Abstract
Vernal keratoconjunctivitis (VKC) is an allergic eye disease and an important cause of hospital referral among children in Africa and Asia. Hospital-based studies have suggested a role for parasites in its pathogenesis. To determine the prevalence and risk factors for VKC in Central Africa, we conducted a nested population-based case control study in Rwanda, involving randomly selected primary schools from different environments (rural/urban) and climate. A prevalence of VKC of 4.0% (95% confidence interval 3.3–4.7%) was found among 3,041 children studied (participation rate 94.7%). The intestinal parasitic burden was not related to VKC. Besides hot dry climate (odds ratio [OR] = 1.5, P = 0.05) and male gender (OR = 1.7, P = 0.005), multivariate analysis identified higher economic status as a risk for VKC (OR = 1.4, P = 0.005). The effect on VKC of higher economic status appears not to act through differences in parasitic intestinal load.
Introduction
Vernal keratoconjunctivitis (VKC) is a chronic, bilateral allergic disease of the external eye that leads to chronic irritation, watering, and discharge. It occurs universally but is more common in hot and dry environments. It typically affects children in their first two decades and although the majority of cases have a good prognosis and resolve spontaneously after puberty, potentially sight-threatening corneal changes occur in up to 10% of patients (Figure 1).1–4 Vernal keratoconjunctivitis is an important cause of hospital referral among children in many parts of Africa,3,5,6 Asia,7 and the Middle East1 with a prevalence of 5% reported for children in their first decade in Chad and Djibouti.8 The pathogenesis of VKC is complex and involves environmental, endocrine, racial, and genetic factors.3,9 Many studies of allergic diseases show a rural environment to be protective but the mechanism of this effect is unknown.10,11 There are conflicting reports on the effect of parasitic intestinal load in the pathogenesis of atopy,12,13 and whether helminthiasis is protective14,15 or a risk factor for allergy.16 Anti-parasitic treatment was reported to improve the course of VKC in two case series,8,17 and a hospital-based study in Nigeria showed that patients with VKC had a larger intestinal parasite load in their stools than unaffected controls.18 These findings suggest that parasites play a role in the pathogenesis of VKC and that treatment of parasitic infestation may help control VKC.
Figure 1.
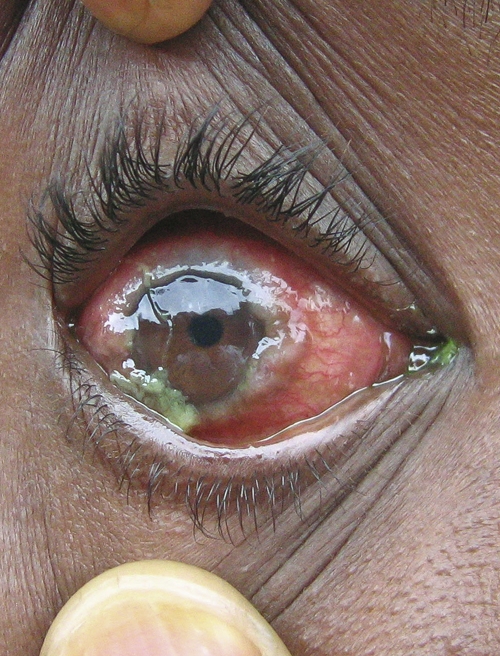
The 360° limbal papillae are potentially sight-threatening in this case of severe vernal keratoconjunctivitis.
Because there has been relatively little work on the prevalence and risk factors for developing VKC in tropical countries, we undertook a nested population-based case control study of primary school children in Rwanda (Central Africa). The purpose was to assess the prevalence of VKC and the role of potential risk factors for development of disease such as gender, gastrointestinal parasitic burden, climate, socio-economic level, and sensitization to locally relevant allergens.
Methods
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Rwandan National Ethics Committee. The study was performed during the dry season between August and September 2007. The sample size calculation, questionnaire, data recording instruments, and logistics were based on an earlier pilot study. The questionnaire, information sheet, and consent form were translated from English into the local language, Kinyarwanda, and back-translated by two different translators.
Sample size calculation.
A case control study with three controls recruited for each case of VKC would require 107 cases (and 321 controls) to detect an odds ratio (OR) of 2.0 at the 95% confidence level and with 80% power, assuming 40% of cases are exposed to the risk factor of interest. Expecting a prevalence of VKC of 3.5%, 3,057 children would need to be examined to generate 107 cases.
Sampling strategy.
Four different environments were identified: two in districts with a dry hot climate (urban Kicukiro and rural Bugesera), and two in a district with a humid moderate climate (urban and rural Muhanga).19 Primary schools located in these environments were then randomly selected from the district school lists, using a random digit generator sheet and according to probability proportional to size. Because the selected schools in the moderate environment were small, an additional two schools had to be selected there, resulting in six schools chosen to have equal numbers of children from the different environments. For each selected school an alphabetical list of school children between 7 and 14 years of age was generated based on the enrollment documents. Although in Rwanda primary school education is compulsory, we also included and traced 18 children who had abandoned school, and who were identified following a comparison of the current school registers and the previous ones until 6 years before the study. Starting from the top of each list children were recruited until similar numbers of participants were invited from each environment. The selected children and their families were then invited to participate by public announcements and during a parents meeting at the school. The study took place on the school premises and on the study day the identity of the children was checked against their vaccination certificate, medical insurance, or identity card, and only those whose identity was confirmed were included. An information sheet was read to the parents or guardians in Kinyarwanda, after which they were asked to sign a consent form.
Interview and examination of all children.
