Abstract
A dengue vaccine effective against all four serotypes is urgently needed. However, safety and immunogenicity could be affected by prior exposure to flaviviruses. This open, controlled, phase IIa study was conducted in 35 healthy adults who had received monovalent, live attenuated Vero cell-derived dengue vaccine against dengue virus 1 (VDV1) or 2 (VDV2) or yellow fever (YF) vaccine 1 year before or who were flavivirus-naïve. All participants received one subcutaneous injection of tetravalent dengue vaccine (TDV) and were followed for 180 days. Previous vaccination did not increase reactogenicity, laboratory abnormalities, or incidence of vaccine viremia, but it did increase the neutralizing antibody response to dengue virus that persisted at day 180. There was no increase in YF antibodies in participants previously immunized with YF vaccine. Prior exposure to YF or monovalent dengue vaccines had no adverse effects on the safety or incidence of viremia associated with this TDV, but it increased immunogenicity.
Introduction
Dengue virus is a member of the positive-strand RNA flavivirus genus that includes yellow fever (YF) and other arthropod-borne viruses that cause human disease. Infection with any of the four antigenically distinct dengue serotypes generally leads to a mild, if temporarily debilitating, self-limiting disease, but it can result in dengue hemorrhagic fever and subsequently, dengue shock syndrome. Although infection with one serotype confers immunity to subsequent infection with the same serotype, it does not provide durable protection against infection with other serotypes.1 Thus, epidemics of different serotypes can circulate simultaneously, and an individual can suffer secondary and tertiary dengue infections. Moreover, progression from dengue fever to dengue hemorrhagic fever or dengue shock syndrome seems to be facilitated by a prior infection with a different serotype.2
In the absence of an effective specific treatment of dengue, control of the disease relies on suppression of the main arthropod vector Aedes aegypti (although Ae. albopictus is an increasingly important vector in the Americas) or the development of appropriate vaccines. No dengue vaccines are currently available; however, given the global scale of the dengue problem and the expense of mosquito prevention measures, vaccine development has become a public health priority worldwide. Sanofi Pasteur has developed a live attenuated tetravalent dengue vaccine (TDV) using recombinant technology. This vaccine contains four recombinant viruses, each of which has the genes encoding for dengue pre-membrane and envelope proteins (the two main antigens) of one of four dengue serotypes and the genes encoding the non-structural and capsid proteins of the attenuated YF 17D vaccine virus.3–5 The resulting viruses possess the antigenicity of the parental dengue virus and the well-characterized replication ability of the YF 17D strain. A previous trial6 evaluated the safety profile of three doses of a TDV that contains 5 log10 cell culture infectious dose (CCID50) of each recombinant dengue serotype in flavivirus-naive adults. This TDV was shown to be well-tolerated, and it induced high immunogenicity against all four serotypes after three doses.
Target populations for the dengue vaccine could have been exposed to flaviviruses through infection or vaccination. YF is present in South America in the same endemic areas as dengue, and YF immunization is routine in some countries. Prior flavivirus exposure may impact the infectivity, safety, and immunogenicity of TDV. Before the first clinical trial with TDV, a preliminary trial with the monovalent dengue virus-2 vaccine, produced with the same technology as that used for TDV, suggested that prior YF vaccination can induce a slight increase in immune response and vaccine virus viremia as well as cross-neutralizing antibodies against other serotypes.7 Moreover, carrier-induced suppression might also occur given the shared genetic sequence between YF 17D and the recombinant dengue vaccine viruses, although this was not observed in the trial described above or in a phase I trial of a Japanese encephalitis vaccine using the same YF 17D backbone.8
We further investigated the effect of previous immunization with an investigational, live attenuated, whole-virion monovalent dengue vaccine or YF vaccine on infectivity, safety, and humoral immunogenicity of a single injection of TDV in healthy young adults. The effect on cell-mediated immunity has been reported previously.9
Methods
This open, controlled, phase IIa study was conducted in a single center in Australia.9 The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonization guidelines, and European Directive 2001/20/EC as well as national and local requirements regarding ethical conduct. The study documents were approved by the Royal Adelaide Hospital Research Ethics Committee before the start of the trial. All participants provided written informed consent.
Participants.
Three groups of participants were recruited. Volunteers who had previously received one injection of an investigational monovalent, live attenuated Vero cell-derived dengue vaccine to dengue virus-1 (VDV1) or dengue virus-2 (VDV2; VDV group) or one injection of YF vaccine (Stamaril; Sanofi Pasteur, Lyon, France; YF group) 1 year before in a phase 1 clinical trial were compared with flavivirus-naive participants confirmed by a negative serological test (naive group). The VDV vaccine approach has been previously described.10 VDV1 and VDV2 are two live attenuated whole-virion vaccines cultured on Vero cells. All VDV1 and VDV2 recipients except one and all YF recipients seroconverted (neutralizing antibody titer ≥ 10) to homologous dengue strain or YF after the previous vaccination.
Healthy men and women aged 18–40 years were eligible for participation. Women were required to not be pregnant (negative pregnancy test) or breastfeeding and be using an effective method of contraception means of birth control from ≥ 4 weeks before vaccination until 4 weeks after vaccination. People with a history of thymic pathology, thymectomy, myasthenia gravis, immunodeficiency or immunosuppressive therapy, systemic hypersensitivity to any study injection or component, or flavivirus infection or vaccination (except those administered in the previous dengue trial) and people planning flavivirus vaccination during the study period or travel during the present trial period to areas with high dengue endemicity were excluded. Those with comorbid conditions that could interfere with trial conduct or completion, pre-defined laboratory abnormalities at screening, or seropositive tests for HIV or hepatitis B or C were also ineligible.
