Abstract
Rocky Mountain spotted fever, Lyme disease, and ehrlichiosis are tick-borne diseases that are reported annually in Kentucky. We conducted a survey to describe infection prevalence of tick-borne pathogens in Amblyomma americanum and Dermacentor variabilis ticks collected in Kentucky. During 2007–2008, we collected 287 ticks (179 D. variabilis and 108 A. americanum) from canine, feral hog, horse, raccoon, white-tailed deer, and human hosts in six counties in Kentucky. Ticks were screened for Rickettsia spp., Borrelia spp., and Ehrlichia spp. by using polymerase chain reaction. Forty-one (14.3%) ticks (31 A. americanum and 10 D. variabilis) were polymerase chain reaction–positive for a Rickettsia spp. Fourteen (4.9%) ticks (6 A. americanum and 8 D. variabilis) were positive for E. chaffeensis, and 4 A. americanum (1.4%) were positive for E. ewingii. One (0.4%) A. americanum was positive for Borrelia lonestari. Although Rocky Mountain spotted fever is diagnosed in Kentucky, no R. rickettsii was found in ticks in this study.
Introduction
Tick-borne rickettsioses are of public health importance because they are a substantial cause of morbidity and mortality worldwide.1 Ticks are responsible for transmitting most vector-borne diseases in the United States. Ticks are capable of transmitting a variety of pathogens, which cause diseases such as spotted fever rickettsiosis, Lyme disease, and human ehrlichiosis.2 In the United States, the most commonly reported rickettsial human pathogen is the obligate intracellular bacteria Rickettsia rickettsii, the causative agent of Rock Mountain spotted fever (RMSF).3 The vectors that are historically attributed in the transmission of R. rickettsii are the American dog tick (Dermacentor variabilis) in the eastern United States and the Rocky Mountain wood tick (Dermacentor andersoni) in the western United States.4 Rock Mountain spotted fever is characterized by a fever, rash, and other possible complications such as encephalitis, coagulopathy, and respiratory disorders.5 There were 15 cases of RMSF reported in Kentucky during 2004–2008.6
In addition to R. rickettsii, other rickettsial species may cause morbidity. There is serologic evidence that another spotted fever group Rickettsia (SFGR) (Rickettsia amblyommii) may also be a human pathogen.7,8 In North Carolina, serum samples from patients that had probable cases of RMSF were tested and had higher end-point titers to R. amblyommii than to R. rickettsii.9 In 2006, an adult Amblyomma americanum tick infected with R. amblyommii was removed from a North Carolina patient with a macular rash at the tick attachment site.10 Amblyomma americanum ticks have been found to be infected with R. amblyommii in the southeastern United States and in the lower midwestern and coastal New England regions.11–14 Rickettsia parkeri, another SFGR, has also been associated with human illness. The principle vector for R. parkeri is Amblyomma maculatum, the Gulf Coast tick.3 The first confirmed human case of spotted fever rickettsiosis caused by R. parkeri was reported in 2004.15 In 2007, Whitman and others detected and isolated R. parkeri from an eschar that developed after a tick bite from a patient in Virginia.16
The causative agent of Lyme disease is a gram-negative bacterial spirochete (Borrelia burgdorferi), which is spread by the tick Ixodes scapularis in the eastern United States and the tick Ixodes pacificus in the western United States. The most common characteristic of early stage Lyme disease is an erythema migrans rash accompanied by nonspecific symptoms such as fever, malaise, fatigue, headache, myalgia, and arthralgia.17 Other major symptoms include arthritis and regional lymphadenopathy.18 In Kentucky, 18 cases of Lyme disease were reported in 2006–2008,6 with an average incidence of 0.5 per 100,000 persons during 1992–2006.19 An additional Borrelia species (Borrelia lonestari), has been tentatively associated with a Lyme borreliosis–like disease in the United States, which is sometimes referred to as southern tick–associated rash illness (STARI).20–23 It is believed that the symptoms of STARI are less severe than those of Lyme disease.24 Borrelia lonestari was identified and characterized in A. americanum ticks in 1995 and was first isolated in 2004.20,23,25 Borrelia lonestari has additionally been identified in A. americanum ticks that were removed from humans, including patients from Kentucky.26
Ehrlichia chaffeensis is a gram-negative obligate intracellular bacterium and the etiologic agent of human monocytotrophic ehrlichiosis.27–29 Ehrlichia chaffeensis is maintained in a zoonotic cycle involving its principal reservoir, the white-tailed deer (Odocoileus virginianus) and A. americanum ticks.30–33 This disease is characterized by fever, headache, myalgia, thrombocytopenia, leukopenia, and increased liver enzyme levels. Most cases cause only mild illness, although more serious complications, including death, can occur. Human monocytotrophic ehrlichiosis is most commonly reported from the southeastern and south central United States.34 The A. americanum tick is also a vector for Ehrlichia ewingii,35,36 the cause of granulocytic ehrlichiosis in humans.37–39 Thirteen cases of ehrlichiosis were reported in Kentucky in 2008, a 325% increase over 2007.6 In this study, we used molecular methods to determine the infection prevalence of ehrlichial, rickettsial, and borrelial species in A. americanum and D. variabilis ticks collected from a variety of wildlife and domestic hosts from six counties in Kentucky.
