 probable cases of APSGN reported that were excluded from analysis of incidence. The demographic details of the study population are displayed in Table 1. The majority of cases occurred in Indigenous Australian children who lived in remote locations. Figure 1 shows the age distribution of cases with 386 (88.1%) occurring in children < 15 years of age and an overall age range of 0–54 years (median 7 years). Figure 2 is a map of the local community incidence of cases in children 0–14 years of age, which shows geographical distribution of cases within the NT with a predominance seen in the Top End.
Table 1.
Demographic characteristics of study population
| Characteristic | Confirmed (N = 415) (%) | Probable (N = 23) (%) | Total (%) |
|---|---|---|---|
| Median age (range) | 7 (0–54) | 5 (1–11) | 7 (0–54) |
| Sex | |||
| Male | 206 (49.6) | 17 (73.9) | 226 (51.6) |
| Female | 209 (50.4) | 6 (24.1) | 215 (48.4) |
| Indigenous status | |||
| Indigenous | 392 (94.5) | 23 (100) | 415 (94.7) |
| Non-indigenous | 16 (3.9) | 0 | 16 (3.7) |
| Unknown | 7 (1.7) | 0 | 7 (1.6) |
| Remote location of residence | 405 (97.6) | 23 (100) | 428 (97.7) |
Figure 1.
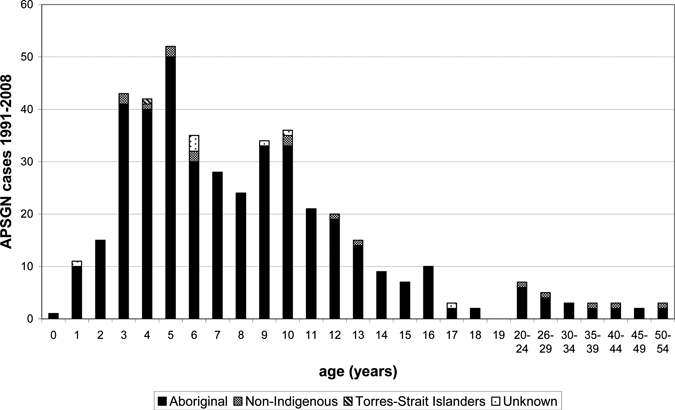
Age distribution of cases of acute post-streptococcal glomerulonephritis (APSGN).
Figure 2.
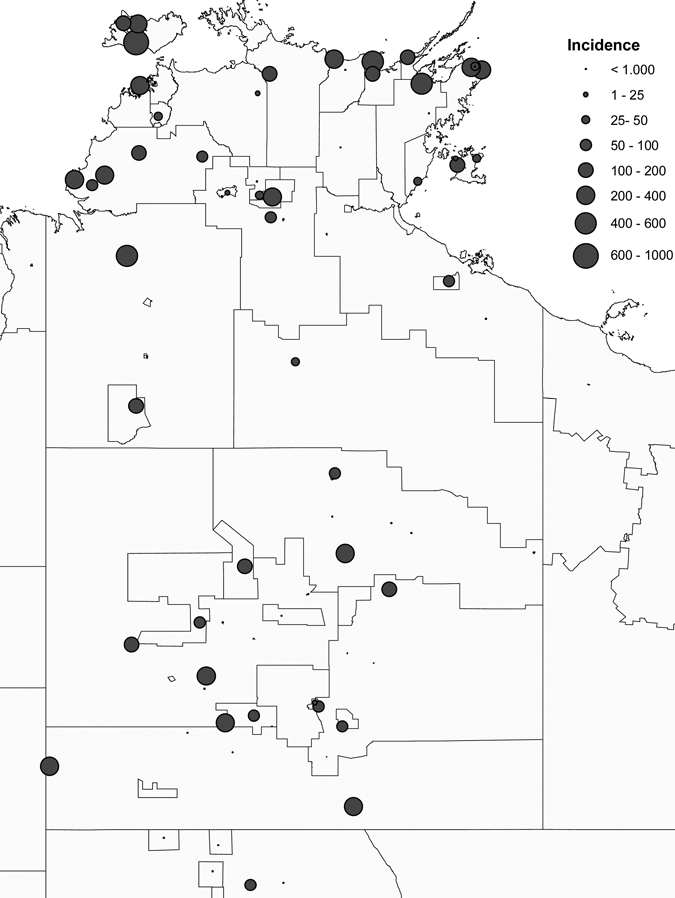
Local community incidence of acute post-streptococcal glomerulonephritis (APSGN) in children 0–14 years of age (annual incidence/100,000 person-years).
