Abstract
Outbreaks of Mayaro fever have been associated with a sylvatic cycle of Mayaro virus (MAYV) transmission in South America. To evaluate the potential for a common urban mosquito to transmit MAYV, laboratory vector competence studies were performed with Aedes aegypti from Iquitos, Peru. Oral infection in Ae. aegypti ranged from 0% (0/31) to 84% (31/37), with blood meal virus titers between 3.4 log10 and 7.3 log10 plaque-forming units (PFU)/mL. Transmission of MAYV by 70% (21/30) of infected mosquitoes was shown by saliva collection and exposure to suckling mice. Amount of viral RNA in febrile humans, determined by real-time polymerase chain reaction, ranged from 2.7 to 5.3 log10 PFU equivalents/mL. Oral susceptibility of Ae. aegypti to MAYV at titers encountered in viremic humans may limit opportunities to initiate an urban cycle; however, transmission of MAYV by Ae. aegypti shows the vector competence of this species and suggests potential for urban transmission.
Introduction
Mayaro virus (MAYV) is a mosquito-borne Alphavirus in the Family Togaviridae. Since it was first identified from the blood of five febrile cases in Trinidad in 1954,1 MAYV has been isolated or its presence has been implicated by antibody surveys in countries throughout tropical South America and into Central America and the Caribbean.2–9 MAYV causes a self-limited illness characterized by fever, rash, and severe arthralgia, which is similar to chikungunya virus and other members of the Semliki Forest antigenic complex.10 Outbreaks of Mayaro fever recognized to date have been small and associated with rural communities,1,11,12 likely reflecting spillover infections from a sylvatic (enzootic) transmission cycle of MAYV. Because of limited surveillance and diagnostic laboratory facilities in much of the endemic region, many Mayaro fever cases are doubtlessly undiagnosed, leading to a significant underrecognition of disease. One long-term study, however, provides an estimate of Mayaro fever in the urban area of Iquitos in the Amazon Basin of northeastern Perú. Through this clinic-based surveillance system, active since 1990, approximately seven cases of Mayaro fever have been identified per year by virus isolation, reverse transcription polymerase chain reaction (RT-PCR), or serology.13 Surrounded by tropical forest and supporting a large (~380,000), dynamic populace, Iquitos presents a porous front between a sylvan habitat for alphaviruses14–19 and an urban environment that maintains endemic, Aedes aegypti-vectored transmission of dengue virus.20
Our understanding of the arthropod vectors that naturally transmit MAYV comes from a small number of ecological studies. Aitken and others21 first isolated MAYV from a pool of Mansonia venezuelensis collected in the Rio Grande Forest in Trinidad in 1957. Isolates have subsequently been obtained from small numbers of Ae. (Ochlerotatus) serratus, Psorophora ferox/albipes, Sabethes spp., Culex spp., and more commonly, Haemagogus janthinomys.8,22,23 In a well-described, concurrent outbreak of Mayaro fever and yellow fever in Belterra, Pará, Brazil in 1978, nine isolates of MAYV were recovered from mosquito specimens.24 All isolates were from Hg. janthinomys.
Experimental studies to determine laboratory vector competence of mosquito species for MAYV, likewise, have been limited. Aitken and Anderson25 intrathoracically inoculated MAYV into mosquitoes from seven species found in Trinidad, including Ae. aegypti, and assessed the potential for each species to transmit the virus to exposed chicks.25 Transmission was shown from only one of these species—a single representative of Ae. scapularis. Evidence of MAYV replication in Ae. aegypti was not presented.25 Although Aitken and Anderson25 cite personal communication indicating that MAYV prototype strain TRVL 4675 replicates in experimentally infected Ae. aegypti and Anopheles quadrimaculatus, there are no published studies that show laboratory vector competence of Ae. aegypti for MAYV. Smith and Francy,26 however, assessed vector efficiency of a Brazilian strain of Ae. albopictus that fed on a viremic hamster with a MAYV titer of 5.3 log10 plaque-forming units (PFU)/mL, and found that infection ranged from 9% to 16% (13 of 111 total, days 6–20 post-infection) of the engorged mosquitoes, and that approximately half of the infected mosquitoes in the study transmitted virus when tested by salivation in capillary tubes (5/11) and feeding on newborn mice (3/6). Although Smith and Francy26 termed this Brazilian strain of Ae. albopictus relatively refractory to infection with MAYV, they suggested that this species may be sufficiently susceptible to serve as a secondary vector in the context of an outbreak or as a bridging vector between transmission cycles of distinct ecologies.26
Recent, large epidemics of the closely related, primarily enzootic chikungunya virus in the Indian Ocean and South Asia27 raise the question of whether other enzootic arboviruses possess a similar potential to emerge in an urban cycle.28 Mayaro fever cases in Iquitos, although they may not represent infection events within the city, demonstrate the opportunity for viremic people to contact urban vectors. Furthermore, reports in 2010 of travelers returning to France and the Netherlands with serological evidence of recent MAYV infection29,30 illustrate opportunities for export of the virus beyond its endemic range and additional contact between this virus and atypical, urban vector species.
To assess the potential for an urban cycle of MAYV to be initiated and sustained, the vector competence of anthropophilic, urban-dwelling mosquito species for MAYV should be evaluated first in the laboratory. Because of the paucity of published studies of MAYV in Ae. aegypti, the abundance of this mosquito species in urban areas of tropical South America, and the importance of this species in serving as a vector for dengue virus transmission in the region, we evaluated the laboratory vector competence of an Iquitos strain of Ae. aegypti for MAYV. Here, we report the efficiency of oral infection, dissemination, and transmission of MAYV in this species. To establish field relevance of these data, the viral loads in acute sera from human Mayaro fever cases in Peru and Bolivia were quantified and compared with the experimentally determined threshold of MAYV infection in Ae. aegypti.
Materials and Methods
Virus.
MAYV strain IQT4235 was inoculated into Vero 76 cells at a multiplicity of infection of approximately 0.01, and cells were harvested at 36 hours post-infection in preparation for mosquito feeding experiments. This strain was initially isolated from the serum of a febrile patient (case 2)10 in Iquitos in 1997, and had been passaged one time in C6/36 cells and three times in Vero 76 cells.
Mosquitoes.