Materials and Methods
Tick collection and identification.
Adult ticks were collected from May through August 2008 by the United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services (USDA APHIS-WS) from six counties in Kentucky. Ticks were collected as a convenience sample in routine USDA APHIS-WS nuisance calls. Ticks were collected from personal pets, feral hogs, canines, horses, a raccoon, a white-tailed deer, and a human. All ticks were sent to the Tennessee Department of Health Vector-Borne Diseases Laboratory and were identified to species and life stage based on morphologic criteria.40
Isolation of DNA.
Ticks were individually homogenized with metal beads and resuspended in 225 μL of phosphate-buffered saline. DNA was extracted from 200 μL of the homogenate by using a 5 PRIME Manual Ready PCR DNA Column Kit (5 PRIME Inc., Gaithersburg, MD) according to the manufacturers' instructions.
Identification of rickettsiae.
Extracted DNA was initially screened by using a real-time polymerase chain reaction (PCR) to amplify the 17-kDa gene of all SFGR.41 For species identification, a conventional PCR specific for the outer membrane protein A gene of SFGR was conducted on positive samples as described by using primers Rr190.602n and Rr190.70p.42 The PCR products were detected by electrophoresis of 10 μL of product on a 2% agarose gel containing ethidium bromide. Field isolates of R. amblyommii from A. americanum ticks and double-distilled water were used as positive and negative controls, respectively, for all real-time and conventional PCRs. The outer membrane protein A amplicons from PCR-positive samples were further subjected to a restriction fragment length polymorphism assay by digestion with Rsa1 (Promega, Madison, WI) and Pst1 (Fermentas, Glen Burnie, MD) at 37°C for 1 hour.42 Digested fragments were subjected to electrophoresis on 10% polyacrylamide gels. A subset of restriction fragment length polymorphism samples were verified by sequence analysis with a 3130x1 Genetic Sequencer (Applied Biosystems, Foster City, CA) and BigDye Terminator (Applied Biosystems).
Identification of ehrlichiae.
Extracted tick DNA was screened for Ehrlichia chaffeensis by using a nested PCR assay to amplify a segment of the variable length PCR target (VLPT gene).43 Extracted tick DNA was screened for Ehrlichia ewingii by using a nested, species-specific conventional PCR specific for the 16S ribosomal RNA gene.37 Deer blood specimens positive for E. chaffeensis and E. ewingii, as confirmed by sequence analysis, were used as positive controls, and double-distilled water was used as a negative control. The PCR products were detected by electrophoresis of 10 μL of product on 2% agarose E-gels (Invitrogen, Carlsbad, CA) and visualized under ultraviolet light. All positive samples were verified by sequence analysis.
Identification of Borrelia spp.
Extracted tick DNA was screened for Borrelia spp. by using a nested PCR assay to amplify the 16S–23S ribosomal RNA gene intergenic spacer region as described by Bunikis and others.44 This more conserved region enables detection of other Borrelia species circulating such as B. lonestari. Ticks were also screened by using a PCR assay to detect Borrelia burgdorferi specifically. Forward primer SL1 and reverse primer SL2 were used as described.45,46 A standard laboratory strain B. burgdorferi isolate from Shelter Island, New York (strain B31) was used as a positive control, and double-distilled water was used as a negative control in all reactions. All positive samples were sequenced to determine and confirm species.
Statistical analysis.
Pearson chi-square tests were used for statistical analyses.
Results
During May–August 2008, 287 adult ticks were collected: 108 (37.6%) A. americanum and 179 (62.4%) D. variabilis. Of the A. americanum ticks collected, 75 (69.4%) were female and 33 (30.6%) were male. Of the D. variabilis ticks collected, 101 (56.4%) were female and 78 (43.6%) were male. Immature ticks were not collected in this study because of the ease at which adult ticks can be identified and removed relative to immature ticks in a quick convenience sampling.
Forty-one (14.3%) ticks were infected with a Rickettsia spp. One A. americanum tick was infected with B. lonestari (0.35%). Fourteen (4.88%) ticks were infected with E. chaffeensis and 4 (1.39%) A. americanum ticks were infected with E. ewingii. None of the ticks were infected with B. burgdorferi (Table 1). The overall infection prevalence for A. americanum was significantly higher than that for D. variabilis (39% versus 10%; P < 0.0002). Among A. americanum ticks, male infection prevalence was significantly higher than female infection prevalence (45% versus 29%; P < 0.0336).
Table 1.
Prevalence of Rickettsia spp., Borrelia lonestari, Ehrlichia chaffeensis, and Ehrlichia ewingii in ticks from Kentucky, by species and sex
| Tick | Sex | Rickettsia spp., no. positive/no. tested (%) | B. lonestari, no. positive/no. tested (%) | E. chaffeensis, no. positive/no. tested (%) | E. ewingii, no. positive/no. tested (%) | Total, no. positive/no. tested (%) |
|---|---|---|---|---|---|---|
| Amblyomma americanum | 31/108 (28.7) | 1/108 (0.9) | 6/108 (5.6) | 4/108 (3.7) | 42/108 (38.9) | |
| M | 13/33 (39.4) | 0/33 | 3/33 (9.1) | 2/33 (6.1) | 15/33 (45.5)* | |
| F | 18/75 (24) | 1/75 (1.3) | 3/75 (4) | 2/75 (2.7) | 22/75 (29.3)* | |
| Dermacentor variabilis | 10/179 (5.6) | 0/179 | 8/179 (4.5) | 0/179 | 18/179 (10.1) | |
| M | 6/78 (7.7) | 0/78 | 4/78 (5.13) | 0/78 | 10/78 (12.8) | |
| F | 4/101 (4) | 0/101 | 4/101 (4) | 0/101 | 8/101 (7.92) | |
| Totals | 41/287 (14.3) | 1/287 (0.4) | 14/287 (4.9) | 4/287 (1.4) | 60/287 (20.9) |
Ticks were co-infected with multiple species.