The incidence rates for different populations within the NT are shown in Table 2. The highest incidence of 94.3 cases/100,000 person-years occurred in Indigenous children < 15 years of age. The rate ratio of cases in Indigenous Australians to non-Indigenous Australians was 53.6 (95% CI = 32.6–94.8).
Table 2.
Incidence rates of confirmed acute post-streptococcal glomerulonephritis (APSGN) in Northern Territory, Australia*
| Age | Population estimates (2007)† | Person-years | Number of confirmed cases notified 1992–2007 | Annual incidence/100,000 persons | 95% CI | |
|---|---|---|---|---|---|---|
| Total population | All ages | 214,929 | 3,067,895 | 383 | 12.5 | (11.3–13.8) |
| 0–14 years | 51,765 | 800,283 | 331 | 41.4 | (37.0–46.1) | |
| > 14 years | 163,164 | 2,267,612 | 48 | 2.1 | (1.6–2.8) | |
| Indigenous | All ages | 65,159 | 906,670 | 360 | 39.7 | (35.7–44.0) |
| 0–14 years | 22,540 | 332,981 | 314 | 94.3 | (84.2–105.3) | |
| > 14 years | 42,619 | 573,689 | 42 | 7.3 | (5.3–9.9) | |
| Non-indigenous | All ages | 149,770 | 2,161,225 | 16 | 0.74 | (0.42–1.2) |
| 0–14 years | 29,225 | 467,302 | 11 | 2.3 | (1.2–4.2) | |
| > 14 years | 120,545 | 1,693,923 | 5 | 0.30 | (0.095–0.69) |
CI = confidence interval.
Sum of annual estimated NT population (Department of Health Gains Planning).
The incidence of APSGN varied from year to year reflecting the occurrence of yearly sporadic cases and small clusters, with the occurrence of larger more widespread outbreaks approximately every 5 years (see Figure 3). These outbreaks predominantly affect those < 15 years of age. During the study period there were peaks of notifications in 1995 (N = 68), 2000 (N = 55), and 2005 (N = 87 [confirmed cases]).
Figure 3.
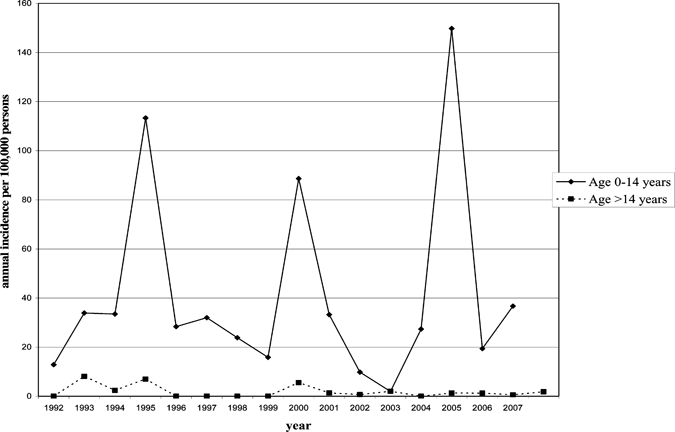
Annual incidence of acute post-streptococcal glomerulonephritis (APSGN) in the Northern Territory (NT)
Seasonal distribution.
In the Top End there was a peak of notifications from April to June in the early dry season, whereas in Central Australia there was no significant seasonal change (see Figure 4). In the Top End 64.7% of cases occurred in the dry and 35.3% in the wet (P < 0.0001). This seasonal distribution within the Top End may reflect a larger number of outbreaks occurring at this time.
Figure 4.