Participating children and their parents or guardians were interviewed about demographic, socio-economic, and environmental factors of potential etiological relevance. Dust exposure was defined as reported dust on plants surrounding the house during at least one season. Symptoms in relation to VKC were also questioned, and the findings will be published separately. Socio-economic status was evaluated in different ways. First, methods from the World Bank's Living Standards Measurement Study20 were used as the basis of assessment because they had already been validated in Rwanda.21 Seven locally relevant items were chosen that best reflected household expenditure in Rwanda for monthly household expenditure questions (Table 1). Possession of the following assets was also ascertained: bench, table, chair, cupboard, sofa or armchair, and telephone and the possession of any luxury items. Furthermore, questions were asked about the materials used for the construction of the walls, floor, and roof of their house. A total economic parameter was then calculated based on monthly expenditure for the seven selected items, housing construction materials and possession of the mentioned assets. Second, the value of the family house was estimated by the monthly rent as if rented to someone else. Other measures of socio-economic status included the occupation of the head of household and the number of rooms in the house. Higher educational level of the head of the household was defined as at least 1 year of secondary school completed.
Table 1.
Questions on monthly household expenditure for seven selected items and family house value
| Public transport, taxi-minibus |
| Sugar |
| Personal care items (haircuts, cosmetics, soap, shampoo) |
| Clothing or shoes for adults and children (including school uniform) |
| Telephone (including cell phone) |
| Water and household energy (firewood, charcoal, cooking gas, petrol, kerosene, candle, electricity) |
| Fish, meat |
| Value of the family house estimated by the monthly rent as if let to someone else |
All children had a general examination by a physician, an eye examination by an ophthalmologist using a portable slit lamp, and a skin examination by a dermatologist. Vernal keratoconjunctivitis was defined as the presence of conjunctival papillae > 1 mm diameter over the upper tarsal plate and/or limbal papillae. The presence of > 6 clock hours of limbal papillae was classified as severe VKC. Asthma was diagnosed on the basis of wheezing on auscultation after exercise challenge entailing an intensive pumping maneuver.22 Eczema was defined using the criteria of Hanifin and Rajka.23
Investigation of cases and controls.
The following tests were performed on cases of VKC and controls. Controls for these tests were the first three children without VKC seen by the ophthalmologist after a case. 1) A blood sample was taken, kept on ice, and transported to King Faisal Hospital Laboratory, Kigali, Rwanda, where the sera were centrifuged and stored frozen at −18 °C before transfer. Specific serum immunoglobulin E (IgE) concentrations (CAP250, Phadia, Uppsala, Sweden) for Timothy-grass, Sorghum, Maize corn, mosquito (Aedes communis), Eucalyptus, Acacia, and Ascaris lumbricoides were measured in the Clinical Biology Laboratory (Ghent University Hospital, Belgium) and Strongyloides antibodies were determined by the Institute of Tropical Medicine (ITM, Antwerp, Belgium) by an enzyme-linked immunosorbent assay (ELISA) (IVD Research, Inc., Carlsbad, CA). 2) A stool sample was collected. To avoid sample contamination the traditional toilet was cleaned before use and children were given detailed instructions on how to deliver and collect a sample, which was then labeled immediately and kept on ice. The stool specimens were transported to King Faisal Hospital Laboratory, where they underwent fixation (formalin) procedures. The preserved stool specimens were sent to ITM where the samples were processed for microscopic detection of ova both qualitatively and quantitatively (number of helminthes eggs calculated/gram of feces) using the ether sedimentation technique adapted from Loughlin and Spitz.24 3) Immediate hypersensitivity was assessed by skin prick tests (SPTs) using commercially available allergens considered common in Africa: goat and cow hair (Allergen Therapeutics Ltd., Worthing, UK), pig and cow dander, Johnson grass and Acacia (Lofarma, Milan, Italy), Maize corn, Dermatophagoides (D) pteronyssinus, German cockroach, mosquito Aedes communis, Aspergillus, dog hair, with positive (histamine 10 mg/dL) and negative (saline) controls (Stallergenes, Antony France).Cases and controls were tested on the volar surface of both forearms using disposable 1 mm prick lancets (Stallergenes). The reaction was recorded after 15 minutes by the dermatologist. The wheal size was calculated as the mean of the longest and perpendicular wheal diameters. A positive reaction was defined as either a wheal size of ≥ 5 mm, or between 3 and 5 mm if this was at least equal to 70% of the wheal of the positive control. The IgE-atopy and SPT-sensitization were defined as a positive IgE-test or SPT, respectively, for at least one tested allergen other than Ascaris. All questionnaires, pediatric and dermatological examinations, SPTs, and laboratory investigations were undertaken in a masked fashion without knowledge of the child's VKC status.
Statistical analysis.
Data were double-entered in a Microsoft Access database (Redmond, WA) and logistic regression models (STATA software version 9.2, College Station, TX) were fitted to calculate the OR. Risk factors were introduced into the model starting with the variables with the strongest correlation with VKC (highest Pearson's χ2 value). Significance levels of 0.05 and 0.01 were used in the evaluation of risk factors and possible interaction terms, respectively. The likelihood ratio test was used to decide which risk factors contribute significantly in describing the variation in the data. The goodness of fit of the logistic model was assessed using the Pearson's χ2 test, comparing the observed frequencies with the expected frequencies predicted by the model.
Results
Prevalence of VKC.
From a total of 3,447 children invited to attend this study, 169 could not be traced, 3 withheld consent, 219 children were outside the age limit according to their identity cards (contrary to school records), and an additional 7 children were excluded because it was not possible to confirm their identity. Therefore, a total of 3,049 children were recruited for this study with a participation rate among potentially eligible children of 94.7%. Of the 3,049 eligible children assessed a further 8 were excluded because of missing data. A total of 121 children were diagnosed with VKC giving a prevalence of 4.0% (95% confidence interval [CI] = 3.3% to 4.7%), with severe VKC in 39 of the 121 cases (32.2%). The total study population had an equal distribution by gender, climate, and urban background (Table 2). Cases and controls for the tests had a similar distribution of baseline characteristics, except for gender with fewer boys among the controls (P = 0.002) (Table 3).