Thirty-two (11.1%) ticks were infected with R. amblyommii. Eight (2.8%) D. variabilis ticks were infected with R. montana, and 1 (0.4%) adult male D. variabilis was infected with R. parkeri. No A. americanum ticks were infected with R. montana or R. parkeri (Table 2). There were significantly more R. amblyommii infections than either R. montana or R. parkeri infections (P < 0.0002). Overall, A. americanum ticks were infected with Rickettsia spp. at a significantly higher prevalence than were D. variabilis ticks (28% versus 6.2%; P < 0.0002). Additionally, male A. americanum ticks were infected with Rickettsia spp. at a significantly higher prevalence than female A. americanum ticks (39% versus 23%; P < 0.0184) (Table 2).
Table 2.
Prevalence of Rickettsia species in ticks by species and sex host, Kentucky
| Tick | Sex | Rickettsia amblyommii, no. positive/no. tested (%) | Rickettsia Montana, no. positive/no. tested (%) | Rickettsia parkeri, no. positive/no. tested (%) | Total Rickettsia spp., no. positive/no. tested (%) |
|---|---|---|---|---|---|
| Amblyomma americanum | 30/108 (27.8) | 0/108 | 0/108 | 30/108 (27.8) | |
| M | 13/33 (39.4) | 0/33 | 0/33 | 13/33 (39.4) | |
| F | 17/75 (22.7) | 0/75 | 0/75 | 17/75 (22.7) | |
| Dermacentor variabilis | 2/179 (1.1) | 8/179 (4.5) | 1/179 (0.6) | 11/179 (6.2) | |
| M | 1/78 (1.3) | 4/78 (5.1) | 1/78 (1.3) | 6/78 (7.7) | |
| F | 1/101 (1) | 4/101 (4) | 0/101 | 5/101 (5) | |
| Totals | 32/287 (11.1) | 8/287 (2.8) | 1/287 (0.4) | 41/287 (14.3) |
Four A. americanum ticks were co-infected with two bacterial species. Two ticks were co-infected with R. amblyommii and E. ewingii. One tick was co-infected with R. amblyommii and E. chaffeensis and one tick was co-infected with R. amblyommii and B. lonestari.
Ticks were collected from 6 counties; most (231 ticks, 80.5%) were collected in Warren County. The remaining ticks were collected from five counties in central and western Kentucky. Ticks were removed from a variety of wildlife and domestic hosts; 80 from canines, 33 from feral hogs, 165 from horses, 4 from raccoons, 1 from a white-tailed deer, 1 from a human, and 3 from unknown hosts (Table 3). The ticks removed from the human, raccoons, and unknown hosts were not infected with any of the bacteria tested. The one tick removed from the white-tailed deer was infected with R. amblyommii. Ticks removed from feral hogs had the highest infection prevalence (11 of 33, 33%). Thirty-two (19%) ticks removed from horses and 16 (20%) ticks removed from canines were infected with one or more of the bacterial species tested (Figure 1).
Table 3.
Number of adult ticks collected from a variety of vertebrate hosts by tick species, Kentucky
| Host | Total ticks | Amblyomma americanum | Dermacentor variabilis |
|---|---|---|---|
| Canine | 80 | 19 | 61 |
| Feral hog | 33 | 31 | 2 |
| Horse | 166 | 57 | 109 |
| Human | 1 | 1 | 0 |
| Raccoon | 4 | 0 | 4 |
| White-tailed deer | 1 | 1 | 0 |
| Not documented (unknown) | 3 | 0 | 3 |
Figure 1.
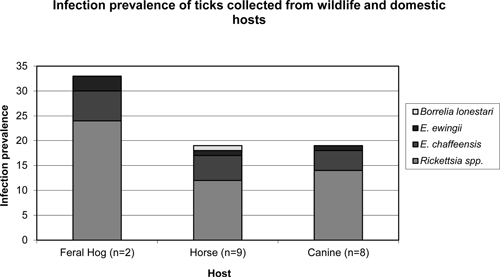
Infection prevalence of ticks collected from the three most common wildlife and domestic hosts in this study, Kentucky.
Discussion
No R. rickettsii, the etiologic agent of RMSF, was found in this study. Cases of RMSF are reported annually across Kentucky. This finding is similar to recent tick surveys in Tennessee, which has one of the highest incidences of RMSF in the United States, where no R. rickettsii was found in more than 1,200 ticks tested.11 Cases reported as RMSF may be caused by other rickettsial species. Disease caused by different rickettsial species may have different presentations and severity. These findings could have important clinical implications in regions where multiple rickettsial species are found.