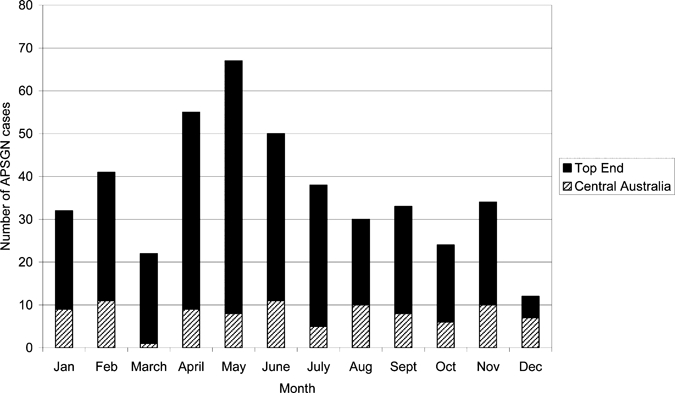
Notifications/month in Top End and Central Australian region
Emm sequence types.
Cross-referencing between the Menzies School of Health Research streptococcal isolate database, the NTCDC notification database, and hospital clinical records identified isolates associated with 20 confirmed cases. Seven cases from 1991 and 1992 included in the analysis were identified from the MSHR streptococcal database and confirmed by clinical records but had not been notified to NTCDC. All GAS emm types found were represented in skin sore isolates. In two APSGN cases the same GAS emm type was cultured from throat swabs and from a skin sore swab; emm55.0 in one case and emm68.2 in the other. There were no GAS isolates found only in throat swabs, although throat swabs were not collected in all cases. Table 3 shows the emm types isolated during the study.
Table 3.
The emm sequence type of APSGN isolates
| Year | emmST | Outbreak cases | Sporadic cases | Community | Other/community screening |
|---|---|---|---|---|---|
| 1991 | 57.2 | 1 | 2* | A (outbreak case), B, C | |
| 91.0 | 1 | A | |||
| 1992 | 55.0 | 1 | D | ||
| 98.1 | 1 | E | |||
| 68.2 | 1 | F | |||
| 1995 | 19.7 | 1 | G | 1 unconfirmed case community G† | |
| 1–4.0 | 1 | H | |||
| 2000 | 3.22 | 3 | I, J, K | 1 unconfirmed in community J† | |
| 49.3 | 1 | L | |||
| 70.0 | 1 | I | |||
| 85.0 | 1 | L | |||
| 2001 | 105.0 | 1 | L | ||
| 2005 | 55.0 | 4 | L, J, C, N | 1 subclinical case in community J 4 case contacts from community L |
Limited data/case reporting thus unable to confirm whether outbreak/sporadic.
Additional isolates from possible cases in communities with outbreaks that have not been able to be confirmed retrospectively.
Emm55.0 GAS was isolated from four cases during the 2005 outbreak from four geographically isolated communities within the NT. In addition, emm55.0 GAS was isolated from four of five case contacts in 2005. Emm55.0 was also isolated from a confirmed case in 1992.
In 1995, the year of the other large outbreak, emm19.7 GAS was isolated from a confirmed case in the community with the most cases. Emm19.7 was also isolated from a suspected case that could not be confirmed retrospectively. Emm1-4.0 GAS was isolated in the same year from a sporadic case. It is not known whether that person had been in contact with people from communities involved in the outbreak.
From the outbreak in 2000 emm3.22 GAS was isolated from three different communities in one district. Emm70.0 GAS was also isolated from a case in one of these communities. From a community in a different district that had 13 confirmed cases, emm49.3 and emm85.0 GAS were isolated.