Ae. aegypti eggs were collected in six residential sites in the Punchana and Iquitos Districts of Maynas Province, Peru. Eggs were stored at 20°C in a Biosafety Level 2 (BSL-2) insectary until they were hatched (F0 generation), reared to adult stage, and allowed to oviposit. Adults of the F1 generation were maintained on a 10% sucrose solution in preparation for feeding experiments. At 5–9 days post-eclosion, mosquitoes were sorted by sex, and females were deprived of sucrose for 48 hours before experimental feeding.
Mosquito infection.
In preparation for feeding mosquitoes by artificial blood meal, MAYV was 10-fold serially diluted in sterile phosphate-buffered saline (PBS) and mixed 1:1 with defibrinated sheep blood (Colorado Serum Company, Denver, CO). One milliliter of each infectious blood meal was stored at −80°C for titration, and the remaining ~2 mL were introduced into a Hemotek arthropod feeder (Discovery Workshops; Accrington, Lancashire, United Kingdom) that was heated to 37°C and covered with fresh mouse skin for exposure to mosquitoes. Mosquitoes were allowed to feed for ~1 hour. Engorged mosquitoes were maintained in an environmental chamber (Model 818; Precision, Winchester, VA) at 28°C with a 14-hour to 10-hour light–dark cycle until harvested for virus testing.
In preparation for feeding mosquitoes on viremic mice, adult female CD-1 mice (Charles River Laboratories, Wilmington, MA) were injected subcutaneously in the back with 5.7 log10 PFU MAYV strain IQT 4235 between 24 and 48 hours before mosquito feeding. Immediately preceding the feed, mice were anesthetized with pentobarbital, and a blood sample was taken for virus titration. Mosquitoes were allowed access to the mice for ~1 hour.
Mosquito transmission.
Transmission potential of Ae. aegypti was assessed by two established techniques. First, we used the capillary tube saliva collection method modified by Vanlandingham and others.31 Each virus-exposed mosquito was chilled on ice, legs and wings were removed, and proboscis was inserted into mineral oil in a capillary tube for ~1 hour. Individual saliva samples in oil were expressed into microcentrifuge tubes containing 100 µL minimum essential medium (MEM) (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS) and 0.5% Amphotericin-B (Sigma Aldrich, St. Louis, MO). Matched saliva samples and mosquito bodies were stored at −80°C until tested for virus. To assess natural transmission, individual 3-day-old mice were exposed to a single, previously blood meal-engorged mosquito for ~1 hour. All mice were held for 4 days to allow the development of viremia, at which time blood was collected and stored at −80°C until tested along with whole mosquito bodies.
Cytopathic effect assay.
One milliliter of MEM with 5% FBS and 1% Amphotericin-B and one zinc-coated steel grinding ball (Daisy, Rogers, AR) were added to each cryotube containing bodies or legs and wings of a single mosquito. Specimens were triturated mechanically in a Mixer Mill model MM301 (Retsch, Haan, Germany) and centrifuged for clarity. Supernatant (100 μL) and an equal volume of Vero 76 cell suspension, sufficient for a confluent monolayer in 24 hours, were added to each well of a 96-well microplate. Plates were maintained at 37°C and 5% CO2 for cell growth and observation for cytopathic effect assay (CPE) over the next 5 days. All CPE-positive mosquito specimens, undiluted, were plaque assayed as described below to confirm the presence of virus.
Virus titration by plaque assay.
For titration of MAYV in mosquito blood meals and in mosquito specimens, 100 µL sample from serial 10-fold dilutions were inoculated in duplicate onto a confluent monolayer of Vero 76 cells in 12-well tissue culture plates. Virus was allowed to adsorb for 1 hour at 37°C, an overlay of 0.4% agarose in MEM with 2% FBS was added, and plates were held at 37°C and 5% CO2 for 62–68 hours. After this procedure, 10% formaldehyde (~0.5 mL) was introduced into each well, agarose plugs were removed, and cells were covered with a crystal violet stain (70% water, 30% methanol, and 0.25% crystal violet) to visualize plaques. For titration of saliva samples, 50 µL saliva-infused MEM were inoculated into 24-well plates and assayed as above. In virus-positive samples, 25 μL remaining sample were diluted for optimal reading, and plaque assay was repeated to confirm results. Quantity of virus was expressed as the number of PFU per 1 mL in the case of infectious dose and as the number of PFU per sample in the case of saliva virus titers.
RNA extraction and quantitative real-time RT-PCR.
Serum was obtained from human Mayaro fever cases in Peru and Boliva between 2006 and 2009, with the presence of MAYV confirmed by immunofluorescence assay after culture in Vero 76 cells. RNA was extracted from 35 µL serum diluted in 105 μL PBS using the QIAamp Viral RNA Minikit (Qiagen, Valencia, CA) using the manufacturer's protocol. Primers and probe for quantitative real-time RT-PCR (qRT-PCR) were designed using VisualOMP (DNA Software, Ann Arbor, MI) to amplify and detect a region of the E2 gene between nucleotides 8690 and 8817, and consist of the following: forward primer (5′-GTGGTCGCACAGTGAATCTTTC-3′), reverse primer (5′-CAAATGTCCACCAGGCGAAG-3′), and probe (carboxyfluorescein [FAM]-5′-ATGGTGGTAGGCTATCCGACAGGTC-3′-carboxytetramethylrhodamine [TAMRA]). Reverse transcription and quantitative PCR steps were performed with an iScript One-Step RT-PCR Kit for Probes (Bio-Rad, Hercules, CA) using the manufacturer's protocol and an iCycler (Bio-Rad, Hercules, CA). For absolute quantification, a standard curve was constructed with 10-fold dilutions of serum from an experimentally MAYV-infected mouse with a viremic titer of 8.3 log10 PFU/mL determined by plaque assay in Vero 76 cells. Amplification efficiency of the reaction was 101.7%, with a correlation coefficient of 0.98. All samples were run in triplicate in the same plate as controls and PFU-quantified standards. Specificity was determined by including RNA from the closely related Una virus (UNAV; strain PE10800, Peru, 1998) in the sample plate. No UNAV RNA was detected with this primer/probe set. qRT-PCR results are presented in PFU equivalents/mL.
Statistical analyses.