Volunteers for the flavivirus-naive control group were screened using two epitope-blocking enzyme-linked immunosorbent assay (ELISA) tests developed at the Arbovirus and Emerging Disease Unit of Westmead Hospital (Westmead, Australia): (1) a pan flavivirus ELISA covering detected antibodies to all members of the flavivirus family, including dengue 1, 2, 3, and 4, Japanese encephalitis, and YF (1) and (2) a dengue-specific antibody test. In both assays, total antibody regardless of antibody class, directed against a viral antigen, is measured by combining the test sample with a pre-determined amount of conjugate (in this case, a monoclonal antibody directed against the antigen) and incubating the mixture with the antigen-coated solid phase. Specific antibody, if present, competes with the conjugated monoclonal antibody for binding sites on the solid phase, leading to a reduction in signal. The signal generation is inversely proportional to the amount of antibody present.
Treatment.
All participants received a single injection of the TDV (Sanofi Pasteur). The vaccine was stored frozen at < −70°C. Each dose contained approximately 5 log10 cell culture infectious dose (CCID50) of each vaccine virus. TDV is formulated in minimum essential medium containing human serum albumin (2.5%; United States Pharmacopeia) and lactose (7.5%). All vaccines were supplied by Sanofi Pasteur (Lyon, France). The vaccine was administered subcutaneously to the deltoid region of the upper arm in a volume of 0.5 mL.
Outcome measures.
Assessments were performed at screening, on the day of vaccination, and 7, 14, 21, 28, 60, and 180 days after vaccination.
Safety.
Study personnel assessed safety for 30 minutes after each vaccination. Participants used specially provided diary cards to record solicited injection-site reactions of pain, erythema, and swelling for 7 days and systemic reactions of fever (oral), headache, malaise, myalgia, and asthenia for 14 days after injection. Spontaneously reported adverse events occurring up to 28 days after injection were also recorded. Serious adverse events were recorded throughout the study. At each visit, the investigator interviewed the participants concerning any solicited reactions and unsolicited adverse events recorded on the diary card as well as any other adverse events that may have occurred, and performed a clinical examination when necessary. Biochemistry and hematology analyses were performed on blood collected before vaccination and on days 7, 14, and 28. These time points were chosen based on the findings of similar analyses performed during phase I on blood collected on days 4, 8, 12, 16, and 28.6 A pre-defined severity scale was used to categorize laboratory parameters and solicited reactions as mild, moderate, or severe (Table 1). The investigator assessed the severity of adverse events and determined their relationship to study injection based on clinical findings. By convention, solicited adverse events were considered to be related to the vaccine and are referred to as solicited reactions.
Table 1.
Severity scales used to categorize laboratory parameters and solicited reactions
| Mild | Moderate | Severe | |
|---|---|---|---|
| Pain | Easily tolerated | Sufficiently discomforting to interfere with normal behavior or activities | Incapacitating, unable to perform usual activities, may have and/or require medical care or absenteeism |
| Erythema or edema | < 2.5 cm | ≥ 2.5 to < 5 cm | ≥ 5 cm |
| Fever | ≥ 37.5°C to 38°C oral | ≥ 38.1°C to 39.0°C oral | ≥ 39.1°C oral |
| Headache, malaise, myalgia, or asthenia | Noticeable but does not interfere with daily activities | Interferes with daily activities | Prevents daily activities |
| Hb decrease (female; g/dL) | ≥ 9.4 to ≤ 10.9 | ≥ 8.4 to < 9.4 | < 8.4 |
| Hb decrease (male; g/dL) | ≥ 11.3 to ≤ 12.8 | ≥ 10.3 to < 11.3 | < 10.3 |
| WBC decrease (cells/mm3) | < LLN to ≥ 3,000 | < 3,000 to ≥ 2,000 | < 2,000 |
| Lymphocytes decrease (cells/mm3) | < LLN to ≥ 700 | < 700 to ≥ 500 | < 500 |
| Neutrophils decrease (cells/mm3) | < LLN to ≥ 1,500 | < 1,500 to ≥ 1,000 | < 1,000 |
| Platelets decrease (cells/mm3) | < LLN to ≥ 100,000 | < 100,000 to ≥ 75,000 | < 75,000 |
| ALT (increase by factor) | > ULN to < 2.5 × ULN | ≥ 2.5 to < 4.0 × ULN | ≥ 4.0 × ULN |
| AST (increase by factor) | > ULN to < 2.5 × ULN | ≥ 2.5 to < 4.0 × ULN | ≥ 4.0 × ULN |
| Serum creatinine (increase by factor) | > ULN to < 1.5 × ULN | ≥ 1.5 to < 2.0 × ULN | ≥ 2.0 × ULN |
| CPK (increase by factor) | > ULN to < 2.5 × ULN | ≥ 2.5 to < 4.0 × ULN | ≥ 4.0 × ULN |
| Bilirubin total (increase by factor) | > ULN to < 1.5 × ULN | ≥ 1.5 to < 2.0 × ULN | ≥ 2.0 × ULN |
ULN = upper limit of normal; LLN = lower limit of normal.
Viremia.