Discussion
This study demonstrates that despite a decline in the incidence of APSGN seen in many parts of the industrialized world, rates of APSGN in the NT remain high in the Indigenous Australian population. Recent studies have estimated an annual incidence of APSGN in developing countries of between 9.3 and 28.5 cases/100,000 population.1,7 This is comparable to our overall estimated incidence in the NT of 12.5 cases/100,000 person-years. However, the rates in the Indigenous Australian population are significantly higher. The incidence in Indigenous Australian children in the Northern Territory < 15 years of age of 94.3 cases/100,000 is almost double the rate of 50.5 cases/100,000 reported in Maori children in New Zealand in the 1980s,20 and is more than three times the estimated rate for children in the developing world calculated by Carapetis and others.7 In one small Aboriginal community the incidence in children < 15 years of age over the study period was 891 cases/100,000 person-years, accepting that the denominator is small in calculating this figure. Indigenous Australians have also previously been reported to have extremely high rates of rheumatic heart disease.21
The short term prognosis for children with APSGN is generally good with a mortality of < 0.5% and fewer than 2% progressing to end-stage renal failure.22 The long-term implications of APSGN are less clear with studies reporting mixed outcomes.23 Although many studies have reported favorable outcomes, some are less reassuring, especially in the Indigenous Australian population. A recent study has found significantly higher rates of albuminuria in Indigenous Australians with previous APSGN compared with controls.24 Given albuminuria is a marker of early chronic kidney disease, this suggests that APSGN may be contributing to the extremely high rates of chronic renal failure seen in Indigenous Australian adults.25 The incidence rates in this study included only notified confirmed cases. Case ascertainment and reporting is likely to be incomplete. For instance, since 2005, 23 cases have been notified on the basis of laboratory criteria alone (subclinical or probable cases) and these were not included in this analysis. The true incidence of APSGN is therefore likely to be considerably higher than that calculated in this study.
Although we were unable to definitively ascertain the site of underlying streptococcal infection in cases in this study, in previous reports of APSGN outbreaks within the NT the majority of cases have been attributed to impetigo. During the outbreak in 2000, 87% of cases were reported to have skin sores and 40% had scabies.12 Group A Streptococcus were isolated from 26% of cases, all of which were from skin sores. The prevalence of impetigo has been documented in up to 70% of children in Indigenous communities.26 In contrast, pharyngitis has been shown to be uncommon in indigenous communities.27 However, although we documented a seasonal distribution of APSGN in the Top End of the NT with a peak in the early dry season from April to June (generally corresponding to Top End outbreaks), there is not a clearly recognized seasonal distribution of pyoderma in this region. Although one previous study showed a peak in the dry season in one community,27 a three year surveillance study, as part of the “Healthy Skin Program,” did not show an overall seasonal change in prevalence of pyoderma or scabies.28
Although APSGN occurs after a streptococcal infection, isolation of GAS from confirmed cases was uncommon in this study and in part reflects a generally low rate of skin sore sampling in cases. Additional limitations to attributing APSGN to a specific emm type include the potential for simultaneous carriage of multiple types21 and the rapid clearance of specific types from households.14 Nevertheless, isolation of GAS from confirmed cases is generally regarded as evidence of a causative link, with isolation from other community members at the time of an outbreak providing circumstantial evidence of the potentially nephritogenic S. pyogenes.
In this study, 20 GAS isolates representing 12 different emm types from confirmed APSGN cases were identified. Sequence type emm55 GAS was identified as being associated with an outbreak and has also been reported from a previous APSGN outbreak in the NT in 198310 and from outbreaks in Trinidad29 and cases in Ethiopia.30 A prospective household study swabbing throats and skin sores for GAS in two of the outbreak communities,14 performed during the period of the APSGN outbreak in 2005, showed a peak of isolation of emm55 GAS corresponding with the outbreak. This study showed that emm55 GAS was the predominant type isolated in the communities during this time over a 3-month period and emm55 GAS was not isolated prior, or subsequently. This supports the theory that the nephritogenic GAS type introduced to the communities spreads quickly and then disappears, possibly corresponding with the development of type-specific immunity in the local population.
Other epidemic types found in this study include emm3.22 GAS, which was isolated from skin sores in three cases during the 2000 outbreak in three different communities in one district. Serotype M3 GAS has previously been associated with pharyngitis-associated APSGN in other countries31 but not definitively with pyoderma-associated APSGN. Serotype M3 GAS has also been recognized as having a predilection for throat rather than for skin. It was also the most common type isolated from the throat of 500 school staff and children screened in New Zealand in 1983 in response to a cluster of cases of acute rheumatic fever.32 However, all the emm3.22 GAS isolates in our collection were isolated from the skin sores. Emm49.0, emm70.0, and emm85.0 GAS were also isolated from individual cases in outbreak communities during 2000. Serotype M49 GAS has been widely reported as a causative strain of pyoderma-associated APSGN and has been responsible for previous outbreaks.20,33,34 Emm70 and emm85 GAS have not been previously reported to be associated with APSGN. It is possible that unlike the outbreak in 2005, which appears to be related to one type of GAS, there may have been multiple types responsible for the outbreak in 2000. There was only one isolate from a confirmed outbreak case in 1995, which was emm19.7 GAS that has not previously been reported in association with APSGN.