Statistical analyses were performed using GraphPad Prism version 5.0c (GraphPad Software, La Jolla, CA). Curve fitting of normalized data was used to establish the 50% oral infectious dose (OID50) for each infection method, and these doses were compared by a two-tailed student t test. A P value of < 0.05 was considered statistically significant.
Results
Infection and dissemination of MAYV.
Females of the F1 generation of an Iquitos strain of Ae. aegypti that engorged on artificial blood meals with doses of MAYV ranging from 3.4 to 7.3 log10 PFU/mL showed dose-dependent infection at day 14 post-feeding (Table 1). Dose-dependent infection was observed also in mosquitoes feeding on viremic mice. However, at comparable doses, the percentage of mosquitoes that became infected was higher when fed on a viremic mouse than on an artificial blood meal (Table 1). To compare the susceptibility of Ae. aegypti with MAYV by feeding method, we determined the OIDs needed to infect 50% of mosquitoes (OID50). The OID50 was 6.65 log10 PFU/mL when mosquitoes ingested virus by artificial feeding and 6.08 log10 PFU/mL when mosquitoes fed on viremic mice, showing a statistically significant difference in the proportions of mosquitoes infected by feeding method (Figure 1). Overall, at the highest titered dose, 84% of mosquitoes were infected with MAYV, whereas none of the mosquitoes that fed on infectious doses between 3.4 and 5.5 log10 PFU/mL were infected. One exception in this dose range was a single infected mosquito (1/32) that fed on a mouse with a viremic titer of 4.3 log10 PFU/mL. Percent dissemination based on leg/wing assays ranged independently of blood meal dose from 71% to 100% of the infected mosquitoes (Table 1).
Table 1.
Percentage of Ae. aegypti showing infection, dissemination (legs and wings from infected bodies), and transmission of MAYV after exposure to an artificial blood meal at various dilutions or viremic adult mice
| Blood meal titers (PFU/mL) | Percent infected of total (bodies) | Percent disseminated of infected (legs and wings) | Percent transmitting of infected (oil droplet or blood sample from mouse)* | ||
|---|---|---|---|---|---|
| Artificial feeder | Viremic mouse | Capillary tube | Suckling mouse | ||
| 7.34† | 84 (31/37) | 94 (29/31) | 88 (7/8) | 67 (2/3) | |
| 6.57‡ | 32 (10/31) | 80 (8/10) | 43 (3/7) | NT | |
| 6.28† | 41 (13/32) | 92 (12/13) | 100 (2/2) | NT | |
| 6.08† | 55 (16/29) | 94 (15/16) | 67 (2/3) | NT | |
| 5.85† | 44 (14/32) | 71 (10/14) | 100 (2/2) | NT | |
| 5.59‡ | 24 (7/29) | 71 (5/7) | 100 (3/3) | NT | |
| 5.57§ | 3 (1/32) | 100 (1/1) | 0 (0/2) | NT | |
| 5.45§ | 0 (0/29) | 0 | NT | NT | |
| 5.11† | 0 (0/22) | 0 | NT | NT | |
| 4.61§ | 0 (0/32) | 0 | NT | NT | |
| 4.36‡ | 0 (0/22) | 0 | NT | NT | |
| 4.30‡ | 0 (0/24) | 0 | NT | NT | |
| 4.26§ | 3 (1/32) | 100 (1/1) | NT | NT | |
| 3.73† | 0 (0/32) | 0 | NT | NT | |
| 3.36‡ | 0 (0/31) | 0 | NT | NT | |
| ND (< 2.00)‡ | 0 (0/15) | 0 | NT | NT | |
NT = not tested; ND = not detected.
Ten specimens from mosquitoes fed at each of seven infectious doses were sampled at 14 days post-feeding for a separate transmission study. Specimens were distinct from those specimens used to calculate the infection and dissemination rates.
One independent day of mosquito infection.
A second independent day of mosquito infection.
A third independent day of mosquito infection.
Figure 1.
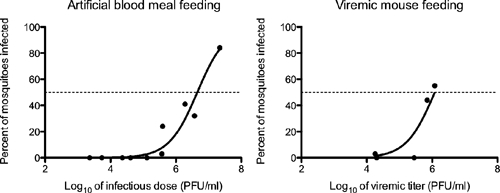
Comparison of OID50 in Ae. aegypti by feeding method. The MAYV titer at which 50% of Ae. aegypti were infected was 6.65 log10 PFU/mL when mosquitoes were fed by artificial blood meal and 6.08 log10 PFU/mL when fed on a viremic mouse. The difference in OID50s was significant by unpaired t test (P value = 0.0044).
To assess replication in the insects over time, we harvested mosquitoes that had fed on a virus dose of 7.3 log10 at days 0, 1, 3, 7, and 14 post-artificial blood meal feeding and titered bodies and legs/wings of specimens separately. The titer of MAYV in mosquito bodies decreased between days 0 and 3 and then increased above the quantity ingested to an average virus titer of ~7.0 log10 PFU/mL (Figure 2) at days 7 and 14, a pattern indicative of MAYV replication. Dissemination of virus to legs and wings was observed in 1 of 8 (13%) mosquitoes at day 3 post-infection, 11 of 11 (100%) mosquitoes at day 7, and 13 of 13 (100%) mosquitoes at day 14. Titers of virus in legs and wings increased between days 3 and 7, and at day 14, they remained approximately 2 logs lower than mean body titer.
Figure 2.
Replication of MAYV in bodies and legs/wings of orally infected Ae. aegypti. Bodies and legs/wings of mosquitoes that fed on 7.3 log10 PFU/mL MAYV were screened for the presence of virus by cytopathic effect assay, and positive specimens were virus-titered by plaque assay.
Transmission of MAYV.
Transmission potential of artificially infected mosquitoes was assessed by salivation into oil in capillary tubes. Pooled across infectious doses, 19 of 27 (70%) experimentally infected mosquitoes had MAYV in their saliva (Table 1), suggesting their potential to transmit the virus. MAYV titers in saliva varied independently of blood meal dose and method of feeding from 2 to 600 PFU/sample (range = 0.3–2.8 log10 PFU/mL, mean = 1.9 log10 PFU/mL) (Figure 3). To determine whether Ae. aegypti was capable of transmitting MAYV naturally, mosquitoes (10 total, fed on an artificial blood meal with a virus titer of 7.3 log10 PFU/mL) were exposed, individually, to 3-day-old suckling mice at day 14 post-feeding. Mosquitoes were observed probing on mice, but none were found blood-engorged after exposure. All mosquitoes and mice were assayed for the presence of virus, and two of three (67%) mice probed by infected mosquitoes were viremic at 4 days post-exposure (Table 1). MAYV was not detected in legs or wings of the third infected mosquito, suggesting that the inability to transmit virus was because virus failed to disseminate from the midgut (data not shown).