The occurrence and quantification of the four dengue vaccine serotypes were determined in sera before vaccination and on days 7, 14, and 21. These time points were chosen based on the findings of similar analyses performed during phase I on blood collected every 2 days for 20 days after vaccination, showing that viremia occurred at low levels and mainly on days 8 and 14.6 Sera were first tested for non-serotype–specific TDV viremia by reverse transcriptase-polymerase chain reaction (RT-PCR) with primers from a YF virus-specific 5′-end gene sequence targeting the YF genes common to all vaccine serotypes. Results were quantified by a nucleic acid-based assay and expressed as a concentration of log10 plaque forming unit (PFU) equivalents/mL (lowest level of quantitation [LLOQ] = 1.46 log10 PFU/mL). Positive samples were tested by serotype-specific quantitative RT-PCR using primers spanning the junction between the specific inserted envelope sequence and the YF NS-1 downstream sequence and quantitative tissue culture by plaque assay.11,12 This RT-PCR only detected viremia caused by recombinant rather than wild-type virus. RT-PCR results for infections were expressed as concentrations in log10 genome equivalents (GEQ) per milliliter; LLOQs were 4.83, 4.74, 4.97, and 4.99 log10 GEQ/mL for dengue virus-1 to -4, respectively (< 1.7 log10 PFU/mL). Virus titer was also assessed using a standard plaque assay with Vero cells and specific immunostaining for each serotype, with results expressed as log10 PFU/mL (with a LLOQ of 1.6 log10 PFU/mL). These analyses were performed at the sponsor's Global Clinical Immunology Laboratory (Swiftwater, PA).
Immunogenicity.
Neutralizing antibody levels against the four dengue serotypes (parental wild-type strains of the vaccine)6,7 were measured by plaque reduction neutralization tests (PRNT50) before vaccination and on days 28, 60, and 180.13 Serial twofold dilutions of heat-inactivated serum starting at 1:10 were mixed with a challenge dose of each dengue serotype (1–4) and inoculated in duplicate into 24-well plates of confluent Vero cells. After adsorption, the cell monolayers were overlaid and incubated for a few days. The plaques were then visualized by immunostaining with dengue serotype-specific monoclonal antibodies. The neutralizing antibody titer based on the initial serum dilution was expressed as the reciprocal serum dilution reducing the mean plaque count by 50% compared with dengue antibody-negative control serum. The LLOQ was 10. Neutralizing antibody levels against the YF 17D strain were also measured by PRNT80 before vaccination and on day 28. The LLOQ was 5. These analyses were performed at the sponsor's Global Clinical Immunology Laboratory (Swiftwater, PA).
Statistical analyses.
All analyses were descriptive and conducted by group with 95% confidence intervals (CIs) for the main parameters. To calculate geometric mean neutralizing antibody titers (GMTs), samples with titers below the assay's LLOQ were assigned the value LLOQ/2 (i.e., 5). Participants with values ≥ 10 were considered seropositive for the corresponding serotype. The primary analyses considered VDV1- and VDV2-primed participants as a single group. Exploratory analyses were performed on VDV1-primed and VDV2-primed subgroups.
Statistical analyses were performed using SAS software (version 8.2 or later).
Results
Forty-two participants were screened; 40 were considered eligible for inclusion, and 35 were enrolled and received a single injection of TDV. Of these 35 participants, 15 were in the VDV-primed group (VDV1 subgroup = 7 and VDV2 subgroup = 8), 8 were in the YF-primed group, and 12 were in the naïve group. All 35 completed the study. The demographic characteristics were comparable across groups (Table 2).
Table 2.
Demographic characteristics
| VDV1-primed (N = 7) | VDV2-primed (N = 8) | All VDV-primed (N = 15) | YF-primed (N = 8) | Naive (N = 12) | |
|---|---|---|---|---|---|
| Male/female (n) | 3/4 | 5/3 | 8/7 | 4/4 | 5/7 |
| Mean ± SD age (years) | 23.7 ± 2.8 | 25.4 ± 6.7 | 24.6 ± 5.2 | 25.9 ± 6.6 | 27.4 ± 6.4 |
| Mean ± SD weight (kg) | 73.0 ± 12.5 | 76.7 ± 13.5 | 75.0 ± 12.7 | 78.3 ± 17.6 | 83.0 ± 14.5 |
| Mean ± SD height (cm) | 175.6 ± 13.9 | 173.3 ± 4.9 | 174.3 ± 9.8 | 176.4 ± 10.9 | 177.1 ± 9.6 |
| Mean ± SD BMI (kg/m2) | 23.6 ± 2.4 | 25.7 ± 5.3 | 24.7 ± 4.2 | 24.9 ± 3.2 | 26.4 ± 3.8 |
BMI = body mass index.
At baseline, a few samples from VDV-primed participants showed a neutralizing response against the dengue vaccine serotypes 1, 2, and 3 (Figure 1), and none of these participants was seropositive to YF. Three of seven participants primed with VDV1 and zero of those primed with VDV2 were seropositive against serotype 1, and seven of eight participants primed with VDV2 and zero of those primed with VDV1 were seropositive against serotype 2 at baseline. In contrast, all eight YF-primed participants were seropositive for YF, and one was also seropositive for the dengue serotype 2. None of the participants enrolled in the naïve group had detectable antibodies against YF or any of the four dengue serotypes, confirming their naïve status.
Figure 1.