Of the GAS types found in sporadic cases of APSGN, emm57 is well recognized to cause pyoderma-associated APSGN.20,35 Emm68.2, emm91.0, emm98.1, and emm105.0 GAS have not previously been reported in association with APSGN, although it is possible that these more recently described types were not able to be identified by the serotyping methods used in older studies.
Of the 12 GAS emm types reported in this study, only two would be covered by the 26-valent M-protein-based GAS vaccine that has been under clinical investigation.36 A review of the global distribution of emm types by Steer and others37 found that this vaccine would provide poor coverage of emm types seen in Africa and the Pacific, highlighting the need for further molecular epidemiological data from these regions.
Although APSGN has classically been associated with infection with GAS, it has occasionally been reported to occur after infection with other streptococcal species.1 Lancefield group C and G streptococci (GCS/GGS) have been associated with APSGN, in particular Streptococcus zooepidemicus, which has been responsible for a number of APSGN outbreaks associated with unpasteurized milk.38,39 A recent surveillance study we undertook showed that GCS/GGS, especially Streptococcus dysgalactiae subsp. equisimilis are frequently isolated from the throats of Indigenous Australians in our region, although this represented asymptomatic carriage, with the study finding little evidence of GCS/GGS pharnygitis.27,40 The GCS/GGS, with the exception of S. zooepidemicus, can produce hemolysins that cross-react with streptolysins produced by GAS and can cause elevations of ASO.41 It is therefore possible that in some cases of reported APSGN in this study, elevations of streptococcal serology may be affected by the presence of GCS/GGS, although the lack of clinical pharyngitis from GCS/GGS makes this generally unlikely. The role if any of GCS/GGS as a potential causative agent of APGSN in this setting remains unclear but warrants further investigation. Nevertheless, given that the vast majority of APSGN cases in our region have been associated with pyoderma12 and that GAS has been the dominant Streptococcus species in pyoderma, with GCS/GGS mostly restricted to throat isolates,27,40 it is very likely that GAS accounts for the vast majority of APSGN cases and in particular the epidemics.
APSGN remains a significant health problem in Northern Australia. The rates of APSGN in the Indigenous Australian population are among the highest documented worldwide, accepting that in many countries case ascertainment and reporting is limited. In Australia, this condition predominantly affects Indigenous Australian children living in remote Indigenous communities in the sub-tropical and semi-arid areas of Australia. In these communities living conditions are often poor, with marked overcrowding of housing42 and often inadequate sanitation facilities,43 which together facilitate the rapid spread of GAS.44 Improving living conditions, housing, and education in remote communities is thus essential to reduce the rates of APSGN and other sequelae of GAS infection. Reporting of these rates has been made possible by the presence of a developed public health surveillance network in Australia. It is likely that the high rates of APSGN reported in this study do not reflect a problem only present in remote Indigenous Australian communities, but instead a global problem that is present in many developing countries but is underrecognized and underreported.
ACKNOWLEDGMENTS
We thank the staff at the Northern Territory Centre for Disease Control and the Royal Darwin Hospital Microbiology Laboratory for their work.
Footnotes
Financial support: This work was support by the National Health and Medical Research Council (grant no. 436009) and Menzies School of Health Research (treatise scholarship awarded to C.S.M.).
Authors' addresses: Catherine S. Marshall and Allen C. Cheng, Infectious Diseases Unit, Alfred Hospital, Level 2, Burnet Institute, Melbourne, Victoria, Australia, E-mails: casmarshall@hotmail.com and allen.cheng@med.monash.edu.au. Peter G. Markey, Lesley Scott, and Vicki L. Krause, Centre for Disease Control, Northern Territory Department of Health and Families, Building 4 Royal Darwin Hospital, Northern Territory, Australia, E-mails: peter.markey@nt.gov.au, lesley.scott@nt.gov.au, and vicki.krause@nt.gov.au. Rebecca J. Towers, Leisha J. Richardson, and Bart J. Currie, Tropical and Emerging Infectious Diseases Division, Menzies School of Health Research, Casuarina, Northern Territory, Australia, E-mails: rebecca.towers@menzies.edu.au, leisha.richardson@menzies.edu.au, and bart@menzies.edu.au. Peter K. Fagan, Serology Division, Pathology Department, Royal Darwin Hospital, Tiwi, Northern Territory, Australia, E-mail: peter.fagan@nt.gov.au.