Figure 3.
Number of PFUs of MAYV expressed per Ae. aegypti saliva sample. MAYV was detected in 19 saliva samples from 27 MAYV-infected mosquitoes. Titers of virus in saliva were not associated with blood meal titers or method of feeding (a = artificial feeding; m = viremic mouse feeding).
Quantification of MAYV in febrile cases.
Viral RNA in acute sera from human Mayaro fever cases was quantified by qRT-PCR testing. Among 22 samples with RNA quantities above the limit of detection, viremias ranged from 2.7 to 5.3 log10 PFU equivalents/mL (PFUe/mL), with a geometric mean of 4.2 log10 PFUe/mL (95% confidence interval [CI] = 3.9–4.5 log10 PFUe/mL) (Table 2).
Table 2.
Quantity of MAYV RNA in acute serum from Mayaro fever cases from 2006 to 2009
| Sample code | qRT-PCR (log10 PFU equivalents/mL) |
|---|---|
| FCB0587 | 5.34 |
| IQE6594 | 4.90 |
| IQE6601 | 4.88 |
| FMD0181 | 4.79 |
| FSL1307 | 4.79 |
| FSB0319 | 4.77 |
| IQD5316 | 4.70 |
| FVB0250 | 4.62 |
| IQD7395 | 4.52 |
| FSB0719 | 4.48 |
| FSB1131 | 4.43 |
| IQD7337 | 4.32 |
| OBT2191 | 4.30 |
| IQD5364 | 4.08 |
| FSB0311 | 3.95 |
| FMD1068 | 3.89 |
| FSC0497 | 3.58 |
| MFI0231 | 3.54 |
| FVB0241 | 3.46 |
| IQD4881 | 3.43 |
| IQE7750 | 2.98 |
| IQE1750 | 2.70 |
| FSC0498 | < LOD |
| IQD7408 | < LOD |
| IQD7446 | < LOD |
LOD = limit of detection.
Discussion
The primary objective of this study was to examine the laboratory vector competence of a common urban species of mosquito for MAYV. Specifically, we determined the capacity of Ae. aegypti to be infected by and to transmit MAYV, in order to explore the possibility that this species could maintain a human–mosquito–human cycle of the virus within Iquitos, Perú, a large urban area surrounded by vast tropical forest, Hg. janthinomys,32 and active, enzootic transmission of MAYV. To minimize colonization effects33–35 and population variability36–39 that could interfere with applicability of these results to the specific geographic context, all studies were conducted with an F1 generation of an Iquitos strain of Ae. aegypti.
Overall, the percentage of Ae. aegypti infected with MAYV was found to increase with dose above a certain threshold, which has been described for other virus–vector systems.40 Because method of feeding may impact the ability of virus to infect mosquitoes, and therefore, results vary in their applicability to natural transmission, we directly compared two methods—artificial and viremic mouse feeding. Artificial feeding allowed infectious doses over a range that might be comparable with human viremias; viremic mouse feeding is expected to better represent natural vertebrate to vector transmission. We found that the difference in infectivity of MAYV was approximately five times greater when mosquitoes were fed on a viremic animal rather than an artificial blood meal, a difference that has been recognized to various degrees in previous studies.41–44 The threshold of oral infection of Ae. aegypti with MAYV is estimated to be between 5.0 and 5.5 log10 PFU/mL, with 0–24% of mosquitoes becoming infected at artificial blood meal doses ranging from 5.1 to 5.6 log10 PFU/mL. Additional data points from mosquitoes fed on viremic mice at titers between 4.0 and 6.0 log10 PFU/mL would refine this threshold. The efficiency of dissemination and the amount of virus in saliva did not differ by method of infection (Table 1 and Figure 3), similar to the findings of Smith and others44 with Venezuelan equine encephalitis virus infection in Ae. (Ochlerotatus) taeniorhynchus and Ae. albopictus.
MAYV was transmitted efficiently by Ae. aegypti, with our evaluation of natural transmission of MAYV to suckling mice showing results similar to the results ascertained by saliva collection in capillary tubes (67% and 70%, respectively), although only a small number of infected mosquitoes were exposed to mice in this trial. Furthermore, MAYV was successfully transmitted to mice after probing alone without apparent blood engorgement by mosquitoes, adding to previous reports of extravascular inoculation as an important route of arbovirus transmission.45,46 Based on the replication kinetics shown here, the extrinsic incubation period of MAYV in Ae. aegypti may be short. Dissemination to the hemocoel, a pre-requisite for infection of the salivary glands and presentation of virus to a vertebrate host, occurs as early as 3 days post-infection according to the presence of virus in legs and wings of mosquitoes. Titers of MAYV in saliva of Ae. aegypti, determined by in vitro collection, were low relative to West Nile virus in Cx. pipiens pipiens31 but similar to West Nile virus in Cx. tarsalis,47 eastern equine encephalitis virus in Culiseta melanura,48 and Venezuelan equine encephalitis virus in Ae. albopictus and Ae. taeniorhynchus,49 with the last of these viruses representing the most comparable virus–vector systems. However, titers determined by in vitro versus in vivo methods vary,47,50 and how accurately these results translate to the quantity of virus transmitted to a vertebrate host is uncertain. However, these data unequivocally show that MAYV can be transmitted from infected Ae. aegypti, reducing the importance of this potential barrier in limiting an urban cycle of MAYV transmission.