Table 2.
Distribution of demographic, socio-economic, and medical characteristics of the study population and their univariate association (odds ratio [OR], P value, 95% confidence intervals [CI]) with vernal keratoconjunctivitis (VKC)
| Risk factor for VKC | % | OR | P value | CI |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Male gender | 51.0 | 1.78 | 0.003 | 1.22–2.61 |
| Living in hot dry climate | 51.0 | 1.6 | 0.014 | 1.10–2.32 |
| Urban environment | 50.3 | 1.37 | 0.086 | 0.95–1.96 |
| Age group | ||||
| 7–9 years | 44.0 | 1.0 | ||
| 10–11 years | 31.2 | 0.88 | 0.574 | 0.57–1.37 |
| 12–14 years | 24.9 | 1.08 | 0.722 | 0.70–1.69 |
| Trend | 1.03 | 0.811 | 0.82–1.28 | |
| Socio-economic characteristics | ||||
| Total economic level | ||||
| Poor | 19.4 | 1.0 | ||
| Middle | 47.1 | 1.44 | 0.214 | 0.81–2.59 |
| Rich | 33.5 | 2.14 | 0.009 | 1.20–3.84 |
| Trend | 1.45 | 0.004 | 1.12–1.87 | |
| House rent | ||||
| Low | 58.6 | 1.0 | ||
| Middle | 26.0 | 1.21 | 0.4 | 0.78–1.89 |
| High | 15.4 | 2.11 | 0.001 | 1.35–3.31 |
| Trend | 1.48 | 0.002 | 1.16–1.89 | |
| Professional level of head of household | ||||
| Subsistence agriculture or unemployed | 66.3 | 1.0 | ||
| Craftsmen, vocational | 17.2 | 1.38 | 0.182 | 0.86–2.22 |
| Office, civil servant, business | 16.5 | 1.76 | 0.012 | 1.12–2.75 |
| Trend | 1.37 | 0.01 | 1.08–1.74 | |
| Increasing number of rooms in the house | ||||
| 1 to 3 rooms | 55.8 | 1.0 | ||
| 4 rooms | 28.7 | 1.19 | 0.434 | 0.77–1.84 |
| 5 or more rooms | 15.5 | 2.07 | 0.001 | 1.31–3.25 |
| Trend | 1.45 | 0.003 | 1.14–1.85 | |
| Sleeping on foam mattress | 42.2 | 1.41 | 0.063 | 0.98–2.03 |
| House floor made of earth/clay | 62.9 | 0.65 | 0.021 | 0.45–0.94 |
| Close daily contact with animals | 41.2 | 0.67 | 0.043 | 0.45–0.99 |
| Medical characteristics | ||||
| Asthma | 0.9 | 4.7 | 0.002 | 1.6–14.1 |
| Eczema | 0.8 | 1.1 | 0.94 | 0.1–8.6 |
Table 3.
Distribution of baseline characteristics of vernal keratoconjunctivitis cases and controls with their differences, expressed in P value
| Cases (N = 121) | Controls (N = 363) | P value | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age | |||||
| 7–9 years | 33 | 27.3 | 96 | 26.5 | |
| 10–11 years | 34 | 28.1 | 117 | 32.2 | |
| 12–14 years | 54 | 44.6 | 150 | 41.3 | 0.773 |
| Male gender | 78 | 64.5 | 174 | 48.1 | 0.002 |
| Urban environment | 65 | 53.7 | 195 | 53.7 | 1 |
| Hot climate | 75 | 62.0 | 225 | 62.0 | 1 |
| Total economic level | |||||
| Poor | 15 | 12.4 | 55 | 15.36 | |
| Middle | 52 | 42.98 | 153 | 42.74 | |
| Rich | 54 | 44.63 | 150 | 41.9 | 0.442 |
| House rent | |||||
| Low | 58 | 48.33 | 191 | 52.62 | |
| Middle | 31 | 25.83 | 109 | 30.03 | |
| High | 31 | 25.83 | 63 | 17.36 | 0.12 |
| Higher educational level | 31 | 25.62 | 78 | 21.55 | 0.355 |
Univariate analysis of risk factors for VKC.
Demographic, socio-economic, and medical characteristics of the study population and their association with VKC are shown in Table 3. The risk factors associated with the presence of VKC were socio-economic characteristics, male gender, and residence in a hot dry climate (Table 2). Living in an urban environment and sleeping on a foam mattress (as opposed to hay) were of borderline positive significance. Protective associations were a house floor made of earth or clay and close daily contact with animals. There was no association between VKC and the family smoking habits, reported food allergies of the child, number of siblings, educational level of the head of the household, kitchen fumes, insecticide use, and the construction materials of the house. Although age was not a significant overall risk factor, VKC was significantly more frequent in older girls, 12–14 years of age (OR = 1.5, P = 0.025). We found no significant interaction between age and gender. There was a significant association between VKC and asthma (OR = 4.7, P = 0.002), but not with eczema (P = 0.94).
Results of investigations.