References
- 1.Rodriguez-Iturbe B, Musser JM. The current state of poststreptococcal glomerulonephritis. J Am Soc Nephrol. 2008;19:1855–1864. doi: 10.1681/ASN.2008010092. [DOI] [PubMed] [Google Scholar]
- 2.Svartman M, Finklea JF, Earle DP, Potter EV, Poon-King T. Epidemic scabies and acute glomerulonephritis in Trinidad. Lancet. 1972;1:249–251. doi: 10.1016/s0140-6736(72)90634-4. [DOI] [PubMed] [Google Scholar]
- 3.Berrios X, Lagomarsino E, Solar E, Sandoval G, Guzman B, Riedel I. Post-streptococcal acute glomerulonephritis in Chile–20 years of experience. Pediatr Nephrol. 2004;19:306–312. doi: 10.1007/s00467-003-1340-9. [DOI] [PubMed] [Google Scholar]
- 4.Yap HK, Chia KS, Murugasu B, Saw AH, Tay JS, Ikshuvanam M, Tan KW, Cheng HK, Tan CL, Lim CH. Acute glomerulonephritis–changing patterns in Singapore children. Pediatr Nephrol. 1990;4:482–484. doi: 10.1007/BF00869825. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Shen Y, Feld LG, Stapleton FB. Changing pattern of glomerular disease at Beijing Children's Hospital. Clin Pediatr (Phila) 1994;33:542–547. doi: 10.1177/000992289403300906. [DOI] [PubMed] [Google Scholar]
- 6.Coppo R, Gianoglio B, Porcellini MG, Maringhini S. Frequency of renal diseases and clinical indications for renal biopsy in children (report of the Italian National Registry of Renal Biopsies in Children). Group of Renal Immunopathology of the Italian Society of Pediatric Nephrology and Group of Renal Immunopathology of the Italian Society of Nephrology. Nephrol Dial Transplant. 1998;13:293–297. doi: 10.1093/oxfordjournals.ndt.a027821. [DOI] [PubMed] [Google Scholar]
- 7.Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- 8.Blyth CC, Robertson PW, Rosenberg AR. Post-streptococcal glomerulonephritis in Sydney: a 16-year retrospective review. J Paediatr Child Health. 2007;43:446–450. doi: 10.1111/j.1440-1754.2007.01109.x. [DOI] [PubMed] [Google Scholar]
- 9.Streeton CL, Hanna JN, Messer RD, Merianos A. An epidemic of acute post-streptococcal glomerulonephritis among aboriginal children. J Paediatr Child Health. 1995;31:245–248. doi: 10.1111/j.1440-1754.1995.tb00795.x. [DOI] [PubMed] [Google Scholar]
- 10.Gogna NK, Nossar V, Walker AC. Epidemic of acute poststreptococcal glomerulonephritis in aboriginal communities. Med J Aust. 1983;1:64–66. doi: 10.5694/j.1326-5377.1983.tb136039.x. [DOI] [PubMed] [Google Scholar]
- 11.Evans C. Acute post sreptococcal glomerulonephritis in the Northern Territory 1980–2000. NT Dis Control Bull. 2001;8:1–40. [Google Scholar]
- 12.Kearns T, Evans C, Krause V. Outbreak of acute post streptococcal glomerulonephritis in the Northern Territory—2000. NT Dis Control Bulletin. 2001;8:6–14. [Google Scholar]
- 13.Rammelkamp CH, Jr, Weaver RS. Acute glomerulonephritis, the significance of the variations in the incidence of the disease. J Clin Invest. 1953;32:345–358. doi: 10.1172/JCI102745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald MI, Towers RJ, Andrews R, Benger N, Fagan P, Currie BJ, Carapetis JR. The dynamic nature of group A streptococcal epidemiology in tropical communities with high rates of rheumatic heart disease. Epidemiol Infect. 2007;136:529–539. doi: 10.1017/S0950268807008655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald MI, Towers RJ, Fagan P, Carapetis JR, Currie BJ. Molecular typing of Streptococcus pyogenes from remote Aboriginal communities where rheumatic fever is common and pyoderma is the predominant streptococcal infection. Epidemiol Infect. 2007;135:1398–1405. doi: 10.1017/S0950268807008023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Australian Institute of Health and Welfare Australia's Health 2006. 2006. http://www.aihw.gov.au/publications/index.cfm/title/10321 AIHW, ed. cat. no. AUS 73: AIHW. Available at. Accessed December 8, 2008.