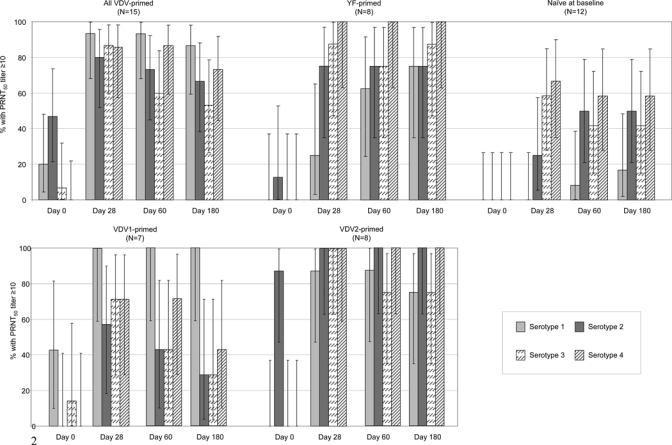
Seropositivity rate against each dengue serotype (parental dengue strain) before and 28, 60, and 180 days after a single injection of tetravalent dengue vaccine. Error bars indicate 95% confidence intervals.
Safety.
There were no serious adverse events, immediate systemic adverse events, or other significant adverse events. There was no notable effect of prior vaccination with either VDV or YF on the reactogenicity of TDV compared with the reactogenicity profile observed in the baseline-naïve participants (Table 3). The proportion of participants reporting each category of adverse event seemed comparable between the three groups, and the most commonly reported reaction in each group was headache (i.e., did not seem to be higher in the VDV- and YF-primed groups than in the naïve group). Most reactions were mild or moderate (Table 3). No severe injection-site reaction was reported. Two participants presented severe systemic reactions: one VDV1-primed participant experienced headache on day 13 after vaccination, and one YF-primed participant experienced malaise concomitantly with a severe upper respiratory tract infection (unrelated to vaccination) on day 10 after vaccination. Both reactions resolved after 1 day. Although systemic reactions were solicited for a 2-week period, most occurred within the first 7 days of vaccination and lasted no more than 3 days. Pyrexia was observed in one participant in the VDV-primed group and three participants in the naïve group. Only one (a naive-group participant) had a case that lasted longer than 1 day.
Table 3.
Adverse reactions (solicited adverse reaction or unsolicited adverse event considered as related to the vaccine) reported after a single tetravalent dengue vaccine injection by Medical Dictionary for Regulatory Activities (MedDRA) system organ class and preferred term and severity*
| VDV-primed (N = 15) | YF-primed (N = 8) | Naive (N = 12) | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Any adverse reaction | 10 | 67 | 6 | 75 | 11 | 92 |
| Any systemic reaction | 8 | 53 | 6 | 75 | 9 | 75 |
| Any injection-site reaction | 6 | 40 | 0 | 5 | 42 | |
| Severe adverse reactions | 1 | 7 | 1 | 13 | 0 | |
| Severe systemic reactions | 1† | 7 | 1‡ | 13 | 0 | |
| Severe injection-site reactions | 0 | 0 | 0 | |||
| Abnormal laboratory parameter rated clinically significant by the investigator | 1 | 7 | 3 | 38 | 2 | 17 |
| Adverse reactions by system organ class and preferred term | ||||||
| Blood and lymphatic system disorders | 1 | 7 | 0 | 0 | ||
| Lymphadenopathy | 1 | 7 | 0 | 0 | ||
| General disorders and administration site conditions | 9 | 60 | 4 | 50 | 10 | 83 |
| Injection-site erythema: any severity | 6 | 40 | 0 | 2 | 17 | |
| Moderate | 1 | 7 | 0 | 0 | ||
| Malaise: any severity | 3 | 20 | 4 | 50 | 5 | 42 |
| Moderate | 1 | 7 | 1 | 13 | 0 | |
| Severe | 0 | 1 | 13 | 0 | ||
| Asthenia | 2 | 13 | 1 | 13 | 5 | 42 |
| Injection-site induration | 1 | 7 | 0 | 0 | ||
| Injection-site edema | 1 | 7 | 0 | 0 | ||
| Injection-site pruritus | 1 | 7 | 0 | 0 | ||
| Pyrexia: any severity | 1 | 7 | 0 | 3 | 25 | |
| Moderate | 0 | 0 | 1 | 8 | ||
| Injection-site hemorrhage: any severity | 0 | 0 | 1 | 8 | ||
| Moderate | 0 | 0 | 1 | 8 | ||
| Injection-site pain: any severity | 0 | 0 | 2 | 17 | ||
| Moderate | 1 | 8 | ||||
| Laboratory investigations | 1 | 7 | 1 | 13 | 1 | 8 |
| Neutrophil count decreased | 1 | 7 | 0 | 1 | 8 | |
| White blood cell count decreased | 1 | 7 | 0 | 1 | 8 | |
| Alanine aminotransferase increased | 0 | 1 | 13 | 0 | ||
| Musculoskeletal and connective tissue disorders | 3 | 20 | 2 | 25 | 4 | 33 |
| Myalgia: any severity | 3 | 20 | 2 | 25 | 4 | 33 |
| Moderate | 1 | 7 | 0 | 0 | ||
| Nervous system disorders | 7 | 47 | 5 | 63 | 8 | 67 |
| Headache: any severity | 7 | 47 | 5 | 63 | 8 | 67 |
| Moderate | 1 | 7 | 3 | 38 | 3 | 25 |
| Severe | 1 | 7 | 0 | 0 | ||
Except where specified, all reactions were reported as mild.
Headache.
Malaise.