Forty-one (14.3%) ticks collected in this study were positive for Rickettsia spp. Similar results were found in Mississippi where 26% of A. americanum ticks were infected with Rickettsia spp.47 However, infection prevalence in ticks collected in nearby Tennessee was higher with 44% of A. americanum and 14% of D. variabilis collected infected with a Rickettsia spp. Concomitantly, there are significantly more human RMSF cases reported in Tennessee (224 cases reported in 2008) than in Kentucky.11
Rickettsii amblyommii was the most common pathogen found, which is consistent with findings in other studies, which have found a high prevalence of R. amblyommii in A. americanum ticks in the southeastern and midwestern United States. In a survey conducted in nine states during 1998–2005, A. americanum ticks were infected with R. amblyommii at a prevalence ranging from 0% to 84% and an average infection prevalence of 41.2%.13 These findings were similar to those in a study showing that 40% of A. americanum ticks collected in Tennessee were infected with R. amblyommii.11 Amblyomma americanum ticks removed from humans in Kentucky were shown to be infected with R. amblyommii at a prevalence of 65%.48
Although when originally isolated R. amblyommii was not known to be pathogenic to laboratory animals,49 there have since been several studies possibly linking the bacterial species to human illness. It has been speculated that bites from R. amblyommii infected ticks may be one cause of high seroprevalence of antibodies to SFGR.12 Rickettsia amblyommii has also been temporally associated a macular rash when an engorged A. americanum tick infected with R. amblyommii was removed from a patient.10 In a 2008 study, three of six probable RMSF cases demonstrated a ≥ 4-fold increase in IgG titers between acute-phase and convalescent-phase serum samples to R. amblyommii antigens, and not to R. rickettsii antigens, suggesting that some reported RMSF cases may be caused by infection with R. amblyommii rather than infection with R. rickettsii.9
One male D. variabilis tick removed from a dog was positive for R. parkeri. Recently, one A. americanum adult was found to be infected with R. parkeri in Tennessee.3 Rickettsia parkeri has been recognized as a human pathogen since 2004 after being isolated from an eschar on a man in Virginia15 and was the likely cause of a death in Ohio in 1990.50 Rickettsii parkeri has caused a confirmed human case of rickettsiosis in Mercer County in Kentucky in 2006.51 These studies suggest that R. parkeri, similarly to R. amblyommii, may be the cause of some disease that is reported as RMSF in Kentucky.
Rickettsia montana is a non-pathogenic rickettsiae52 that was found in eight D. variabilis ticks in this study, which is consistent with results of previous studies.53,54 In Tennessee R. montana has been found at higher prevalences in D. variabilis ticks than in A. americanum ticks (10% and 0.3%, respectively).11 This finding is consistent with the trend we found in Kentucky; 4.47% of D. variabilis and no A. americanum being infected. Rickettsia montana has been shown to interfere with the transmission of pathogenic rickettsiae, including R. rickettsii, in D. variabilis ticks experimentally55 and may partly explain the lack of R. rickettsii found in Kentucky.
Borrelia lonestari has been tentatively associated with a Lyme-like illness referred to as STARI in the United States. In this study, one A. americanum (0.9%) tick was infected with B. lonestari. Borrelia lonestari has been found in A. americanum ticks parasitizing humans along the eastern coast and in southeastern and midwestern regions, including 13 ticks from Kentucky from during 2001–2002.26 A study in Missouri found 5.6% of A. americanum ticks to be infected with B. lonestari,56 and Mixson and others found infection prevalence ranging from 0% to 12.2% and an average infection prevalence of 2.5% in nine states.13 Borrelia burgdorferi was not found in any of the ticks we tested, although we did not collect its principal vector (I. scapularis).
Ehrlichia chaffeensis and E. ewingii infection prevalences in this study were similar to those in a survey conducted by Mixson and others.13 In Tennessee, 2.6% of A. americanum ticks were found to be infected with E. chaffeensis, and 0.8% were infected with E. ewingii, and no other Amblyommma, Ixodes, or Dermacentor ticks tested were positive for any Ehrlichia spp.36 In a study conducted during 1996–2001, white-tailed deer from eight states were tested for E. ewingii by PCR. It was found that 5.5% were positive for E. ewingii infection, including one deer from Kentucky. White-tailed deer are an important reservoir for E. chaffeensis and E. ewingii in the southeastern and south central United States and are an abundant wildlife species in Kentucky.37,57
Four (1.4%) ticks were co-infected with two species of bacteria. All of these ticks were A. americanum and were infected with R. amblyommii and one additional bacterial species. Although the pathogenicity of R. amblyommii has not been confirmed, persons bitten by co-infected A. americanum ticks may be at risk of disease complications because of multiple pathogens, although they may not all be causing symptoms. Identifying pathogens in a tick removed from a patient does not identify which organism is causing disease. Amblyomma americanum ticks are aggressive biters58 and are a vector for multiple pathogens, which increases the risk of patients with co-infection. This risk increases as the populations of A. americanum ticks rise and spread throughout the United States. Amblyomma americanum tick populations have been expanding from the southeastern United States into the northeastern and midwestern United States partly because of the increased density of their common host, the white-tailed deer57,59–61 and as such are an emerging threat to public health.