- 17.GISCA About ARIA+ (Accessibility/Remoteness Index of Australia) 2008. http://www.gisca.adelaide.edu.au/products_services/ariav2_about.html Available at. Accessed October 20, 2008.
- 18.Centers for Disease Control and Prevention . Streptococcus pyogenes emm sequence database. Atlanta, GA: 2011. http://www.cdc.gov/ncidod/biotech/strep/strepblast.htm Available at. Accessed June 13, 2011. [Google Scholar]
- 19.Australian Bureau of Statistics Australia's Population: Australian Bureau of Statistics. 2009. http://www.abs.gov.au/websitedbs/D3310114.nsf/home/Home Available at. Accessed August 30, 2009.
- 20.Lennon D, Martin D, Wong E, Taylor LR. Longitudinal study of poststreptococcal disease in Auckland; rheumatic fever, glomerulonephritis, epidemiology and M typing 1981–86. N Z Med J. 1988;101:396–398. [PubMed] [Google Scholar]
- 21.Carapetis JR, Wolff DR, Currie BJ. Acute rheumatic fever and rheumatic heart disease in the top end of Australia's Northern Territory. Med J Aust. 1996;164:146–149. doi: 10.5694/j.1326-5377.1996.tb122012.x. [DOI] [PubMed] [Google Scholar]
- 22.Holm SE, Nordstrand A, Stevens DL, Norgren M. In: Streptococcal Infections: Clinical Aspects, Microbiology and Molecular Patheogenesis. Stevens DL, Kaplan EL, editors. New York: Oxford University Press; 2000. pp. 152–162. (Acute postreptococcal glomerulonephritis). [Google Scholar]
- 23.Eison TM, Ault BH, Jones DP, Chesney RW, Wyatt RJ. Post-streptococcal acute glomerulonephritis in children: clinical features and pathogenesis. Pediatr Nephrol. 2011;26:165–180. doi: 10.1007/s00467-010-1554-6. [DOI] [PubMed] [Google Scholar]
- 24.White AV, Hoy WE, McCred DA. Childhood post-streptococcal glomerulonephritis as a risk factor for chronic renal disease in later life. MJA. 2001;174:492–496. doi: 10.5694/j.1326-5377.2001.tb143394.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoy WE, Mathews JD, McCredie DA, Pugsley DJ, Hayhurst BG, Rees M, Kile E, Walker KA, Wang Z. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int. 1998;54:1296–1304. doi: 10.1046/j.1523-1755.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- 26.Carapetis JR, Connors C, Yarmirr D, Krause V, Currie BJ. Success of a scabies control program in an Australian aboriginal community. Pediatr Infect Dis J. 1997;16:494–499. doi: 10.1097/00006454-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 27.McDonald MI, Towers RJ, Andrews RM, Benger N, Currie BJ, Carapetis JR. Low rates of streptococcal pharyngitis and high rates of pyoderma in Australian aboriginal communities where acute rheumatic fever is hyperendemic. Clin Infect Dis. 2006;43:683–689. doi: 10.1086/506938. [DOI] [PubMed] [Google Scholar]
- 28.Andrews R, Kearns T, Connors C, Parker C, Carville K, Currie B, Carapetis J. A regional initiative to reduce skin infections among aboriginal children living in remote communities of the Northern Territory, Australia. PLoS Negl Trop Dis. 2009;3:e554. doi: 10.1371/journal.pntd.0000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid HF, Bassett DC, Gaworzewska E, Colman G, Poon-King T. Streptococcal serotypes newly associated with epidemic post-streptococcal acute glomerulonephritis. J Med Microbiol. 1990;32:111–114. doi: 10.1099/00222615-32-2-111. [DOI] [PubMed] [Google Scholar]