A few out of range laboratory values were noted. Among these, two YF-primed participants presented higher than normal aspartate aminotransferase (AST) levels on day 28 (46 and 66 U/L). Above normal alanine transaminase (ALT) levels were detected in one VDV-primed participant (57 U/L on day 14), two YF-primed participants (63 U/L on day 7 in one case and 61–76 U/L on day 28 in the second case), and one naïve participant (59 U/L on day 7). One of these (one YF-primed participant) occurred in a participant who already had an above normal value at baseline. Lower than normal WBC counts were presented by four participants in the VDV-primed group on day 7 or 14 (range = 3.6–3.9 × 109/L) and two naive participants on day 7 (3.2–3.5 × 109/L), one of whom already had a lower than normal WBC count at baseline. Below normal neutrophil counts were presented on day 7 or 14 by three VDV-primed participants (range = 1.56–1.64 × 109/L) and two naïve participants (range = 1.1–1.56 × 109/L), one of whom already had a lower than normal level at baseline. One YF-primed participant had a higher than normal neutrophil count. A few of these abnormal laboratory parameters were considered as clinically significant by the investigator: one VDV2-primed participant had low white blood cell count (WBC; 3.6 × 109/L) and neutrophils (1.56 × 109/L) 7 days after the first vaccination, but values had returned to within the normal range by day 14. In the YF-primed group, one participant had elevated ALT, one had elevated creatinine phosphokinase (CPK) and AST, and one had elevated CPK. None of these abnormalities were severe. Among naive participants, one had reduced WBC, neutrophils, and monocytes, and another had elevated AST and CPK that was rated as severe (elevated CPK of 980 U/L on day 14) but unrelated to vaccination.
Viremia.
Non-serotype–specific vaccine virus viremia was detected in some samples but at levels below the RT-PCR assay's LLOQ in most cases (< 1.46 log10 PFU/mL). Results are, therefore, expressed as percentage of participants with detectable viremia (i.e. ≥ limit of detection). The number and percentage of participants with detectable viremia at any time point after vaccination seemed lower in the VDV-primed group than in either YF-primed or naive control groups (Table 4). Non-serotype–specific viremia could be quantified (≤ 1.83 log10 PFU/mL) in only two (13%) VDV-primed participants (both VDV1-primed), one (13%) YF-primed participant, and two (17%) naive participants. Viremia was observed mainly on day 7 and occasionally, on day 14 but not on day 21 in any group. Serotype 4 viremia was detected most often and was followed by serotype 3 (Table 4). Samples that were positive in the non-serotype–specific RT-PCR were submitted to a plaque assay to detect viral replication. Only three samples were positive by plaque assay (all for serotype 4 with a maximum value of ≤ 2.4 log10 PFU/mL observed in a baseline-naive participant).
Table 4.
Tetravalent dengue vaccine (TDV) virus viremia expressed in terms of the number and proportion of participants with virus detected by RT-PCR on day 7, 14, or 21 after dengue vaccination or quantified by plaque assay
| VDV1-primed (N = 7) | VDV2-primed (N = 8) | All VDV-primed (N = 15) | YF-primed (N = 8) | Naive (N = 12) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| RT-PCR | ||||||||||
| Any serotype non-serotype–specific TDV viremia | 3 | 43 | 4 | 50 | 7 | 47 | 7 | 88 | 10 | 83 |
| Serotype 1 | 0 | 0 | 0 | 0 | 0 | |||||
| Serotype 2 | 0 | 0 | 0 | 0 | 0 | |||||
| Serotype 3 | 0 | 1 | 13 | 1 | 7 | 3 | 38 | 2 | 17 | |
| Serotype 4 | 1 | 14 | 2 | 25 | 3 | 20 | 5 | 63 | 6 | 50 |
| Plaque assay | ||||||||||
| Serotype 1 | 0 | 0 | 0 | 0 | 0 | |||||
| Serotype 2 | 0 | 0 | 0 | 0 | 0 | |||||
| Serotype 3 | 0 | 0 | 0 | 0 | 0 | |||||
| Serotype 4 | 0 | 0 | 2 | 25 | 1 | 8 | ||||
No severe reactions were reported in any of the participants with quantified viremia.
Immunogenicity.
Four weeks after TDV vaccination, a higher proportion of participants in the VDV-primed and YF-primed groups than in the naive group was seropositive to the four serotypes (Table 5 and Figure 1). However, differences in the serotype-specific seropositivity rate on day 28 between the three groups were significant for serotypes 1 (P < 0.0001; Fisher's exact test) and 2 (P = 0.0126) only, and the priming effect of VDV2 seemed greater than VDV1 (Figure 1). Serotype-specific seroconversion rates were 80% or higher in the VDV-primed group (≥ 57%, four of seven in the VDV1 subgroup; ≥ 87.5%, seven of eight in the VDV2 subgroup) and 25% or higher in the YF-primed group (Figure 1). In contrast, no seroconversions against serotype 1 occurred after the single dose of TDV in the naïve participants. Some 67% (10/15) of VDV-primed participants seroconverted to the four dengue serotypes within 28 days of vaccination compared with 25% (2/8) of the YF-primed participants and 0% (0/12) of the naïve participants (Table 5).
Table 5.
