Abstract
To assess immunity after yellow fever (YF) 17D live-attenuated vaccination, we measured the antibody levels before vaccination and at 21 days and 8 months after vaccination in YF-naïve travelers. Thirty subjects were enrolled in the study, with 100% providing sera at 21 days and 86.6% providing sera at 8 months. All subjects seroconverted by day 21, and the geometric mean titers of the anti-YF antibodies decreased between day 21 and month 8 from 6,451 to 1,246. This study corroborates the high rates of seroconversion achieved by the live-attenuated YF vaccine.
Approximately 22 million travelers visit yellow fever (YF) -endemic countries annually.1 An unimmunized traveler visiting an active epidemic area in West Africa for a period of 2 weeks has an estimated risk of 1 to 267 of YF virus disease, a mosquito-borne flavivirus.2 Furthermore, YF vaccine requirements are in place and enforceable by International Health Regulations (IHR) to prevent the spread of YF by viremic individuals. Therefore, it is important for travelers to consider vaccination against YF before travel in addition to practicing vector avoidance during travel.
The YF 17D live-attenuated vaccine, developed in 1936, is generally considered to lead to seroconversion when anti-YF antibody titers determined by plaque reduction neutralization tests (PRNT) are at least 20, although no study has specifically determined a seroprotective PRNT level.3,4 Past studies have shown rapid seroconversion in adults from non-endemic regions, with antibodies appearing within 10 days and peaking 3–4 weeks after vaccination.5,6 Our research goal was to assess seroconversion at 21 days and 8 months after YF vaccination in healthy adult travelers, and it was designed to be a potential comparator for a separate group of subjects receiving an investigational inactivated YF vaccine.
YF-naïve subjects aged 18–49 years who were evaluated pre-travel at Mount Auburn Hospital and needed YF vaccination were recruited prospectively. Serum samples were obtained before vaccination and on day 21 (±1 day) and month 8 (±30 days) after vaccination with YF-VAX (Sanofi-Pasteur Inc., Swiftwater, PA). Our exclusion criteria were those subjects that had prior YF vaccination, resided in a YF-endemic country, served in the US military, or received the Japanese encephalitis vaccine within the previous 30 days. Serum samples were analyzed for anti-YF antibodies at Focus Diagnostics (Cypress, CA) using a qualified PRNT with a 50% endpoint.7 We defined seroconversion as at least a fourfold increase in the neutralizing antibody titer from before YF vaccination.7
Thirty subjects were successfully enrolled, with a median age of 29.5 years (range = 18–49 years); 60% were female. All 30 subjects provided serum samples before vaccination and on day 21, and 26 subjects (86.6%) returned to the clinic to provide a third serum sample at month 8. These 26 returning subjects traveled between day 21 and month 8 to 12 different YF-endemic countries, with Ethiopia, Benin, and Ghana being the most frequently visited destinations. Before vaccination, all subjects had an antibody titer < 1:10. By day 21 post-vaccination, 100% of subjects had seroconverted, and the geometric mean titer (GMT) was 6,451. At month 8, all subjects were still seropositive, and the GMT declined to 1,246 (Figure 1).
Figure 1.
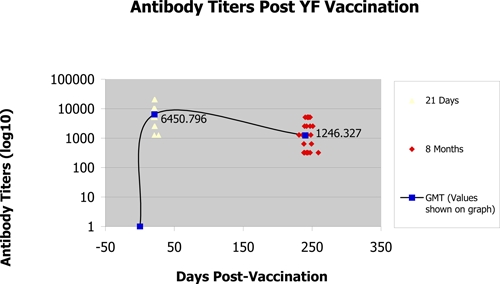
Antibody titers from subjects on day 21 and month 8 post-vaccination (the line denotes an estimate of GMT levels based on the available data on days 0 and 21 and month 8).
Prior studies have found that Caucasians, males, and people with a history of military service had higher antibody titers than people of African-American or Hispanic background, females, or people without a history of service duty.4,8 Our sample size was too small to infer any significant differences regarding ethnicity. The antibody titers were slightly lower in our male subjects than females subjects, but this difference from results of earlier studies could be a sampling bias because of our small sample size. Our female subjects maintained higher antibody titers at month 8 (GMT = 1,538) than male subjects (GMT = 934) or 23% and 17% of their day 21 GMTs, respectively. Subjects who traveled to South America (N = 7) maintained a higher GMT at month 8 compared with subjects who traveled to Africa (N = 19). Trip duration did not influence the month 8 titer. Our study excluded people with military service history, and we found that age did not seem to have a significant effect on immune response.
In other studies, seroconversion occurred in 90% of subjects by day 10 and > 99% as early as 21 days post-vaccination.5,9 This study similarly found high rates and levels of seroconversion, with 100% seroconversion by day 21. The durability of neutralizing antibodies after vaccination is well-known, and it has been suggested that immunity may be life-long.10 Our data confirm the maintenance of high antibody levels 8 months after YF vaccination, although follow-up at a longer interval would be desirable to determine the rate of antibody loss. Revaccination is recommended at intervals of 10 years. However, in a recent study of laboratory workers who were required to maintain immunity above a PRNT80 titer of 40, the majority of subjects had very low titers and required a booster dose within 3–5 years of the prior YF vaccination.4
A limitation of this study is the small sample size, which led to difficulty with deriving statistical significance. Another limitation is the possibility that the subjects could have been exposed to a flavivirus infection during travel, which may have affected the titers at month 8. Previous studies of individuals who had received YF vaccine and were subsequently immunized with a heterologous flavivirus, such as tick-borne encephalitis (TBE), Japanese encephalitis (JE), or dengue, have shown broadening of the antibody response because of anamnestic responses against cross-reactive epitopes, and a boost in YF antibodies in such cases may occur.11,12 However, these studies have not shown high levels of YF antibodies resulting from heterologous flavivirus infection, and moreover, neutralizing antibodies for YF are considered to be distinct from other flaviviruses.4,5 Finally, with respect to the risk of exposure to heterologous flaviviruses, in Africa, annual rates of endemic flavivirus infection in indigenous populations are approximately 1%.13 One of the most prevalent heterologous flavivirus infections in YF-endemic areas is dengue. Clinical dengue infection of travelers has been reported to occur in approximately 5 per 100,000 travellers14; although subclinical infections undoubtedly occur at a higher incidence, it is unlikely based on these considerations that exposure to flaviviruses influenced our study results.