Amblyomma americanum ticks had higher infection prevalence than D. variabilis ticks, potentially because of their aggressive biting behavior. In addition, A. americanum ticks have a broader host range than D. variabilis, which may also contribute to the higher infection prevalence. Also, A. americanum ticks are the vector for the most abundant pathogen found in this study (R. amblyommii) (Tables 1 and 2). Additionally, males had a higher Rickettsia spp. infection prevalence in A. americanum and D. variabilis ticks (Tables 1 and 2). This prevalence is potentially caused by the life cycle of male and female ticks. Female ticks take large blood meals to support egg production. After all eggs are deposited, the female dies. Males may live longer and take several blood meals from multiple hosts, which increases the potential for contracting and transmitting bacterial pathogens.62
Most (80.5%) of the ticks were collected in Warren County in Kentucky. There are 120 counties in Kentucky, of which 12 counties, dispersed throughout the state, where 16 RMSF cases have been diagnosed during 2006–2010. Although there were no cases of RMSF diagnosed in Warren County in this five-year period, bordering counties have had diagnosed RMSF cases. There were 27 cases of Lyme disease reported in Kentucky during 2005–2010. These cases, similar to reported cases of RMSF, were distributed throughout the state. There were 16 counties that reported Lyme disease during 2005–2010; we collected ticks from 3 of these counties and from bordering counties. There was no significant difference between tick densities found on the variety of hosts among all six counties. Because ticks were collected as a convenience sampling on USDA APHIS-WS nuisance calls, no host-seeking ticks were collected. Ticks removed from feral hogs had the highest infection prevalences. This finding may be potentially caused by their lifestyle because it has been documented that they migrate through many different types of habitats and spend approximately half of their time grazing in shrub lands that may be heavily tick infested.63
The absence of R. rickettsii in this study should be investigated further. If ticks are co-infected with multiple rickettsial species, R. rickettsii may not be amplified by our molecular methods. Rhipicephalus sanguineus ticks have recently been found to be a vector for R. rickettsii in Arizona, but no R. sanguineus ticks were collected in this study.64 Serologic studies should be conducted in RMSF patients to determine whether other SFGR, such as R. amblyommii and R. parkeri, are causing disease in humans. There should be regular surveys to monitor Ehrlichia infection prevalences in ticks over time to determine if increases in human ehrlichiosis cases in Kentucky are related to increased infection in the tick populations. Lastly, additional studies should be conducted to determine what species of Borrelia are causing the Lyme disease cases reported annually in Kentucky.
In this study, we found multiple pathogens that are known or suspected causes of human illness. Physicians should be aware of the common tickborne diseases in their area of practice and include them in the differential diagnosis for patients with a febrile illness. It is important for physicians to be aware that multiple species of Rickettsiae, Borrelia and Ehrlichiae can cause tick-borne diseases.
Footnotes
Authors' addresses: Charissa M. Fritzen, Junjun Huang, Kathleen Westby, John R. Dunn, Timothy F. Jones, and Abelardo C. Moncayo, Tennessee Department of Health, Vector-Borne Diseases Section, Communicable and Environmental Diseases, Nashville, TN, E-mails: cmn37@cornell.edu, Junjun.Huang@tn.gov, kmwest2@ilstu.edu, John.Dunn@tn.gov, Tim.F.Jones@tn.gov, and Abelardo.Moncayo@tn.gov. James D. Freye and Brett Dunlap, United States Department of Agriculture, Animal and Plant Health Inspection Service, Wildlife Services, Madison, TN, E-mails: James.D.Freye@aphis.usda.gov and Brett.G.Dunlap@aphis.usda.gov. Michael J. Yabsley, Southeastern Cooperative Wildlife Disease Study, University of Georgia, Athens, GA, E-mail: myabsley@uga.edu. Mike Schardein, Kentucky Department for Public Health, Frankfort, KY, E-mail: Mike.Schardein@ky.gov.