- 30.Tewodros W, Kronvall G. M protein gene (emm Type) analysis of group A beta-hemolytic streptococci from Ethiopia reveals unique patterns. J Clin Microbiol. 2005;43:4369–4376. doi: 10.1128/JCM.43.9.4369-4376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wannamaker LW. Differences between streptococcal infections of the throat and of the skin (second of two parts) N Engl J Med. 1970;282:23–31. doi: 10.1056/NEJM197001082820206. [DOI] [PubMed] [Google Scholar]
- 32.Martin DR, Meekin GE, Finch LA. Streptococci–are they different in New Zealand? N Z Med J. 1983;96:298–301. [PubMed] [Google Scholar]
- 33.Anthony B, Kaplan E, Chapman S, Quie P, Wannamaker L. Epidemic acute nephritis with reappearance of type 49 Streptococcus. Lancet. 1967;2:787–790. [Google Scholar]
- 34.Majeed HA, Yousof AM, Rotta J, Havlickpva H, Bahar G, Bahbahani K. Group A streptococcal strains in Kuwait: a nine-year prospective study of prevalence and associations. Pediatr Infect Dis J. 1992;11:295–300. [PubMed] [Google Scholar]
- 35.Potter EV, Ortiz JS, Sharrett AR, Burt EG, Bray JP, Finklea JF, Poon-King T, Earle DP. Changing types of nephritogenic streptococci in Trinidad. J Clin Invest. 1971;50:1197–1205. doi: 10.1172/JCI106597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, Baxendale DM, Reddish MA, Hu MC, Stroop SD, Linden J, Fries LF, Vink PE, Dale JB. Safety and immunogenicity of 26-valent group a Streptococcus vaccine in healthy adult volunteers. Clin Infect Dis. 2005;41:1114–1122. doi: 10.1086/444458. [DOI] [PubMed] [Google Scholar]
- 37.Steer AC, Law I, Matatolu L, Beall BW, Carapetis JR. Global emm type distribution of group A streptococci: systematic review and implications for vaccine development. Lancet Infect Dis. 2009;9:611–616. doi: 10.1016/S1473-3099(09)70178-1. [DOI] [PubMed] [Google Scholar]
- 38.Balter S, Benin A, Pinto SW, Teixeira LM, Alvim GG, Luna E, Jackson D, LaClaire L, Elliott J, Facklam R, Schuchat A. Epidemic nephritis in Nova Serrana, Brazil. Lancet. 2000;355:1776–1780. doi: 10.1016/s0140-6736(00)02265-0. [DOI] [PubMed] [Google Scholar]
- 39.Barnham M, Thornton TJ, Lange K. Nephritis caused by Streptococcus zooepidemicus (Lancefield group C) Lancet. 1983;1:945–948. doi: 10.1016/s0140-6736(83)92078-0. [DOI] [PubMed] [Google Scholar]
- 40.McDonald M, Towers RJ, Andrews RM, Carapetis JR, Currie BJ. Epidemiology of Streptococcus dysgalactiae subsp. tropical communities, Northern Australia. Emerg Infect Dis. 2007;13:1694–1700. doi: 10.3201/eid1311.061258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams GS. Group C and G streptococci infections: emerging challenges. Clin Lab Sci. 2003;16:209–213. [PubMed] [Google Scholar]
- 42.Bailie RS, Stevens M, McDonald E, Halpin S, Brewster D, Robinison G, Guthridge S. Skin infection, housing and social circumstances in children living in remote Indigenous communities: testing and methodological approaches. BMC Public Health. 2005;5:128. doi: 10.1186/1471-2458-5-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gracey M, Williams P, Houston S. Environmental health conditions in remote and rural aboriginal communities in western Australia. Aust N Z J Public Health. 1997;21:511–518. doi: 10.1111/j.1467-842x.1997.tb01744.x. [DOI] [PubMed] [Google Scholar]
- 44.Currie BJ, Carapetis JR. Skin infections and infestations in Aboriginal communities in northern Australia. Australas J Dermatol. 2000;41:139–143. doi: 10.1046/j.1440-0960.2000.00417.x. [DOI] [PubMed] [Google Scholar]