We collected stool samples from 120 (99.2%) cases and 360 (99.2%) controls. Neither the presence of Strongyloides antibodies nor the helminthic burden in the stools (for the individual helminthes and in total) was related to the presence of VKC, even after stratification and correction for anti-parasitic medication taken before the study (Table 4). There was also no significant difference in the number of helminthic eggs between cases and controls (P = 0.851). Vernal keratoconjunctivitis was not related to the level of any specific IgE concentration. The proportion of participants that reacted to none of the SPTs was 53.8% and 57.8% for cases and controls, respectively, without a statistically significant difference (P = 0.46) between the two groups. Although there were three positive SPT results for Maize corn, an OR could not be calculated because there were no responders among the controls. Blood samples and SPTs were performed in 100% and 96.7% of cases and 99.5% and 97.5% of controls, respectively.
Table 4.
Results of additional tests (qualitative stool examination, Strongyloides antibodies, skin prick tests, and serum immunoglobulin E (IgE) concentrations) and their association with vernal keratoconjunctivitis*
| Additional tests | % | OR | P value | CI |
|---|---|---|---|---|
| Helminthes (eggs) in stools | 23.1 | 1.0 | 0.950 | 0.6–1.7 |
| Stools positive for specific helminthes (eggs) | ||||
| Ancylostomidae | 9.8 | 1.3 | 0.425 | 0.7–2.5 |
| Trichuris trichiura | 5.6 | 0.7 | 0.424 | 0.3–1.8 |
| Ascaris lumbricoides (feconded) | 4.0 | 0.8 | 0.685 | 0.3–2.4 |
| Ascaris lumbricoides (feconded) | 3.1 | 0.7 | 0.650 | 0.2–2.7 |
| Trichostrongylus | 1.7 | 1.8 | 0.411 | 0.4–7.8 |
| Schistosoma mansoni | 1.0 | 0.8 | 0.795 | 0.1–6.8 |
| Enterobius vermicularis | 0.8 | NA | 0.247 | NA |
| Taenia | 0.2 | NA | 0.083 | NA |
| Hymenolepis nana | 0.2 | NA | 0.564 | NA |
| Fasciolidae | 0.2 | NA | 0.564 | NA |
| Dicrocoelium dendriticum | 0.2 | NA | 0.564 | NA |
| Positive Strongyloides antibodies | 3.3 | 0.3 | 0.229 | 0.04–2.4 |
| Skin prick test-sensitization | 43.2 | 1.2 | 0.456 | 0.8–1.8 |
| Specific positive skin prick test | ||||
| Cockroach | 13.6 | 0.8 | 0.555 | 0.4–1.6 |
| Mosquito (Aedes communis) | 13.0 | 1.4 | 0.222 | 0.8–2.6 |
| Goat hair | 12.1 | 1.0 | 0.951 | 0.5–1.9 |
| Acacia | 11.9 | 0.8 | 0.529 | 0.4–1.6 |
| Pig dander | 9.6 | 1.0 | 0.949 | 0.5–2.0 |
| Aspergillus | 9.6 | 1.0 | 0.949 | 0.5–2.0 |
| D. pteronyssinus | 6.4 | 1.6 | 0.266 | 0.7–3.4 |
| Dog hair | 4.3 | 0.8 | 0.609 | 0.2–2.3 |
| Cow dander | 3.6 | 0.9 | 0.899 | 0.3–2.9 |
| Cow hair | 0.9 | 1.0 | 0.994 | 0.1–9.8 |
| Maize corn | 0.6 | NA | 0.003 | NA |
| Johnson grass | 0.2 | NA | 0.082 | NA |
| IgE atopy | 47.9 | 1.3 | 0.285 | 0.8–1.9 |
| Specific positive IgE-concentration | ||||
| Ascaris lumbricoides | 39.5 | 0.8 | 0.421 | 0.6–1.3 |
| Sorghum | 37.4 | 1.1 | 0.560 | 0.7–1.7 |
| Mosquito (Aedes communis) | 34.4 | 1.4 | 0.156 | 0.9–2.1 |
| Timothy-grass | 25.8 | 0.9 | 0.780 | 0.6–1.5 |
| Maize corn | 25.4 | 1.0 | 0.940 | 0.6–1.6 |
| Acacia | 20.3 | 0.8 | 0.520 | 0.5–1.4 |
| Eucalyptus | 19.6 | 0.8 | 0.320 | 0.4–1.3 |
OR = odds ratio; P value; CI = confidence interval (95%); NA = not available.
Univariate analysis of socio-economic factors.
An increase in house value was generally strongly associated with other socio-economic parameters, urban environment, and sleeping on a foam mattress (Table 5). Children living in richer households had significantly fewer helminthes in their stools, less animal contact, and fewer floors made of earth in their houses, and these effects were independent of urban environment.
Table 5.
Significant univariate associations of house value and urban environment*
| Associated factors | OR | |
|---|---|---|
| High house value | Educational level head household† | 10.1 |
| Reported sleeping on foam mattress† | 5.7 | |
| Total economic level† | 5.6 | |
| Urban environment† | 4.7 | |
| Professional level of head of household† | 4.3 | |
| Increasing number of rooms in the house† | 2.7 | |
| House floor made of earth/clay† | 0.2 | |
| Helminthes in stools† | 0.5 | |
| Animal contact† | 0.5 | |
| Skin prick test-sensitization to any of tested allergens‡ | 0.7 | |
| Urban environment | IgE-atopy† | 0.2 |
| Animal contact† | 0.2 | |
| Skin prick test-sensitization to any of tested allergens† | 0.5 |
OR = odds ratio; P value.
Significantly correlated P value < 0.001.
Significantly correlated 0.001 ≤ P value ≤ 0.05.
Multivariate analysis.