To relate human viremias to the experimentally determined threshold of mosquito infection and infer whether Ae. aegypti-vectored transmission could be maintained across this other potentially critical barrier, we quantified MAYV in serum from acutely infected people. Serum samples from febrile cases were tested first by plaque assay in Vero 76 cells shown to be competent for MAYV infection by positive controls included in each cell culture plate. In 8 of 25 samples, 10–100 PFU/mL MAYV were detected, with reduced plaque sizes relative to low-passage stock virus (IQT4235) observed in all positive samples (data not shown). The remaining sera yielded no plaques by 92 hours post-inoculation compared with plaques that developed ~65 hours post-inoculation from stock virus. Because RNA has been shown to be more stable than infectious virus through storage and shipping processes with variable temperature conditions or multiple freeze–thaw cycles,51,52 we also tested samples by qRT-PCR. To obtain a result relevant to this study, we created a standard curve from plaque assay-quantified viremic mouse serum and expressed quantities of MAYV in test samples in terms of PFU equivalents based on the amount of RNA detected. Preparation of this standard with in vivo rather than in vitro replication of the virus, with serum as the virus source and minimal manipulation before quantification, may provide a close approximation of the RNA to PFU ratio for MAYV in acute phase human serum under ideal laboratory conditions. If RNA remained stable despite degradation of infectious virus particles, this method would offer a more reliable, biologically meaningful measure of MAYV for comparison with vector competence data. Of 25 human serum samples tested by qRT-PCR, viral RNA was undetected or outside of the dynamic range of the assay in 3 samples despite previous virus isolation, indicating that the original isolates were recovered from serum with < 2 log10 PFU/mL MAYV. In the remaining sera, a mean of 4.2 log10 PFUe/mL and a maximum of 5.3 log10 PFUe/mL MAYV were detected. Because these samples were acquired through a passive surveillance network, the sampling time in the course of viremia was based solely on when a person came to the study clinic for care; as a consequence, these values are likely to be an underestimate of peak viremic titers (within 24 hours of symptom onset)53 in humans. However, despite this caveat, these results are similar to those results described in earlier studies using different quantitative methods. In the 1978 Belterra outbreak, the highest titer viremia, nearly 4.0 log10 PFU/0.1 mL by plaque assay of whole blood, was captured on the first day of clinical observation.53 In two additional cases, with samples obtained on days 3 and 5 of symptoms, the median infective dose in C6/36 cell culture was approximately 105.0 tissue culture/mL and was determined by immunofluorescence assay.10
Together, these data suggest that there may be a limited opportunity, constrained by viremic time and virus titer, for MAYV to be transmitted between a human host and Ae. aegypti. Although Ae. aegypti could potentially serve as a vector for this virus, the short period of time that a viremic person is able to infect a small percentage of mosquitoes may explain, at least in part, the lack of apparent urban transmission of MAYV in Iquitos, despite the Mayaro fever cases that continue to appear at clinics in the city.13 This low probability of infection could be altered by at least three possible ecologic or genetic changes: (1) increased spatial and temporal overlap between viremic humans and this potential vector, (2) increased oral susceptibility of Ae. aegypti to MAYV, or (3) increased viremic titers and/or duration of viremias in humans.
Aedes species, and specifically, Ae. aegypti, should be considered candidates for a potential urban cycle of MAYV. These species merit concern for at least three reasons. First, the presumed enzootic vector, Hg. janthinomys, is in the same (Aedine) tribe of mosquitoes as Ae. aegypti, and vector cross-over has been accomplished repeatedly by yellow fever virus.54 Dengue and chikungunya viruses, both similarly transmitted by sylvatic species of Aedine mosquitoes, have exploited Ae. aegypti as a primary urban vector with widespread, negative effect. Second, the susceptibility of Aedes species, described in this study and the study by Smith and Francy,26 contrasts sharply with the susceptibility of Cx. pipiens quinquefaciatus, an abundant urban vector in the tropics that we found completely refractory to infection with MAYV, even at a dose of 8.0 log10 PFU/mL (data not shown). Finally, Ae. aegypti and Ae. albopictus have reclaimed and expanded their geographic range in the Americas within the last half century.55 Opportunities for contact alone should warrant additional consideration of these species in the urbanization of arboviruses, including MAYV. Urban areas in the midst of enzootic MAYV circulation offer critical vantage points for observing ecologic change and possible impacts on transmission. Importantly, virus and vector strains, both known to vary geographically, that are found in combinations other than what is offered in Iquitos may demonstrate a more susceptible system and prove to be a better source for the initiation of an urban cycle of MAYV transmission. Vector competence testing with MAYV and Ae. aegypti from other locations in the Amazon Basin would complement the research presented in addressing this potential.
Because Ae. aegypti has been shown in this study to readily disseminate and transmit MAYV after infected, it is valuable to consider the case of two other alphaviruses, both of which have caused large epidemics of human disease. Chikungunya and Venezuelan equine encephalitis viruses provide examples of single amino acid changes that have resulted in increased mosquito infection, either directly through vector (Ae. albopictus) susceptibility in the case of chikungunya virus56 or indirectly through increased titers of viremia in equine hosts in the case of Venezuelan equine encephalitis virus.57 Although urban transmission of both viruses is characterized primarily by sporadic appearances rather than local maintenance, the impact of outbreaks facilitated by increased efficiency of vector infection has been substantial. If a similar advantageous mutation were to be fixed in a population of MAYV, Ae. aegypti could host the virus with an increased possibility of sustaining human–mosquito–human transmission. Understanding the genetic or ecologic barriers that currently constrain MAYV to an enzootic cycle is not only of intrinsic value but also of clear relevance to the broader study of arbovirus emergence.
ACKNOWLEDGMENTS
The authors would like to express appreciation to Jing Huang for expert advice and help in rearing of mosquitoes used in this study. We acknowledge the Dirección General Forestal y de Fauna Silvestre in Peru for allowing collection (#415-2009-AG-DGFFS-DGEFFS) and export (#000703-AG-DGFFS) of Ae. aegypti eggs from Iquitos. The authors thank Carolina Guevara and febrile surveillance study personnel in Peru and Bolivia for identifying Mayaro fever cases and providing serum for this analysis. The authors thank Helvio Astete, Gerson Perez Rodriguez, Hugo Jaba Ruiz, and Manuel Ruiz Rioja of Naval Medical Research Unit Six for collecting Ae. aegypti eggs used to establish our experimental population and Gabriela Vasquez La Torre, Steven Stoddard, and Amy Morrison for arranging for permits and shipping eggs used in this study (in Iquitos). We also thank Douglas Watts, Thomas Ksiazek, and Eric Halsey for reviewing and improving this manuscript.