Geometric mean titers and seropositivity (percent with titer ≥ 10) to at least one, two, three, or all four serotypes before and up to 180 days after a single injection of tetravalent dengue vaccine
| VDV-primed (N = 15) | YF-primed (N = 8) | Naive (N = 12) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D28 | D60 | D180 | D0 | D28 | D60 | D180 | D0 | D28 | D60 | D180 | |
| Seropositive to ≥ 1 serotype | ||||||||||||
| n | 11 | 15 | 15 | 15 | 1 | 8 | 8 | 8 | 0 | 11 | 11 | 11 |
| % | 73 | 100 | 100 | 100 | 13 | 100 | 100 | 100 | 0 | 92 | 92 | 92 |
| 95% CI | 45–92 | 78–100 | 78–100 | 78–100 | 0–53 | 63–100 | 63–100 | 63–100 | 0–27 | 62–100 | 62–100 | 62–100 |
| Seropositive to ≥ 2 serotypes | ||||||||||||
| n | 0 | 15 | 14 | 12 | 0 | 7 | 7 | 7 | 0 | 5 | 6 | 7 |
| % | 0 | 100 | 93 | 80 | 0 | 88 | 88 | 88 | 0 | 42 | 50 | 58 |
| 95% CI | 0–22 | 78–100 | 68–100 | 52–96 | 0–37 | 47–100 | 47–100 | 47–100 | 0–27 | 15–72 | 21–79 | 27–85 |
| Seropositive to ≥ 3 serotypes | ||||||||||||
| n | 0 | 11 | 10 | 8 | 0 | 6 | 7 | 7 | 0 | 2 | 1 | 1 |
| % | 0 | 73 | 67 | 53 | 0 | 75 | 88 | 88 | 0 | 17 | 8 | 8 |
| 95% CI | 0–22 | 45–92 | 38–88 | 27–79 | 0–37 | 35–97 | 47–100 | 47–100 | 0–27 | 2–48 | 0–39 | 0–39 |
| Seropositive to all serotypes | ||||||||||||
| n | 0 | 10 | 8 | 7 | 0 | 2 | 3 | 5 | 0 | 0 | 1 | 1 |
| % | 0 | 67 | 53 | 47 | 0 | 25 | 38 | 63 | 0 | 0 | 8 | 8 |
| 95% CI | 0–22 | 38–88 | 27–79 | 21–73 | 0–37 | 3–65 | 9–76 | 25–92 | 0–27 | 0–27 | 0–39 | 0–39 |
| Geometric mean titer | ||||||||||||
| GMT DENV-1 | 7.02 | 45.8 | 39.1 | 27.2 | 5.00 | 8.40 | 14.9 | 13.4 | 5.00 | 5.00 | 5.38 | 6.19 |
| 95% CI | 4.70–10.5 | 28.5–73.8 | 25.0–61.2 | 16.4–45.0 | 5.00–5.00 | 3.76–18.8 | 6.47–34.1 | 7.45–24.1 | 5.00–5.00 | 5.00–5.00 | 4.58–6.32 | 4.51–8.49 |
| GMT DENV-2 | 13.4 | 52.5 | 50.8 | 41.8 | 5.74 | 28.1 | 29.7 | 25.4 | 5.00 | 7.67 | 11.9 | 13.1 |
| 95% CI | 7.04–25.7 | 21.8–126 | 20.3–127 | 16.8–104 | 4.15–7.94 | 10.8–73.1 | 10.1–87.2 | 8.44–76.3 | 5.00–5.00 | 4.62–12.7 | 6.38–22.0 | 6.74–25.5 |
| GMT DENV-3 | 5.36 | 41.4 | 17.9 | 15.2 | 5.00 | 33.1 | 22.4 | 27.6 | 5.00 | 28.0 | 16.3 | 13.3 |
| 95% CI | 4.62–6.20 | 20.6–83.1 | 9.37–34.4 | 8.25–28.1 | 5.00–5.00 | 13.9–78.7 | 8.05–62.6 | 12.5–60.9 | 5.00–5.00 | 8.95–87.2 | 5.99–44.5 | 5.76–30.6 |
| GMT DENV-4 | 5.00 | 91.1 | 77.3 | 42.5 | 5.00 | 221 | 155 | 124 | 5.00 | 103 | 44.0 | 33.2 |
| 95% CI | 5.00–5.00 | 37.5–222 | 34.5–173 | 18.3–98.6 | 5.00–5.00 | 64.4–762 | 58.6–411 | 54.1–286 | 5.00–5.00 | 20.0–527 | 11.5–169 | 11.1–99.8 |
The GMTs on day 28 against serotypes 1, 2, 3, and 4 were, respectively, 45.8, 52.5, 41.1, and 91.1 in the VDV-primed group compared with 8.4, 28.1, 33.1, and 221 in the YF-primed group and 5.0, 7.7, 28.0, and 103 in the naive group (Table 5). In the VDV1 subgroup, GMTs against serotypes 1, 2, 3, and 4 increased from 10.3, 5.0, 5.79, and 5.0 on day 0 to 64.5, 14.7, 20.1, and 40.3 on day 28. The GMTs in VDV2 subgroup increased from 5.0, 32.0, 5.0, and 5.0 on day 0 to 34.0, 160, 78.0, and 206.
A delayed response was seen in the YF-primed group for serotype 1 with the proportion of positive participants increasing from 25% on day 28 to 63% on day 60 and 75% on day 180. This pattern was not seen for the other three serotypes in this group (Figure 1). In the VDV-primed group, antibody responses tended to decrease against each serotype between days 28 and 180, with the greatest decrease (from 87% on day 28 to 53% on day 180) seen for serotype 3 (Figure 1).
Vaccination with TDV had no discernable effect on the YF antibody response in the YF-primed group or the naive group. All eight YF-primed participants had anti-YF antibody levels of at least 10 both before and 28 days after TDV vaccination, with a GMT of 202 before TDV vaccination and 223 on day 28. Before and after TDV vaccination, 0 of 12 naïve participants displayed an anti-YF antibody response; 0 of 15 VDV-primed participants had an anti-YF response before TDV vaccination. However, one VDV1-primed participant and one VDV2-primed participant (2/15; 13%) seroconverted to YF after TDV vaccination, although titers were low in both cases (≤ 18).