References
- 1.Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spach DH, Liles WC, Campbell GL, Quick RE, Anderson DE, Fritsche TR. Tick borne diseases in the United States. New Engl Med. 1993;329:936–947. doi: 10.1056/NEJM199309233291308. [DOI] [PubMed] [Google Scholar]
- 3.Cohen SB, Yabsley MJ, Garrison LE, Freye JD, Dunlap BG, Dunn JR, Mead DG, Jones TF, Moncayo AC. Rickettsia parkeri in Amblyomma americanum ticks, Tennessee and Georgia, USA. Emerg Infect Dis. 2009;15:1471–1473. doi: 10.3201/eid1509.090330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burgdorfer W. A review of Rocky Mountain spotted fever (tick borne typhus), its agent, and its tick vectors in the United States. J Med Entomol. 1975;12:269–278. doi: 10.1093/jmedent/12.3.269. [DOI] [PubMed] [Google Scholar]
- 5.Demma LJ, Traeger M, Blau D, Gordon R, Johnson B, Dickson J, Ethelbah R, Piontkowski S, Levy C, Nicholson WL, Duncan C, Heath K, Cheek J, Swerdlow DL, McQuiston JH. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector Borne Zoonotic Dis. 2006;6:423–429. doi: 10.1089/vbz.2006.6.423. [DOI] [PubMed] [Google Scholar]
- 6.Kentucky Department for Public Health Five-year reportable disease summary. The Reportable Diseases Summary. 2009:1–25. [Google Scholar]
- 7.Dasch GA, Kelly DJ, Richards AL, Sanchez JL, Rives CC. Western blotting analysis of sera from military personnel exhibiting serological reactivity to spotted fever group rickettsiae. Am Soc Trop Med Hyg. 1993;49:220. [Google Scholar]
- 8.Sanchez JL, Candler WH, Fishbein DB, Green CR, Cote TR, Kelly DJ, Driggers DP, Johnson BJ. A cluster of tick-borne infections: association with a military training and asymptomatic infections due to Rickettsia rickettsii. Trans R Soc Trop Med Hyg. 1992;86:321–325. doi: 10.1016/0035-9203(92)90330-f. [DOI] [PubMed] [Google Scholar]
- 9.Apperson CS, Engber B, Nicholson WL, Mead DG, Engel J, Yabsley MJ, Dail K, Johnson J, Watson DW. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 2008;8:597–606. doi: 10.1089/vbz.2007.0271. [DOI] [PubMed] [Google Scholar]
- 10.Billeter SA, Blanton HL, Little SE, Levy MG, Breitschwerdt EB. Detection of “Rickettsia amblyommii” in association with a tick bite rash. Vector Borne Zoonotic Dis. 2007;7:607–610. doi: 10.1089/vbz.2007.0121. [DOI] [PubMed] [Google Scholar]
- 11.Moncayo AC, Cohen SB, Fritzen CM, Huang E, Yabsley MJ, Freye JD, Dunlap BG, Huang J, Mead DG, Jones TF, Dunn JR. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653–657. doi: 10.4269/ajtmh.2010.09-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stromdahl EY, Vince MA, Billingsley PM, Dobbs NA, Williamson PC. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15–24. doi: 10.1089/vbz.2007.0138. [DOI] [PubMed] [Google Scholar]
- 13.Mixson TR, Campbell SR, Gill JS, Ginsberg HS, Reichard MV, Schulze TL, Dasch GA. Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol. 2006;43:1261–1268. doi: 10.1603/0022-2585(2006)43[1261:poebar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 14.Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 15.Paddock CD, Sumner JW, Comer JA, Zaki SR, Goldsmith CS, Goddard J, McLellan CL, Ohl CA. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:805–811. doi: 10.1086/381894. [DOI] [PubMed] [Google Scholar]
- 16.Whitman TJ, Richards AL, Paddock CD, Tamminga CL, Sniezek PJ, Jiang J, Byers DK, Sanders JW. Rickettsia parkeri Infection after tick bite, Virginia. Emerg Infect Dis. 2007;13:334–336. doi: 10.3201/eid1302.061295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orloski KA, Hayes ED, Campbell GL. Surveillance for lyme disease–United States, 1992–1998. MMWR Surveill Summ. 2000;49:1–11. [PubMed] [Google Scholar]
- 18.Masters EJ. Erythema migrans: early diagnosis and treatment. Postgrad Med. 1993;94:133–142. [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Surveillance for lyme disease—United States, 1992–2006. MMWR Morb Mortal Wkly Rep. 2008;57:1–9. [Google Scholar]
- 20.Varela AS, Luttrell MP, Howerth EW, Moore VA, Davidson WR, Stallknecht DE, Little SE. First culture isolation of Borrelia lonestari, putative agent of southern tick-associated rash illness. J Clin Microbiol. 2004;42:1163–1169. doi: 10.1128/JCM.42.3.1163-1169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burkot TR, Mullen GR, Anderson R, Schneider BS, Happ CM, Zeidner NS. Borrelia lonestari DNA in adult Amblyomma americanum ticks, Alabama. Emerg Infect Dis. 2001;7:471–473. doi: 10.3201/eid0703.010323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James AM, Liveris D, Wormser GP, Schwartz I, Montecalvo MA, Johnson BJ. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J Infect Dis. 2001;183:1810–1814. doi: 10.1086/320721. [DOI] [PubMed] [Google Scholar]
- 23.Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification on an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a lyme-disease like illness. J Infect Dis. 1996;173:403–409. doi: 10.1093/infdis/173.2.403. [DOI] [PubMed] [Google Scholar]