Disclaimer: The views expressed in this article are the views of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or US Government. Tadeusz J. Kochel is in the military service of the US Government. This work was prepared as part of his official duties. Title 17 USC §105 provides that “[c]opyright protection under this title is not available for any work of the United States Government.” Title 17 USC §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person's official duties. The experiments reported herein were conducted in compliance with the Animal Welfare Act and accordance with the principles set forth in “Guide for the Care and Use of Laboratory Animals,” Institute of Laboratory Animals Resources, National Research Council (National Academy Press, 1996). Animal use in this study was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch under Protocol #0003018. Use of human subjects was approved by the Naval Medical Research Center Institutional Review Board (NMRCD.2000.0006 and NMRCD.2000.0008), and use of serum from these subjects was also approved by the University of Texas Medical Branch (Institutional Review Board #95-111), with all protocols in compliance with all applicable federal regulations governing the protection of human subjects.
Footnotes
Financial support: K.C.L. was supported by a National Science Foundation Graduate Research Fellowship and an National Security Education Program David L. Boren Fellowship. This research was funded by Naval Medical Research Center Detachment Work Unit Number 800000.82000.25GB.B0016 and National Institutes of Health Contract N01-AI25489 (to R.B.T.).
Authors' addresses: Kanya C. Long, Department of Entomology, University of California, Davis, Davis, CA, E-mail: kclong@ucdavis.edu. Sarah A. Ziegler, Saravanan Thangamani, Nicole L. Hausser, Stephen Higgs, and Robert B. Tesh, Department of Pathology, University of Texas Medical Branch, Galveston, TX, E-mails: saziegle@utmb.edu, sathanga@utmb.edu, nlhausse@utmb.edu, sthiggs@utmb.edu, and rtesh@utmb.edu. Tadeusz J. Kochel, Viral and Rickettsial Diseases Department, Naval Medical Research Center, Silver Spring, MD, E-mail: tad.kochel@med.navy.mil.
References
- 1.Causey O, Maroja O. Mayaro virus: a new human disease agent. III. Investigation of an epidemic of acute febrile illness on the river Guama in Para, Brazil, and isolation of Mayaro virus as causative agent. Am J Trop Med Hyg. 1957;6:1017–1023. [PubMed] [Google Scholar]
- 2.Talarmin A, Chandler L, Kazanji M, de Thoisy B, Debon P, Lelarge J, Labeau B, Bourreau E, Vie J, Shope R, Sarthou J-L. Mayaro virus fever in French Guiana: isolation, identification, and seroprevalence. Am J Trop Med Hyg. 1998;59:452–456. doi: 10.4269/ajtmh.1998.59.452. [DOI] [PubMed] [Google Scholar]
- 3.Powers A, Aguilar P, Chandler L, Brault A, Meakins T, Watts D, Russell K, Olson J, Vasconcelos P, Travassos da Rosa A, Weaver S, Tesh RB. Genetic relationships among Mayaro and Una viruses suggest distinct patterns of transmission. Am J Trop Med Hyg. 2006;75:461–469. [PubMed] [Google Scholar]
- 4.Torres J, Russell K, Vasquez C, Barrera R, Tesh R, Salas R, Watts D. Family cluster of Mayaro fever, Venezuela. Emerg Infect Dis. 2004;10:1304–1306. doi: 10.3201/eid1007.030860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izurieta R, Macaluso M, Watts D, Tesh R, Guerra B, Cruz L, Galwanker S, Vermund S. Assessing yellow fever risk in the Ecuadorian Amazon. J Glob Inf Dis. 2009;1:7–13. doi: 10.4103/0974-777X.49188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Downs W, Anderson C. Distribution of immunity to Mayaro virus infection in the West Indies. West Indian Med J. 1958;7:190–195. [Google Scholar]
- 7.Galindo P, Srihongse S, de Rodaniche E, Grayson M. An ecological survey for arboviruses in Almirante, Panama, 1959–1962. Am J Trop Med Hyg. 1966;15:385–400. doi: 10.4269/ajtmh.1966.15.385. [DOI] [PubMed] [Google Scholar]
- 8.Groot H, Morales A, Vidales H. Virus isolation from forest mosquitoes in San Vicente de Chucuri, Colombia. Am J Trop Med Hyg. 1961;10:397–402. doi: 10.4269/ajtmh.1961.10.397. [DOI] [PubMed] [Google Scholar]
- 9.Karbaat J, Jonkers A, Spence L. Arbovirus infections in Dutch military personnel stationed in Surinam: a preliminary study. Trop Geogr Med. 1964;4:370–376. [PubMed] [Google Scholar]
- 10.Tesh RB, Watts DM, Russell KL, Damodaran C, Calampa C, Cabezas C, Ramirez G, Vasquez B, Hayes CG, Rossi CA, Powers AM, Hice CL, Chandler LJ, Cropp BC, Karabatsos N, Roehrig JT, Gubler DJ. Mayaro virus disease: an emerging mosquito-borne zoonosis in tropical South America. Clin Infect Dis. 1999;28:67–73. doi: 10.1086/515070. [DOI] [PubMed] [Google Scholar]
- 11.ProMED-mail Mayaro virus disease - Venezuela (03): (Portuguesa). ProMED-mail 2010; 7 Jun: 20100618.2046. 2011. http://www.promedmail.org Available at. Accessed August 15.