The following risk factors remained significantly associated with VKC in multivariate analysis: male gender (OR = 1.7, P = 0.005, 95% CI = 1.2–2.5), hot dry climate (OR = 1.5, P = 0.05, 95% CI = 1.0–2.1), and higher house value (OR = 1.4, P = 0.005, 95% CI = 1.1–1.7). No statistically significant interaction between the retained variables could be found and there was no evidence of a weak fit for the overall model (Pearson's χ2 = 10.1, P = 0.262). When we analyzed cases with severe VKC separately in a multivariate model, the number of rooms as an economic indicator (OR = 1.7, P = 0.005), animal contact (OR = 0.3, P = 0.005), male gender (OR = 2.5, P = 0.011), and dust exposure, (OR = 3.9, P = 0.024) became significant. Dust exposure was highly associated with hot dry climate (OR = 1.66, P < 0.001).
Discussion
Although VKC is rare in temperate regions,25 hospital-based studies from many parts of Africa have identified VKC as an important cause for hospital attendance, with figures ranging from 2.8% to 6% of patients of all ages26 and rising to between 33% and 90% of children and adolescents.5,6 The high participation rate of our population-based study gave sufficient power and precision to provide a more precise estimate of the prevalence of VKC in this region and it enabled us to identify several relevant risk factors. Although sampling of pupils was based on family names, family cluster bias is unlikely because family names are not inherited in Rwanda. We found a population prevalence of 4% for VKC in the susceptible age group of 7 to 14 years, which is similar to the 5% reported in another population-based survey of children from Chad and Djibouti.8 In contrast to Chad and Djibouti, trachoma is uncommon throughout Rwanda and any confusion between a diagnosis of VKC and trachoma is unlikely. By including children who had abandoned their primary school education, we think that our results are representative of the general Rwandese child population. The study was undertaken during the second half of the long dry season when we believe most cases of VKC would have been active. Our results confirm that VKC is a significant public health concern in Rwanda, particularly because there is a pyramidal population structure with nearly half of the population < 17 years of age.27 Rwanda is a landlocked, densely populated country mainly situated more than 1,000 m above sea level with 83% of the population living in rural areas.19,27 Some regions have a drier climate than others. Around the capital Kigali (Kicuciro district) and in the Eastern province (Bugesera district) the annual temperature is above 20.5°C and the annual rainfall is < 1,000 mm/m2. In contrast, Muhanga district, which is situated on the central Plateau, has a more temperate climate with greater rainfall (> 1,200 mm/m2/annum).19
There is to date little information on the specific factors that determine the high prevalence of VKC in Central Africa. We confirmed that VKC is more prevalent in hot dry regions.7 We also identified exposure to dust as an important risk factor for severe disease, in line with other studies that have noted an association of VKC with nonspecific stimuli of conjunctival hyper-reactivity such as sun, dust, and wind.3 However, the mechanism by which this irritation amplifies the signs of allergic eye disease is still unknown.9 To the best of our knowledge this is the first population-based study to link VKC to a higher economic status. Previous case series noted that the majority of cases of VKC came from urban areas,3,17 but selection bias was likely in these hospital-based studies. There have been similar correlations in the literature with other allergic diseases and a “Western” lifestyle,12,28 or urban environment,10,11,14,29,30 although these have been poorly defined. In our study it is unlikely that this link might be biased by misclassification of milder cases of VKC as the association remained significant even for severe cases. Vernal keratoconjunctivitis was associated with different ways of assessing economic status, which gives consistency to the results. Household assets are considered reliable indicators of long-term economic status in low-income countries because they tend to react less to economic fluctuation than other measures of household wealth.21 Economic development has recently increased in Rwanda, especially around the major towns, and an assessment of the influence of growth on the prevalence of VKC would be of interest. Because mattresses are a recognized reservoir for microorganisms,10,12 analysis of mattress dust may help identify the reason for the link between economic status, sleeping on a foam mattress, and VKC.
There is conflicting evidence on whether an individual's parasite burden plays a role in the pathogenesis of atopy (hygiene hypothesis),12,13,31–33 and if so, whether this is protective14,15,34–39 or promotes allergy.16,40–42 A role for parasites in the pathogenesis of VKC was suggested by the observed beneficial effect of anti-parasitic medication on the course of the disease, and the larger gastrointestinal parasite burden found in stools of VKC patients in Nigeria.18 However, controls of these studies were recruited in hospitals, making them less representative of the general population and leading to selection bias. Our study could not confirm this association and we do not think that the effect of higher economic status on VKC acts through differences in intestinal parasites. Recently, the hygiene hypothesis has been broadened to include not only exposure to gastrointestinal infections, but also to several environmental microbial agents and commensal bacteria in the gastrointestinal tract.43 Our study did not investigate differences in commensal bacterial flora in the intestinal tract, but the link between animal contact and the quality of housing with both VKC and economic status is consistent with this wider hygiene hypothesis.
Other large European,44 Asian,7 and South African3 case series have confirmed that male gender is an important risk factor for VKC, with a male/female ratio ranging from 3.2 to 5.2, which is much greater than found in our study. However, other African studies have found no gender difference.5,8,17 Studies from Europe9 and Africa45 have also found that girls are at significantly greater risk of developing VKC in their second decade. This may result from the increased production of androstenedione around puberty in girls.46
Our study found a positive association between VKC and asthma, which is consistent with another population-based study from Djibouti.8 Although VKC tends to be commonly associated with atopy (asthma, eczema) in temperate regions, many hospital-based studies from Europe, Asia, and Africa often found contradictory conclusions.7,17,47,48 These differences in the reported association of VKC and atopy may result from the different methods used to define asthma22 and further studies using better ascertainment of asthma (e.g., peak flow measurements after exercise) are required.