In conclusion, immunization with 17D live-attenuated YF vaccine achieves brisk seroconversion in naive subjects and maintains very high antibody levels 8 months after vaccination. Our results supplement existing data on YF vaccine immunogenicity in travelers from non-endemic regions and provide comparison for development of future YF vaccines.
ACKNOWLEDGMENTS
The authors thank Erika Gleva and Keith Gottlieb for their contributions in study procedures and laboratory analysis.
Footnotes
Financial support: This project was supported by research funding from Xcellerex, Inc., Marlborough, MA.
Disclosure: Some of the authors have received financial compensation from Xcellerex, Inc. This statement is made in the interest of full disclosure and not because the authors consider this compensation to be a conflict of interest.
Authors' addresses: Allison Kay, Mount Auburn Hospital, Cambridge, MA, E-mail: allison.h.kay@gmail.com. Lin H. Chen, Mount Auburn Hospital, Cambridge, MA and Harvard Medical School, Boston, MA, E-mail: lchen@mah.harvard.edu. Maggie Sisti and Thomas P. Monath, Xcellerex Inc., Marlborough, MA, E-mails: maggie@sisticonsulting.com and tmonath@kpcb.com.
References
- 1.World Tourism Organization UNWTO Tourism Highlights. 2010. www.unwto.org/facts/menu.html Available at. Accessed March 28, 2011.
- 2.Monath TP, Cetron MS. Prevention of yellow fever in persons traveling to the tropics. Clin Infect Dis. 2002;34:1369–1378. doi: 10.1086/340104. [DOI] [PubMed] [Google Scholar]
- 3.Monath TP, Fowler E, Johnson C, Balser J, Morin M, Sisti M, Trent D. An inactivated cell-culture vaccine against yellow fever. N Engl J Med. 2011;364:1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- 4.Hepburn MJ, Kortepeter MG, Pittman PR, Boudreau EF, Mangiafico JA, Buck PA, Norris SL, Anderson EL. Neutralizing antibody response to booster vaccination with the 17d yellow fever vaccine. Vaccine. 2006;24:2843–2849. doi: 10.1016/j.vaccine.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 5.Wisseman CL, Jr, Sweet BH, Kitaoka M, Tamiya T. Immunological studies with group B arthropod-borne viruses. I. Broadened neutralizing antibody spectrum induced by strain 17D yellow fever vaccine in human subjects previously infected with Japanese encephalitis virus. Am J Trop Med Hyg. 1962;11:550–561. [PubMed] [Google Scholar]
- 6.Monath TP. Neutralizing antibody responses in the major immunoglobulin classes to YF 17D vaccination of humans. Am J Epidemiol. 1971;93:122–129. doi: 10.1093/oxfordjournals.aje.a121232. [DOI] [PubMed] [Google Scholar]
- 7.Spector S, Tauraso NM. Yellow fever virus. I. Development and evaluation of a plaque neutralization test. Appl Microbiol. 1968;16:1770–1775. doi: 10.1128/am.16.11.1770-1775.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J, Shope RE, Thomas N, Schrader R, Furby D, Bedford P. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg. 2002;66:533–541. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- 9.Lang J, Zuckerman J, Clarke P, Barrett P, Kirkpatrick C, Blondeau C. Comparison of the immunogenicity and safety of two 17D yellow fever vaccines. Am J Trop Hyg. 1999;60:1045–1050. doi: 10.4269/ajtmh.1999.60.1045. [DOI] [PubMed] [Google Scholar]
- 10.Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull World Health Organ. 1981;59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 11.Kayser M, Klein H, Paasch I, Pilaski J, Blenk H, Heeg K. Human antibody response to immunization with 17D yellow fever and inactivated TBE vaccine. J Med Virol. 1985;17:35–45. doi: 10.1002/jmv.1890170106. [DOI] [PubMed] [Google Scholar]
- 12.Rosenzweig EC, Babione RW, Wisseman CL., Jr Immunological studies with group B arthropod-borne viruses. IV. Persistence of yellow fever antibodies following vaccination with 17D strain yellow fever vaccine. Am J Trop Med Hyg. 1963;12:230–235. [PubMed] [Google Scholar]
- 13.Monath TP, Lee VH, Wilson DC, Fagbami A, Tomori O. Arbovirus studies in Nupeko Forest, a possible site of endemic yellow fever transmission in Nigeria. I. Serosurvey of humans and non-human primates. Trans R Soc Trop Med Hyg. 1974;68:30–38. doi: 10.1016/0035-9203(74)90248-x. [DOI] [PubMed] [Google Scholar]
- 14.Vinner L, Domingo C, Ostby AC, Rosenberg K, Fomsgaard A. Cases of travel-acquired dengue fever in Denmark 2001–2009. Clin Microbiol Infect. 2011 doi: 10.1111/j.1469-0691.2011.03543.x. Apr 5. doi: 10.1111/j.1469-0691.2011.03543.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