- 12.LeDuc J, Pinheiro F, Travassos da Rosa A. An outbreak of Mayaro virus disease in Belterra, Brazil. II. Epidemiology. Am J Trop Med Hyg. 1981;30:682–688. doi: 10.4269/ajtmh.1981.30.682. [DOI] [PubMed] [Google Scholar]
- 13.Forshey B, Guevara C, Laguna-Torres A, Cespedes M, Vargas J, Gianella A, Vallego E, Madrid C, Aguayo N, Gotuzzo E, Suarez V, Morales A, Beingolea L, Reyes N, Perez J, Negrete M, Rocha C, Morrison A, Russell K, Blair P, Olson J, Kochel T. Arboviral etiologies of acute febrile illnesses in western South America, 2000–2007. PLoS Negl Trop Dis. 2010;4:e787. doi: 10.1371/journal.pntd.0000787. Group NFSW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turell M, O'Guinn M, Jones J, Sardelis M, Domh D, Watts D, Fernandez R, Travassos da Rosa A, Guzman H, Tesh R, Rossi C, Ludwig G, Mangiafico J, Kondig J, Wasieloski L, Pecor J, Zyzak M, Schoeler G, Mores C, Calampa C, Lee J, Klein T. Isolation of viruses from mosquitoes (Diptera: Culicidae) collected in the Amazon Basin region of Peru. J Med Entomol. 2005;42:891–898. doi: 10.1603/0022-2585(2005)042[0891:IOVFMD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Yanoviak S, Aguilar P, Lounibos L, Weaver S. Transmission of a Venezuelan equine encephalitis complex Alphavirus by Culex (Melanoconion) gnomatos (Diptera: Culicidae) in northeastern Peru. J Med Entomol. 2005;42:404–408. doi: 10.1093/jmedent/42.3.404. [DOI] [PubMed] [Google Scholar]
- 16.Aguilar P, Green I, Coffey L, Medina G, Moncayo A. Endemic Venezuelan equine encepahlitis in northern Peru. Emerg Infect Dis. 2004;10:880–888. doi: 10.3201/eid1005.030634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watts D, Phillips I, Callahan J, Griebenow W, Hyams K. Oropouche virus transmission in the Amazon River basin of Peru. Am J Trop Med Hyg. 1997;56:148–152. doi: 10.4269/ajtmh.1997.56.148. [DOI] [PubMed] [Google Scholar]
- 18.Morrison A, Forshey B, Notyce D, Astete H, Lopez V. Venezuelan equine encephalitis virus in Iquitos, Peru: urban transmission of a sylvatic strain. PLoS Negl Trop Dis. 2008;2:e349. doi: 10.1371/journal.pntd.0000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguilar P, Robich R, Turell M, O'Guinn M, Klein T, Huaman A, Guevara C, Rios Z, Tesh R, Watts D, Olson J, Weaver S. Endemic eastern equine encephalitis in the Amazon region of Peru. Am J Trop Med Hyg. 2007;76:293–298. [PubMed] [Google Scholar]
- 20.Morrison A, Minnick S, Rocha C, Forshey B, Stoddard S, Getis A, Focks D, Russell K, Oslon J, Blair P, Watts D, Sihuincha M, Scott T, Kochel T. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4:e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aitken T, Downs W, Anderson C, Spence L, Casals J. Mayaro virus isolated from a Trinidadian mosquito, Mansonia venezuelensis. Science. 1960;131:986. doi: 10.1126/science.131.3405.986. [DOI] [PubMed] [Google Scholar]
- 22.Karabatsos N. International Catalogue of Arboviruses Including Certain Other Viruses of Vertebrates. San Antonio, TX: American Society of Tropical Medicine and Hygiene; 1985. [DOI] [PubMed] [Google Scholar]
- 23.Pinheiro F, LeDuc J. In: The Arboviruses: Epidemiology and Ecology. Monath T, editor. Boca Raton, FL: CRC Press; 1988. pp. 137–150. (Mayaro virus disease). [Google Scholar]
- 24.Hoch A, Peterson N, LeDuc J, Pinheiro F. An outbreak of Mayaro virus disease in Belterra, Brazil. III. Entomological and ecological studies. Am J Trop Med Hyg. 1981;30:689–698. doi: 10.4269/ajtmh.1981.30.689. [DOI] [PubMed] [Google Scholar]
- 25.Aitken T, Anderson C. Virus transmission studies with Trinidadian mosquitoes: II. Further observations. Am J Trop Med Hyg. 1959;8:41–45. doi: 10.4269/ajtmh.1959.8.41. [DOI] [PubMed] [Google Scholar]
- 26.Smith G, Francy D. Laboratory studies of a Brazilian strain of Aedes albopictus as a potential vector of Mayaro and Oropouche viruses. J Am Mosq Control Assoc. 1991;7:89–93. [PubMed] [Google Scholar]
- 27.Powers A, Logue C. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2376. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 28.Weaver S, Reisen W. Present and future arboviral threats. Antiviral Res. 2009;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Receveur M, Grandadam M, Pistone T, Malvy D. Infection with Mayaro virus in a French traveller returning from the Amazon region, Brazil, January, 2010. Euro Surveill. 2010;15:19563. [PubMed] [Google Scholar]
- 30.Hassing R, Leparc-Goffart I, Blank S, Thevarayan S, Tolou H, van Doornum G, van Genderen P. Imported Mayaro virus infection in The Netherlands. J Infect. 2010;61:343–345. doi: 10.1016/j.jinf.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Vanlandingham D, Schneider B, Klingler K, Fair J, Beasley D, Huang J, Hamilton P, Higgs S. Real-time reverse transcriptase-polymerase chain reaction quantification of West Nile virus transmitted by Culex pipiens quinquefasciatus. Am J Trop Med Hyg. 2004;71:120–123. [PubMed] [Google Scholar]
- 32.Pecor J, Jones J, Turell M, Fernandez R, Carbajal F, O'Guinn M, Sardalis M, Watts D, Zyzak M, Calampa C, Klein T. Annotated checklist of the mosquito species encountered during arboviral studies in Iquitos, Peru (Diptera: Culicidae) J Am Mosq Control Assoc. 2000;16:210–218. [PubMed] [Google Scholar]
- 33.Lorenz L, Beaty B, Aitken T, Wallis G, Tabachnick W. Effect of colonization upon Aedes aegypti susceptibility to oral infection with yellow fever virus. Am J Trop Med Hyg. 1984;33:690–694. doi: 10.4269/ajtmh.1984.33.690. [DOI] [PubMed] [Google Scholar]
- 34.Hardy J, Reeves W, Sjogren R. Variations in the susceptibility of field and laboratory populations of Culex tarsalis to experimental infection with western equine encephalitis virus. Am J Epidemiol. 1976;103:498–505. doi: 10.1093/oxfordjournals.aje.a112251. [DOI] [PubMed] [Google Scholar]