There is a wide range in the reported levels of serum IgE concentrations9 and the results of skin tests47 in patients with VKC, and most series contain unresponsive patients. Cell-mediated immunity to environmental allergens has been described as an important mechanism of VKC besides immediate hypersensitivity.49 It is likely that the allergens responsible are area specific and small case series from Turkey have reported high levels of D. pteronyssinus-specific IgE-concentrations in patients with VKC50 and in Israel, exposure to house dust mite played an important role in the aggravation of symptoms of VKC in patients who were known to be reactive.51 Despite using locally relevant allergens for this study, we could not find a link between the results of the SPT or specific IgE serum tests and the presence of VKC. There are a number of possible explanations for this. It may be that a wide range of untested allergens is responsible for the disease, or that atopy is not required to develop limbal VKC in this population, as suggested by similar proportions of SPT non-responders between VKC-cases and controls. An alternative explanation is that the tests we used did not reflect the activity of allergens active at the conjunctiva, or that non-specific ocular surface irritation has a dominant role.
In conclusion, this population-based study confirms that VKC is an important public health problem in hot and dry regions of Central Africa. Besides the effect of climate and male gender, higher economic status was identified as a risk factor for VKC. If this is true, with the growing economic development in Rwanda an increase in VKC-prevalence is to be expected. The parasitic burden in stools was not related to VKC. Further studies are needed to define the role of atmospheric pollutants in the development of limbal VKC and to identify other immunological, ocular surface, and environmental mechanisms that might determine the difference in prevalence in VKC observed between populations that live in urban or rural communities.
ACKNOWLEDGMENTS
Our gratitude goes to the Rwandan Ministry of Health, the Rwandan Ethics Committee, the authorities in charge of education and health in Muhanga, Kicukiro, and Bugesera district, the Rwanda Meteorological Service, the directors of the schools involved and last but not least the participants and their guardians. We are also grateful for the excellent collaboration with the laboratory of King Faisal Hospital, Kigali (Dr. Vénérand Bigirimana) for the processing and packaging of the samples, with the Institute of Tropical Medicine of Antwerp and with laboratory Clinical Biology, Ghent University Hospital, (Ruth Reunes) for the analysis of the stool and blood samples, respectively.
Footnotes
Financial support: This study was possible thanks to financial support from FRO (Belgian Funds for Research in Ophthalmology), Light for the World Belgium, CBM-International, and PHADIA NV/SA (Brussels, Belgium) and the active participation in the field work of the Kabgayi Eye Unit personnel.
Authors' addresses: Stefan De Smedt and Philippe Kestelyn, Ophthalmology Department, Ghent University Hospital, Belgium, E-mails: stefan.desmedt@ugent.be and philippe.kestelyn@ugent.be. John Nkurikiye and Arjan Hogewoning, Ophthalmology Department, King Faisal Hospital, Kigali, Rwanda, E-mails: jeankur@yahoo.co.uk and arjanhogewoning@yahoo.com. Yannick Fonteyne, Leopoldstraat 36, 2800 Mechelen, Belgium, E-mail: stds@telenet.be. Marjan Van Esbroeck, Department of Clinical Science, Institute of Tropical Medicine, Antwerp, Belgium, E-mail: mvesbroeck@itg.be. Dirk De Bacquer, Department of Public Health, Ghent University, Belgium, E-mail: dirk.debacquer@ugent.be. Stephen Tuft, Moorfields Eye Hospital, National Health Service Foundation Trust, London, UK, E-mail: s.tuft@ucl.ac.uk. Clare Gilbert, International Centre for Eye Health, London School of Hygiene and Tropical Medicine, UK, E-mail: clare.gilbert@lshtm.ac.uk. Joris Delanghe, Clinical Biology Department, Ghent University Hospital, Belgium, E-mail: joris.delanghe@ugent.be.
Reprint requests: Stefan De Smedt, Leopoldstraat 34, 2800 Mechelen, Belgium, E-mail: dr.stefan.desmedt@gmail.com.
References
- 1.Tabbara KF. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. 1999;34:88–92. [PubMed] [Google Scholar]
- 2.Cameron JA. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology. 1995;102:985–993. doi: 10.1016/s0161-6420(95)30925-6. [DOI] [PubMed] [Google Scholar]
- 3.Dahan E, Appel R. Vernal keratoconjunctivitis in the black child and its response to therapy. Br J Ophthalmol. 1983;67:688–692. doi: 10.1136/bjo.67.10.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.BenEzra D, Pe'er J, Brodsky M, Cohen E. Cyclosporine eye drops for the treatment of severe vernal keratoconjunctivitis. Am J Ophthalmol. 1986;101:278–282. doi: 10.1016/0002-9394(86)90819-6. [DOI] [PubMed] [Google Scholar]