- 24.Masters EJ, Grigery CN, Masters RW. STARI, or Masters disease: Lone Star tick-vectored lyme-like illness. Infect Dis Clin North Am. 2008;22:361–376. doi: 10.1016/j.idc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong PM, Rich SM, Smith RD, Hartl DL, Spielman A, Telford SR III. A new Borrelia infecting Lone Star ticks. Lancet. 1996;347:67–68. doi: 10.1016/s0140-6736(96)91604-9. [DOI] [PubMed] [Google Scholar]
- 26.Stromdahl EY, Williamson PC, Kollars TM, Jr, Evans SR, Barry RK, Vince MA, Dobbs NA. Evidence of Borrelia lonestari DNA in Amblyomma americanum (Acari: Ixodidae) removed from humans. J Clin Microbiol. 2003;41:5557–5562. doi: 10.1128/JCM.41.12.5557-5562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olano JP, Walker DH. Human ehrlichioses. Med Clin North Am. 2002;86:375–392. doi: 10.1016/s0025-7125(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 28.Anderson BE, Sumner JW, Dawson JE, Tzianabos T, Greene CR, Olson JG, Fishbein DB, Olsen-Rasmussen M, Holloway BP, George EH. Detection of the etiologic agent of human monocytic ehrlichiosis by polymerase chain reaction. J Clin Microbiol. 1992;30:775–780. doi: 10.1128/jcm.30.4.775-780.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawson JE, Anderson BE, Fishbein DB, Sanchez JL, Goldsmith CS, Wilson KH. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–2745. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockhart JM, Davidson WR, Stallknecht DE, Dawson DE, Howerth EW. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, Fox JC, Kocan KM, Blouin EF. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- 32.Dawson JE, Childs JE, Biggie KL, Moore C, Stallknecht D, Shaddock J, Bouseman J, Hofmeister E, Olson JG. White-tailed deer as a potential reservoir of Ehrlichia spp. J Wildl Dis. 1994;30:162–168. doi: 10.7589/0090-3558-30.2.162. [DOI] [PubMed] [Google Scholar]
- 33.Dawson JE, Stallknecht DE, Howerth EW, Warner C, Biggie K, Davidson WR, Lockhart JM, Nettles VF, Olson JG, Childs JE. Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–2728. doi: 10.1128/jcm.32.11.2725-2728.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McQuiston JH, Paddock CD, Holman RC, Childs JE. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–642. doi: 10.3201/eid0505.990504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anziani O, Ewing S, Barker R. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am J Vet Res. 1990;51:929–931. [PubMed] [Google Scholar]
- 36.Cohen SB, Yabsley MJ, Freye JD, Dunlap BG, Rowland ME, Huang J, Dunn JR, Jones TF, Moncayo AC. Prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in ticks from Tennessee. Vector Borne Zoonotic Dis. 2009;10:435–440. doi: 10.1089/vbz.2009.0058. [DOI] [PubMed] [Google Scholar]
- 37.Yabsley MJ, Varela AS, Tate CM, Dugan VG, Stallknecht DE, Little SE, Davidson WR. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus) Emerg Infect Dis. 2002;8:668. doi: 10.3201/eid0807.020018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikihisa Y, Unver A, Gaudreault-Keener M, Manian FA, Liddell AM, Schmulewitz N, Storch GA. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–155. doi: 10.1056/NEJM199907153410303. [DOI] [PubMed] [Google Scholar]
- 39.Himmel SP, Buller R, Arens M, Gaudreault-Keener M, Storch GA. Proceedings of the First International Conference on Emerging Infectious Diseases. Atlanta, Georgia: Addendum; 1998. p. 4. (Human infection with Ehrlichia ewingii, the agent of Ozark canine granulocytic ehrlichiosis). March 8–11, 1998. (Abstract) [Google Scholar]
- 40.Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida:Ixodoidea), east of the Mississippi River. J Med Entomol. 1989;26:435–448. doi: 10.1093/jmedent/26.5.435. [DOI] [PubMed] [Google Scholar]
- 41.Loftis AD, Reeves WK, Szumlas DE, Abbassy MM, Helmy IM, Moriarity JR, Dasch GA. Surveillance of Egyptian fleas for agents of public health significance: Anaplasma, Bartonella, Coxiella, Ehrlichia, Rickettsia, and Yersinia pestis. Am J Trop Med Hyg. 2006;75:41–48. [PubMed] [Google Scholar]
- 42.Eremeeva M, Yu X, Raoult D. Differentiation among spotted fever group rickettsiae species by analysis of restriction fragment length polymorphism of PCR-amplified DNA. J Clin Microbiol. 1994;32:803–810. doi: 10.1128/jcm.32.3.803-810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sumner JW, Childs JE, Paddock CD. Molecular variation of the Ehrlichia chaffeensis variable length PCR target: an antigen-expressing gene that exhibits interstrain variation. J Clin Microbiol. 1999;37:1447–1453. doi: 10.1128/jcm.37.5.1447-1453.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour A. Typing of Borrelia relapsing fever group strains. Emerg Infect Dis. 2004;10:1661–1664. doi: 10.3201/eid1009.040236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demaerschalck I, Messaoud AB, Kesel MD, Hoyois B, Lobet Y, Hoet P, Bigaignon G, Bollen A, Godfroid E. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J Clin Microbiol. 1995;33:602–608. doi: 10.1128/jcm.33.3.602-608.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sparagano OA, Allsopp MP, Mank RA, Rijpkena ST, Figueroa JV, Jongejan F. Molecular detection of pathogen DNA in ticks (Acari: Ixodidae): a review. Exp Appl Acarol. 1999;23:929–960. doi: 10.1023/a:1006313803979. [DOI] [PubMed] [Google Scholar]