Discussion
This study investigated the effect of pre-existing dengue and YF immunity on the safety, immunogenicity, and infectivity of a candidate tetravalent live attenuated dengue vaccine. Our results show that this pre-existing dengue or YF immunity increased the immune response to a single injection of TDV (particularly against serotypes 1 and 2 for which differences between groups were significant) without any detectable adverse effect on the vaccine's reactogenicity profile and/or the vaccine virus viremia.
A TDV is being developed with the objective of protecting populations living in dengue endemic areas as well as travelers to these areas. In some of these areas, YF occurs, and YF immunization is routine. Dengue vaccine will, therefore, be administered to individuals with some degree of pre-existing immunity against these diseases. Documenting how such individuals respond to TDV is, therefore, an important step in the overall development of this vaccine. In this case, pre-existing immunity was provided by vaccination in a phase 1 trial conducted 1 year previously in which volunteers received either a licensed YF-17D control vaccine or one of two experimental live attenuated, whole-virion, monovalent dengue vaccines against serotypes 1 or 2.9
The small sample size of the phase 1 study from which most of our study population was recruited was a limiting factor and meant that our study could only be descriptive; therefore, observed trends should be interpreted with caution. Despite this limitation, pre-existing flavivirus immunity, particularly against dengue (dengue serotypes 1 and 2), was shown to significantly increase the neutralizing antibody response to dengue serotypes 1 and 2 in the TDV. Consistent with a report of TDV in US adults, one TDV injection in flavivirus-naive individuals resulted in an antibody response directed predominantly against serotypes 3 and 4.6 Morrison and others6 reported that subsequent TDV injections resulted in a balanced immune response against all four serotypes, with 100% seroconversion against all four dengue serotypes observed after three doses of TDV given over the period of 1 year. Compared with the naive group, we observed a relatively homogenous response against the four dengue serotypes after a single TDV injection in those primed with VDV, particularly those primed with VDV2. The priming effect of YF vaccination is in agreement with the phase 1 proof of concept study with monovalent recombinant dengue serotype 2 vaccine.7 This previous study showed that pre-existing YF immunity did not adversely affect the dengue serotype 2 vaccine's immunogenicity but actually increased the cross-neutralizing antibody response to the other three dengue serotypes that were not included in the vaccine. A similar finding in monkeys has also been reported.14 More recently, a study in children and adults in Mexico City found that the immune responses to the four dengue serotypes elicited by a two-dose TDV vaccination regimen in subjects primed with YF were comparable with those elicited by a three-dose TDV vaccination regimen in flavivirus-naïve subjects.15 Another study, also conducted in Mexico, found that previous Japanese encephalitis vaccination could also provide a priming effect, where dengue seropositivity rates and GMTs after one dose of TDV in subjects primed with Japanese encephalitis vaccine were higher than in naïve subjects who received two doses of TDV (Galan JF, and others, unreported data). Pre-existing flavivirus immunity, therefore, consistently increases antibody responses to the tetravalent dengue vaccine. It is important to point out, however, that it is not yet known to what extent the antibody responses reported here as well as those responses reported elsewhere after a multiple-dose TDV vaccination regimen will translate into protection. A phase IIb efficacy and safety study in Thai children and phase III studies in adults and children are currently underway with a three-dose TDV vaccination regimen.
At the three time points considered (on days 7, 14, and 21 after vaccination), previous YF or dengue immunity against serotypes 1 or 2 did not seem to cause greater vaccine viremia after TDV vaccination, and it did not seem to affect the balance between the different serotypes, because serotype 4, followed by 3, viruses were most frequently detected in each group. Furthermore, viremia was detected less frequently in the VDV-primed group than in the other groups and was detected at very low levels in all cases. Of the samples that were positive in the non-serotype–specific RT-PCR, very few were positive when re-analyzed in a plaque assay, indicating that the vaccine viruses have a low degree of replication. The observation of low levels of viremia, mainly of serotypes 3 and 4, is also in agreement with a previous report, and it supports the conclusion that pre-immunity against only one dengue serotype (i.e., serotype 1 or 2) does not enhance subsequent infection with an heterologous vaccine serotype (i.e., serotypes 3 and 4).6 We cannot rule out the possibility that findings would have been different had participants been primed against serotypes 3 or 4 instead of against serotypes 1 or 2 in the phase 1 study. However, viremia and antibody responses after the first injection of TDV are mainly against serotypes 3 and 4, and subsequent TDV vaccinations induce a balanced response against all serotypes, with diminished overall viremia and no increase in reactogenicity.6
There was no indication that YF or dengue priming increased the reactogenicity of TDV or compromised its safety. There were no serious adverse events, withdrawals caused by adverse events, or adverse events of particular clinical significance, and there were no concerns over the biological safety. The reporting rate of the different categories of adverse events showed no marked differences between groups, although the small sample size prevents meaningful comparison.
Cellular immunity was also evaluated in this trial, and the results have been published separately.9 An interferon (IFN)-γ T-helper 1 (Th1) response was induced against each of the four serotypes in the VDV-primed group in agreement with the neutralizing antibody response observed to all four serotypes. In contrast, in the YF and naïve groups, IFN-γ secretion was mostly induced by serotype 4. No marked changes were observed for tumor necrosis factor (TNF)-α and interleukin (IL)-2. These results show that existing dengue immunity, even against only one serotype, primes participants to mount a broader and more balanced Th1 response against all four serotypes after a single TDV immunization.