- 5.Chenge B, Makumyamviri AM, Kaimbo WA, Kaimbo D. Tropical endemic limbo-conjunctivitis in Lúbumbashi, Democratic Republic of the Congo. Bull Soc Belge Ophtalmol. 2003;290:9–16. [PubMed] [Google Scholar]
- 6.Diallo JS. Tropical endemic limboconjunctivitis. Rev Int Trach Pathol Ocul Trop Subtrop. 1976;53:71–80. [PubMed] [Google Scholar]
- 7.Khan MD, Kundi N, Saeed N, Gulab A, Nazeer AF. A study of 530 cases of vernal conjunctivitis from the North West Frontier Province of Pakistan. Pakistan J Ophthalmol. 1986;2:111–114. [Google Scholar]
- 8.Resnikoff S, Cornand G, Filliard G, Hugard L. Limbal vernal conjunctivitis in the tropics. Rev Int Trach. 1988;3-4:53–71. [PubMed] [Google Scholar]
- 9.Bonini S, Bonini S, Lambiase A, Marchi S, Pasqualetti P, Zuccaro O, Rama P, Magrini L, Juhas T, Bucci MG. Vernal keratoconjunctivitis revisited. A case series of 195 patients with long-term followup. Ophthalmology. 2000;107:1157–1163. doi: 10.1016/s0161-6420(00)00092-0. [DOI] [PubMed] [Google Scholar]
- 10.Yemaneberhan H, Bekele Z, Venn A, Lewis S, Parry E, Britton J. Prevalence of wheeze and asthma and relation to atopy in urban and rural Ethiopia. Lancet. 1997;350:85–90. doi: 10.1016/S0140-6736(97)01151-3. [DOI] [PubMed] [Google Scholar]
- 11.Viinanen A, Munhbayarlah S, Zevgee T, Narantsetseg L, Naidansuren T, Koskenvuo M, Helenius H, Terho EO. The protective effect of rural living against atopy in Mongolia. Allergy. 2007;62:272–280. doi: 10.1111/j.1398-9995.2007.01279.x. [DOI] [PubMed] [Google Scholar]
- 12.Warrell DA, Fawcett IW, Harrison BD, Agamah AJ, Ibu JO, Pope HM, Maberly DJ. Bronchial asthma in the Nigerian Savanna Region. Quarterly J Med New Series. 1975;XLIV:325–347. [PubMed] [Google Scholar]
- 13.Carswell F, Merrett J, Merrett TG, Meakins RH, Harland PS. IgE, parasites and asthma in Tanzanian children. Clin Allergy. 1977;7:445–453. doi: 10.1111/j.1365-2222.1977.tb01475.x. [DOI] [PubMed] [Google Scholar]
- 14.Godfrey RC. Asthma and IgE levels in rural and urban communities in The Gambia. Clin Allergy. 1975;5:201–207. doi: 10.1111/j.1365-2222.1975.tb01853.x. [DOI] [PubMed] [Google Scholar]
- 15.Merrett TG, Merrett J, Cookson JB. Allergy and parasites: the measurement of total and specific IgE levels in urban and rural communities in Rhodesia. Clin Allergy. 1976;6:131–134. doi: 10.1111/j.1365-2222.1976.tb01890.x. [DOI] [PubMed] [Google Scholar]
- 16.Dold S, Heinrich J, Wichmann H-E, Wjst M. Ascaris-specific IgE and allergic sensitization in a cohort of school children in the former East Germany. J Allergy Clin Immunol. 1998;102:414–420. doi: 10.1016/s0091-6749(98)70129-0. [DOI] [PubMed] [Google Scholar]
- 17.Sandford-Smith J. Vernal eye disease in Northern Nigeria. Trop Geogr Med. 1979;31:321–328. [PubMed] [Google Scholar]
- 18.Ajaiyeoba A. Vernal keratoconjunctivitis and intestinal parasitic infestations in black children. J Natl Med Assoc. 2005;97:1529–1532. [PMC free article] [PubMed] [Google Scholar]
- 19.Munyakazi A, Ntagaramba JF. Atlas of Rwanda. Oxford: MacMillan; 2005. [Google Scholar]
- 20.Glewwe P, Grosh M. Designing household survey questionnaires for developing countries: lessons from 15 years of the living standards measurement study. Washington, DC: World Bank; 2000. [Google Scholar]
- 21.Rischewski D, Kuper H, Atijosan O, Simms V, Jofret-Bonet M, Foster A, Lavy C. Poverty and musculoskeletal impairment in Rwanda. Trans R Soc Trop Med Hyg. 2008;102:608–617. doi: 10.1016/j.trstmh.2008.02.023. [DOI] [PubMed] [Google Scholar]
- 22.Lee T, Anderson S. Heterogeneity of mechanisms in exercise induced asthma. Thorax. 1985;40:481–487. doi: 10.1136/thx.40.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanifin J, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- 24.Loughlin EH, Spitz SH. Diagnosis of helminthiasis. JAMA. 1949;139:997–1000. doi: 10.1001/jama.1949.02900320027008. [DOI] [PubMed] [Google Scholar]
- 25.Bremond-Gignac D, Donadieu J, Leonardi A, Pouliquen P, Doan S, Chiambarretta F, Montan P, Milazzo S, Hoang-Xuan T, Baudouin C, Aymé S. Prevalence of vernal keratoconjunctivitis: a rare disease? Br J Ophthalmol. 2008;92:1097–1102. doi: 10.1136/bjo.2007.117812. [DOI] [PubMed] [Google Scholar]
- 26.Everaerts M-C, Doutetien C. Chronic tropical endemic limboconjunctivitis (TELC) in southern Benin: epidemiological and meteorological data. Rev Int Trach Pathol Ocul Trop Subtrop Sante Publique. 1993;70:199–214. [PubMed] [Google Scholar]
- 27.The Government of Rwanda K Third General Census of Population and Housing of Rwanda. 2003. Final results: statistical tables.