- 47.Goddard J, Sumner JW, Nicholson WL, Paddock CD, Shen J, Piesman J. Survey of ticks collected in Mississippi for Rickettsia, Ehrlichia and Borrelia species. J Vector Ecol. 2003;28:184–189. [PubMed] [Google Scholar]
- 48.Jiang J, Yarina T, Miller MK, Stromdahl EY, Richards AL. Molecular detection of Rickettsia amblyommii in Amblyomma americanum parasitizing humans. Vector Borne Zoonotic Dis. 2010;10:329–340. doi: 10.1089/vbz.2009.0061. [DOI] [PubMed] [Google Scholar]
- 49.Burgdorfer W, Hayes SF, Thomas LA, Lancaster JL., Jr . In: Rickettsiae and Rickettsial Diseases. Burgdorfer W, Anacker RL, editors. New York: Academic Press; 1981. pp. 595–602. (A new spotted fever group Rickettsia from the lone star tick, Amblyomma americanum). [Google Scholar]
- 50.Ralph D, Pretzman C, Daugherty N, Poetter K. Genetic relationships among the members of the family Rickettsiaceae as shown by DNA restriction fragment polymorphism analysis. Ann N Y Acad Sci. 1990;590:541–552. doi: 10.1111/j.1749-6632.1990.tb42264.x. [DOI] [PubMed] [Google Scholar]
- 51.Paddock CD, Finley RW, Wright CS, Robinson HN, Schrodt BJ, Lane CC, Ekenna O, Blass MA, Tamminga CL, Ohl CA, McLellan SL, Goddard J, Holman RC, Openshaw JJ, Sumner JW, Zaki SR, Eremeeva ME. Rickettsia parkeri rickettsiosis and its clinical distinction from Rocky Mountain spotted fever. Clin Infect Dis. 2008;47:1188–1196. doi: 10.1086/592254. [DOI] [PubMed] [Google Scholar]
- 52.Breitschwerdt EB, Walker DH, Levy MG, Burgdorfer W, Corbett WT, Hurlbert SA, Stebbins ME, Curtis BC, Allen DA. Clinical, hematologic, and humoral immune response in female dogs inoculated with Rickettsia rickettsii and Rickettsia montana. Am J Vet Res. 1988;49:70–76. [PubMed] [Google Scholar]
- 53.Anderson JF, Magnarelli LA, Philip RN, Burgdorfer W. Rickettsia rickettsii and Rickettsia montana from Ixodid ticks in Connecticut. Am J Trop Med Hyg. 1986;35:187–191. doi: 10.4269/ajtmh.1986.35.187. [DOI] [PubMed] [Google Scholar]
- 54.Stromdahl EY, Evans SR, O'Brien JJ, Gutierrez AG. Prevalence of infection in ticks submitted to the human tick test kit program of the U.S. Army Center for Health Promotion and Preventive Medicine. J Med Entomol. 2001;38:67–74. doi: 10.1603/0022-2585-38.1.67. [DOI] [PubMed] [Google Scholar]
- 55.Macaluso KR, Sonenshine DE, Ceraul SM, Azad AF. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J Med Entomol. 2002;39:809–813. doi: 10.1603/0022-2585-39.6.809. [DOI] [PubMed] [Google Scholar]
- 56.Bacon RM, Gilmore RD, Jr, Quintana M, Piesman J, Johnston BJ. DNA evidence of Borrelia lonestari in Amblyomma americanum (Acari: Ixodidae) in southeastern Missouri. J Med Entomol. 2003;40:590–592. doi: 10.1603/0022-2585-40.4.590. [DOI] [PubMed] [Google Scholar]
- 57.Yabsley MJ, Wimberly MC, Stallknecht DE, Little SE, Davidson WR. Spatial analysis of the distribution of Ehrlichia chaffeensis, causative agent of human monocytotropic ehrlichiosis, across a multiple state region. Am J Trop Med Hyg. 2005;72:840–850. [PubMed] [Google Scholar]
- 58.Felz MW, Durden LA. Attachment sites of four tick species (Acari: Ixodidae) parasitizing humans in Georgia and South Carolina. J Med Entomol. 1999;24:178–188. doi: 10.1093/jmedent/36.3.361. [DOI] [PubMed] [Google Scholar]
- 59.Mount GA, Haile DG, Darnerd DR, Daniels E. New version of LSTSIM for computer simulation of Amblyomma americanum (Acari: Ixodidae) population dynamics. J Med Entomol. 1993;30:843–857. doi: 10.1093/jmedent/30.5.843. [DOI] [PubMed] [Google Scholar]
- 60.Felz MW, Durden LA, Oliver JH., Jr Ticks parasitizing humans in Georgia and South Carolina. J Parasitol. 1996;82:505–508. [PubMed] [Google Scholar]
- 61.Means RG, White DJ. New distribution records of Amblyomma americanum (L.) (Acari: Ixodidae) in New York State. J Vector Ecol. 1997;22:133–145. [PubMed] [Google Scholar]
- 62.Goodman JL, Dennis DT, Sonenshine DE. Tick-Borne Diseases of Humans. Washington, DC: American Society for Microbiology Press; 2005. [Google Scholar]
- 63.Cooper SM, Scott HM, de la Garza GR, Deck AL, Cathey JC. Distribution and interspecies contact of feral swine and cattle on rangeland in south Texas: implications for disease transmission. J Wildl Dis. 2010;46:152–164. doi: 10.7589/0090-3558-46.1.152. [DOI] [PubMed] [Google Scholar]
- 64.Demma LJ, Traeger MS, Nicholson WL, Paddock CD, Blau DM, Eremeeva ME, Dasch GA, Levin ML, Singleton J, Jr, Zaki SR, Cheek JE, Swerdlow DL, McQuiston JH. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587–594. doi: 10.1056/NEJMoa050043. [DOI] [PubMed] [Google Scholar]