In conclusion, existing immune responses against dengue or YF had no detectable adverse consequences on the safety of this recombinant live attenuated TDV. Previous exposure to attenuated dengue or YF viruses does, however, have a priming effect on both the humoral and cellular response to subsequent TDV vaccination, suggesting that fewer TDV vaccinations might be needed for effective dengue immunization in flavivirus-exposed populations. These findings support the continued clinical development of this TDV in populations such as children in areas where dengue is endemic or where YF vaccination is routine.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Bruno Guy at Sanofi Pasteur for his critical review of the manuscript and Jane Irons and Grenville Marsh for editorial assistance in the preparation of this manuscript. Dr. Linda Hueston at the Arbovirus Emerging Diseases Unit, CIDMLS-ICPMR for her contribution in dengue testing.
Disclaimers: Remi Forrat, Anh Wartel-Tram, and Jean Lang are employees of Sanofi Pasteur.
Footnotes
Financial support: This study was supported by a grant from Sanofi Pasteur. Editorial assistance for the preparation of this manuscript was funded by Sanofi Pasteur.
Authors' addresses: Ming Qiao, Microbiology and Infectious Diseases, Institute of Medical and Veterinary Science, Adelaide, South Australia, Australia, E-mail: ming.qiao@health.sa.gov.au. David Shaw, Infectious Diseases Unit, Royal Adelaide Hospital, Adelaide, South Australia, Australia, E-mail: david.shaw@health.sa.gov.au. Remi Forrat and Jean Lang, Sanofi Pasteur, Lyon, France, E-mails: remi.forrat@sanofipasteur.com and jean.lang@sanofipasteur.com. Anh Wartel-Tram, Sanofi Pasteur, Thailand, E-mail: wartel-tram.anh@sanofipasteur.com.
References
- 1.Green S, Rothman A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr Opin Infect Dis. 2006;19:429–436. doi: 10.1097/01.qco.0000244047.31135.fa. [DOI] [PubMed] [Google Scholar]
- 2.Kurane I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp Immunol Microbiol Infect Dis. 2007;30:329–340. doi: 10.1016/j.cimid.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Guirakhoo F, Weltzin R, Chambers TJ, Zhang ZX, Soike K, Ratterree M, Arroyo J, Georgakopoulos K, Catalan J, Monath TP. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol. 2000;74:5477–5485. doi: 10.1128/jvi.74.12.5477-5485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guirakhoo F, Arroyo J, Pugachev KV, Miller C, Zhang ZX, Weltzin R, Georgakopoulos K, Catalan J, Ocran S, Soike K, Ratterree M, Monath TP. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J Virol. 2001;75:7290–7304. doi: 10.1128/JVI.75.16.7290-7304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine. 2010;28:632–649. doi: 10.1016/j.vaccine.2009.09.098. [DOI] [PubMed] [Google Scholar]
- 6.Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all four serotypes in Flavivirus-naive adults. J Infect Dis. 2010;201:370–377. doi: 10.1086/649916. [DOI] [PubMed] [Google Scholar]
- 7.Guirakhoo F, Kitchener S, Morrison D, Forrat R, McCarthy K, Nichols R, Yoksan S, Duan X, Ermak TH, Kanesa-Thasan N, Bedford P, Lang J, Quentin-Millet MJ, Monath TP. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: phase I clinical trial for safety and immunogenicity. Effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum Vaccin. 2006;2:60–67. doi: 10.4161/hv.2.2.2555. [DOI] [PubMed] [Google Scholar]
- 8.Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, Marchesani R, Knauber M, Wells KH, Arroyo J, Guirakhoo F. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20:1004–1018. doi: 10.1016/s0264-410x(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 9.Guy B, Nougarede N, Begue S, Sanchez V, Souag N, Carre M, Chambonneau L, Morrisson DN, Shaw D, Qiao M, Dumas R, Lang J, Forrat R. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine. 2008;26:5712–5721. doi: 10.1016/j.vaccine.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez V, Gimenez S, Tomlinson B, Chan P, Thomas N, Forrat R, Chambonneau L, Lang J, Guy B. Innate and adaptive cellular immunity in flavivirus-naive human recipients of a live-attenuated dengue serotype 3 vaccine produced in Vero cells (VDV3) Vaccine. 2006;24:4914–4926. doi: 10.1016/j.vaccine.2006.03.066. [DOI] [PubMed] [Google Scholar]
- 11.Mantel N, Aguirre M, Gulia S, Girerd-Chambaz Y, Colombani S, Moste C, Barban V. Standardized quantitative RT-PCR assays for quantitation of yellow fever and chimeric yellow fever-dengue vaccines. J Virol Methods. 2008;151:40–46. doi: 10.1016/j.jviromet.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Porterfield JS. A plaque technique for the titration of yellow fever virus and antisera. Trans R Soc Trop Med Hyg. 1959;53:458–466. doi: 10.1016/0035-9203(59)90021-5. [DOI] [PubMed] [Google Scholar]
- 13.Roehrig JT, Hombach J, Barrett AD. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008;21:123–132. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
- 14.Guy B, Barban V, Mantel N, Aguirre M, Gulia S, Pontvianne J, Jourdier TM, Ramirez L, Gregoire V, Charnay C, Burdin N, Dumas R, Lang J. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am J Trop Med Hyg. 2009;80:302–311. [PubMed] [Google Scholar]
- 15.Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH. Live-attenuated tetravalent dengue vaccine in dengue-naive children, adolescents, and adults in Mexico City: randomized controlled phase 1 trial of safety and immunogenicity. Pediatr Infect Dis J. 2011;30:e9–e17. doi: 10.1097/INF.0b013e3181fe05af. doi:10.1097/INF.0b013e3181fe05af. [DOI] [PubMed] [Google Scholar]