- 28.Hugg T, Ruotsalainen R, Jaakkloa MS, Pushkarev V, Jaakkola JJK. Comparison of allergic diseases, symptoms and respiratory infections between Finnish and Russian school children. Eur J Epidemiol. 2008;23:123–133. doi: 10.1007/s10654-007-9217-z. [DOI] [PubMed] [Google Scholar]
- 29.Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005;60:1357–1360. doi: 10.1111/j.1398-9995.2005.00961.x. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg EG, Van Niekerk CH, Shore SC, Heese HD, Van Schalkwyk DJ. Prevalence of asthma. Lancet. 1977;2:500. doi: 10.1016/s0140-6736(77)91618-x. [DOI] [PubMed] [Google Scholar]
- 31.Abdurrahman MB, Taqi AM. Childhood bronchial asthma in northern Nigeria. Clin Allergy. 1982;12:379–384. doi: 10.1111/j.1365-2222.1982.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 32.Macfarlane JT, Bachelor M, Ridyard JB, Ball PA. Asthma, IgE and environment in northern Nigeria. Clin Allergy. 1979;9:333–337. doi: 10.1111/j.1365-2222.1979.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 33.Zeyrek CD, Zeyrek F, Sevinc E, Demir E. Prevalence of asthma and allergic diseases in Sanliurfa, Turkey, and the relation to environmental and socioeconomic factors: is the hygiene hypothesis enough? J Investig Allergol Clin Immunol. 2006;16:290–295. [PubMed] [Google Scholar]
- 34.Scrivener S, Yemaneberhan H, Zebenigus M, Tilahun D, Girma S, Ali S, McElroy P, Custovic A, Woodcock A, Pritchard D, Venn A, Britton J. Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. Lancet. 2001;358:1493–1499. doi: 10.1016/S0140-6736(01)06579-5. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell HS. Bronchial asthma in Kenya. East Afr Med J. 1970;47:142–145. [PubMed] [Google Scholar]
- 36.Turton JA. IgE, parasites, and allergy. Lancet. 1976;25:686. doi: 10.1016/s0140-6736(76)92492-2. [DOI] [PubMed] [Google Scholar]
- 37.Hodges MG, Keane-Myers AM. Classification of ocular allergy. Curr Opin Allergy Clin Immunol. 2007;7:424–428. doi: 10.1097/ACI.0b013e3282ef6937. [DOI] [PubMed] [Google Scholar]
- 38.McConchie BW, Norris HH, Bundoc VG, Trivedi S, Boesen A, Urban JF, Keane-Myers AM. Ascaris suum-derived products suppress mucosal allergic inflammation in an interleukin-10-independent manner via interference with dendritic cell function. Infect Immun. 2006;74:6632–6641. doi: 10.1128/IAI.00720-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orren A, Dowdle EB. Effects of allergy, intestinal helminthic infestation and sex on serum IgE concentrations and immediate skin hypersensitivity in three ethnic groups. Int Arch Allergy Appl Immunol. 1975;49:814–830. doi: 10.1159/000231465. [DOI] [PubMed] [Google Scholar]
- 40.Lynch NR, Palenque M, Hagel I, Diprisco MC. Clinical improvement of asthma after anthelminthic treatment in a tropical situation. Am J Respir Crit Care Med. 1997;156:50–54. doi: 10.1164/ajrccm.156.1.9606081. [DOI] [PubMed] [Google Scholar]
- 41.Alshishtawy MM, Abdella AM, Gelber LE, Chapman MD. Asthma in Tanta, Egypt: serologic analysis of total and specific IgE antibody levels and their relationship to parasite infection. Int Arch Allergy Appl Immunol. 1991;96:348–354. doi: 10.1159/000235520. [DOI] [PubMed] [Google Scholar]
- 42.Joubert JR, De Klerk HC, Malan C. Ascaris lumbricoides and allergic asthma. S Afr Med J. 1979;56:599–602. [PubMed] [Google Scholar]
- 43.Von Hertzen L, Haahtela T. Asthma and atopy–the price of affluence? Allergy. 2004;59:124–137. doi: 10.1046/j.1398-9995.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 44.Lambiase A, Minchiotti S, Leonardi A, Secchi A, Rolando M, Calabria G, Orsoni J, Zola E, Ferreri G, Aragona P, Reibaldi A, Chisari G, Bonini S. Prospective, multicenter demographic and epidemiological study on vernal keratoconjunctivitis: a glimpse of ocular surface in Italian population. Ophthalmic Epidemiol. 2009;16:38–41. doi: 10.1080/09286580802573177. [DOI] [PubMed] [Google Scholar]
- 45.Ukponmwan CU. Vernal keratoconjunctivitis in Nigerians: 109 consecutive cases. Trop Doct. 2003;33:242–245. doi: 10.1177/004947550303300419. [DOI] [PubMed] [Google Scholar]
- 46.Divall SA, Radovick S. Endocrinology of female puberty. Curr Opin Endocrinol Diabetes Obes. 2009;16:1–4. doi: 10.1097/med.0b013e3283207937. [DOI] [PubMed] [Google Scholar]
- 47.Tuft SJ, Dart JKG, Kemeny M. Limbal vernal keratoconjunctivitis: clinical characteristics and immunoglobulin E expression compared with palpebral vernal. Eye (Lond) 1989;3:420–427. doi: 10.1038/eye.1989.63. [DOI] [PubMed] [Google Scholar]
- 48.Neumann E, Gutmann M, Blumankrantz N, Michaelson I. A review of four hundred cases of vernal keratoconjunctivitis. Am J Ophthalmol. 1959;47:166–172. doi: 10.1016/s0002-9394(14)76417-7. [DOI] [PubMed] [Google Scholar]
- 49.Tuft SJ, Cree IA, Woods M, Yorston D. Limbal vernal keratoconjunctivitis in the tropics. Ophthalmology. 1998;105:1489–1493. doi: 10.1016/S0161-6420(98)98034-4. [DOI] [PubMed] [Google Scholar]
- 50.Kocabeyoglu S, Bozkurt B, Bilen O, Irkec M, Orhan M. Serum allergen specific immunoglobulin E levels in patients with allergic conjunctivitis. Eur J Ophthalmol. 2008;18:675–679. doi: 10.1177/112067210801800502. [DOI] [PubMed] [Google Scholar]
- 51.Mumcuoglu Y, Zavaro A, Samra Z, Lazarowitz Z. House dust mites and vernal keratoconjunctivitis. Ophthalmologica. 1988;196:175–181. doi: 10.1159/000309896. [DOI] [PubMed] [Google Scholar]