- 35.Gargan T, Bailey C, Higbee G, Gad A, Said S. The effect of laboratory colonization on the vector-pathogen interactions on Egyptian Culex pipiens and Rift Valley fever virus. Am J Trop Med Hyg. 1983;32:1154–1163. doi: 10.4269/ajtmh.1983.32.1154. [DOI] [PubMed] [Google Scholar]
- 36.Tesh R, Gubler D, Rosen L. Variation among geographic strains of Aedes albopictus in susceptibility to infection with Chikungunya virus. Am J Trop Med Hyg. 1976;25:326–335. doi: 10.4269/ajtmh.1976.25.326. [DOI] [PubMed] [Google Scholar]
- 37.Gubler D, Nalim S, Tan R, Saipan H, Sulianti S. Variation in susceptibility to oral infection with dengue viruses among geographic strains of Aedes aegypti. Am J Trop Med Hyg. 1979;28:1045–1052. doi: 10.4269/ajtmh.1979.28.1045. [DOI] [PubMed] [Google Scholar]
- 38.Tabachnick W, Wallis G, Aitken T, Miller B, Amato G, Lorenz L, Powell J, Beaty B. Oral infection of Aedes aegypti with yellow fever virus: geographic variation and genetic considerations. Am J Trop Med Hyg. 1985;34:1219–1224. doi: 10.4269/ajtmh.1985.34.1219. [DOI] [PubMed] [Google Scholar]
- 39.Bennett K, Olson K, de Lourdes Munoz M, Fernandez-Salas I, Farfan-Ale J, Higgs S, Black W, Beatry B. Variation in vector competence for dengue 2 virus among 24 collection of Aedes aegypti from Mexico and the United States. Am J Trop Med Hyg. 2002;67:85–92. doi: 10.4269/ajtmh.2002.67.85. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain R, Sikes R, Nelson D, Sudia W. Studies on the North American arthropod-borne encephalitides. VI. Quantitative determinations of virus-vector relationships. Am J Trop Med Hyg. 1954;60:278–285. doi: 10.1093/oxfordjournals.aje.a119721. [DOI] [PubMed] [Google Scholar]
- 41.Turell M. Reduced Rift Valley fever virus infection rates in mosquitoes associated with pledget feedings. Am J Trop Med Hyg. 1988;39:597–602. doi: 10.4269/ajtmh.1988.39.597. [DOI] [PubMed] [Google Scholar]
- 42.Meyer R, Hardy J, Presser S. Comparative vector competence of Culex tarsalis and Culex quinquefasciatus from the Coachella, Imperial and San Joaquin Valleys of California for St. Louis encephalitis virus. Am J Trop Med Hyg. 1983;32:305–311. doi: 10.4269/ajtmh.1983.32.305. [DOI] [PubMed] [Google Scholar]
- 43.Patrician L, DeFoliart G, Yuill T. Oral infection and transmission of La Crosse virus by an enzootic strain of Aedes triseriatus feeding on chipmunks with a range of viremia levels. Am J Trop Med Hyg. 1985;44:992–998. doi: 10.4269/ajtmh.1985.34.992. [DOI] [PubMed] [Google Scholar]
- 44.Smith DR, Carrara AS, Aguilar PV, Weaver SC. Evaluation of methods to assess transmission potential of Venezuelan equine encephalitis virus by mosquitoes and estimation of mosquito saliva titers. Am J Trop Med Hyg. 2005;73:33–39. [PubMed] [Google Scholar]
- 45.Turell M, Spielman A. Nonvascular delivery of Rift Valley fever virus by infected mosquitoes. Am J Trop Med Hyg. 1992;47:190–194. doi: 10.4269/ajtmh.1992.47.190. [DOI] [PubMed] [Google Scholar]
- 46.Turell M, Tammariello R, Speilman A. Nonvascular delivery of St. Louis encephalitis and Venezuelan equine encephalitis viruses by infected mosquitoes (Diptera: Culicidae) feeding on a vertebrate host. J Med Entomol. 1995;32:563–568. doi: 10.1093/jmedent/32.4.563. [DOI] [PubMed] [Google Scholar]
- 47.Styer L, Kent K, Albright R, Bennett C, Kramer L, Bernard K. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007;3:1262–1270. doi: 10.1371/journal.ppat.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver SC, Scott TW, Lorenz LH. Patterns of eastern equine encephalomyelitis virus infection in Culiseta melanura (Diptera: Culicidae) J Med Entomol. 1990;27:878–891. doi: 10.1093/jmedent/27.5.878. [DOI] [PubMed] [Google Scholar]
- 49.Smith D, Carrara A-S, Aguilar P, Weaver S. Evaluation of methods to assess transmission potential of Venezuelan equine encephalitis by mosquitoes and estimation of mosquito saliva titers. Am J Trop Med Hyg. 2005;73:33–39. [PubMed] [Google Scholar]
- 50.Smith D, Aguilar P, Coffey L, Gromowski G, Wang E, Weaver S. Venezuelan equine encephalitis virus transmission and effect on pathogenesis. Emerg Infect Dis. 2006;12:1190–1196. doi: 10.3201/eid1208.050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guzman H, Ding X, Xiao S-Y, Tesh R. Duration of infectivity and RNA of Venezuelan equine encephalitis, West Nile, and yellow fever viruses dried on filter paper and maintained at room temperature. Am J Trop Med Hyg. 2005;72:474–477. [PubMed] [Google Scholar]
- 52.Mayo D, Beckwith W. Inactivation of West Nile virus during serologic testing and transport. J Clin Microbiol. 2002;40:3044–3046. doi: 10.1128/JCM.40.8.3044-3046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinheiro F, Freitas R, Travassos da Rosa J, Gabbay Y, Mello W, LeDuc J. An outbreak of Mayaro virus disease in Belterra, Brazil. I. Clinical and virological findings. Am J Trop Med Hyg. 1981;30:674–681. doi: 10.4269/ajtmh.1981.30.674. [DOI] [PubMed] [Google Scholar]
- 54.Barrett A, Higgs S. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol. 2007;52:209–229. doi: 10.1146/annurev.ento.52.110405.091454. [DOI] [PubMed] [Google Scholar]
- 55.Lounibos L. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–266. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 56.Tsetsarkin K, Vanlandingham D, McGee C, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anishchenko M, Bowen R, Paessler S, Austgen L, Green I, Weaver S. Venezuelan encephalitis emergence mediated by a phylogenetically predicted viral mutation. Proc Natl Acad Sci USA. 2006;103:4994–4999. doi: 10.1073/pnas.0509961103. [DOI] [PMC free article] [PubMed] [Google Scholar]


