Abstract
To comprehend the molecular processes that lead to the Fas death receptor clustering in lipid rafts, a 21-mer peptide corresponding to its single transmembrane domain (TMD) was reconstituted into mammalian raft model membranes composed of an unsaturated glycerophospholipid, sphingomyelin, and cholesterol. The peptide membrane lateral organization and dynamics, and its influence on membrane properties, were studied by steady-state and time-resolved fluorescence techniques and by attenuated total reflection Fourier transformed infrared spectroscopy. Our results show that Fas TMD is preferentially localized in liquid-disordered membrane regions and undergoes a strong reorganization as the membrane composition is changed toward the liquid-ordered phase. This results from the strong hydrophobic mismatch between the length of the peptide hydrophobic stretch and the hydrophobic thickness of liquid-ordered membranes. The stability of nonclustered Fas TMD in liquid-disordered domains suggests that its sequence may have a protective function against nonligand-induced Fas clustering in lipid rafts. It has been reported that ceramide induces Fas oligomerization in lipid rafts. Here, it is shown that neither Fas TMD membrane organization nor its conformation is affected by ceramide. These results are discussed within the framework of Fas membrane signaling events.
Introduction
Fas (CD95/Apo1), a member of the tumor necrosis factor receptor superfamily, is one of the most studied death receptors (1,2). Together with its ligand, FasL, it constitutes one of the major regulators of cell death and survival in mammalian cells (3). Fas is a 45-kDa type I transmembrane glycoprotein with an N-terminal cysteine-rich extracellular domain (binding domain) and a C-terminal charged cytoplasmatic domain containing an 80-amino-acid region called the death domain that is essential to initiate apoptosis (4). After binding to FasL, preassembled Fas trimers cluster with the Fas-associated death-domain protein (FADD) through their death domains (3,4). Fas-bound FADD associates with procaspase-8, procaspase-10, and the caspase regulator c-FLIP via its death effector domain, forming a protein oligomer called death-inducing signaling complex (DISC) (2). This oligomerization process results in the release of active caspase-8, initiating apoptotic signaling.
In the last few years, lipid rafts have emerged as important mediators of Fas clustering (5–8). Lipid rafts are dynamic membrane regions enriched in cholesterol (Chol) and sphingolipids (9) that, due to their high content in saturated sphingolipids and their tight interactions with Chol, are believed to have properties similar to those of the liquid-ordered (lo) phase (10). Lipid rafts have been involved in several biological processes, ranging from membrane trafficking and organization to signal transduction (9). Fas is recruited and clusters in lipid rafts after ligand-binding, a process that is vital for efficient DISC formation and apoptosis triggering (6,7,11). This observation was sustained by the finding that blocking Fas localization to lipid rafts obstructs its aggregation with intracellular apoptotic proteins, inhibiting apoptosis (5,12,13). It has been reported that Fas oligomerization in lipid rafts is facilitated by the formation of ceramide (Cer) (14,15). After binding to FasL or agonistic molecules, Fas translocation to lipid rafts results in the formation of Cer in those domains by the action of acid sphingomyelinase (14,15). As a result, small rafts coalesce into large signaling platforms where Fas oligomerization and DISC formation is enhanced, triggering an efficient apoptosis signal. Ceramide is the simplest sphingolipid and an established active participant in several biological processes, for example, cell signaling (16). Alterations in membrane biophysical properties caused by Cer seem to be the basis of its biological functions (17). Studies in model membranes have shown that Cer has a strong tendency to form highly ordered gel phases, in a process that is highly dependent on membrane composition (18–20). Cer modifies lipid-raft biophysical properties (21–24), affecting the sorting of molecules into/out of these membrane domains (25). However, the role of Cer in signaling, namely in apoptosis triggering, is still not fully understood.
To better understand which biophysical mechanisms may govern Fas translocation and clustering in lipid rafts and how Cer is able to modulate these processes, we selected the transmembrane domain (TMD) of human Fas receptor and studied its membrane organization in the model raft system POPC/PSM/Chol (26) in the absence and presence of Cer. Taking advantage of Fas TMD intrinsic fluorescence due to the presence of two tryptophan residues (Trp176 and Trp189), steady- and transient-state fluorescence techniques, together with attenuated total reflection Fourier transformed infrared spectroscopy (ATR-FTIR), allowed us to study the peptide lateral distribution, membrane localization, and dynamics in nonraft and raftlike membranes and also to assess Cer effects on those properties. Our results show that Fas TMD conformation and sorting are highly dependent on the membrane state. The peptide presents a strong preference for nonraft membranes (i.e., the liquid-disordered (ld) phase), which persists in the presence of Cer. The biological implications in terms of receptor translocation and clustering to lipid rafts and apoptosis induction are discussed.
Materials and Methods
Materials
A peptide with the sequence n-Ser-Asn-Leu-Gly-Trp-Leu-Cys-Leu-Leu-Leu-Leu-Pro-Ile-Pro-Leu-Ileu-Val-Trp-Val-Lys-Arg-C corresponding to the TMD of Fas (TNFRSF6) (see Supporting Material for further details) was purchased from AnaSpec (Freemont, CA). The peptide was deemed to be >95% pure, as determined by analytical high-performance liquid chromatography and mass spectrometry, and was kept lyophilized at −20°C until use. The lipids and probes 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), N-palmitoyl-sphingomyelin (PSM), and palmitoyl ceramide (PCer) were obtained from Avanti Polar Lipids (Alabaster, AL). Cholesterol (Chol) was from Sigma (St. Louis, MO). Tris-(2-cyanoethyl)-phosphine (TCEP) was purchased from Molecular Probes (Leiden, The Netherlands). All organic solvents were UVASOL grade from Merck (Darmstadt, Germany).
Liposome preparation
Multilamellar vesicles (MLVs) with or without peptide and PCer and composed of POPC/PSM/Chol mixtures that are contained within the tie line that spans the 1:1:1 composition in the POPC/PSM/Chol phase diagram (26) (Fig. S1 in the Supporting Material) were prepared as described previously (23,27). The lipids were suspended in buffer (20 mM HEPES, 10 mM NaCl, 0.1 mM EDTA, 1 mM TCEP, pH 7.4). Addition of the reducing agent TCEP in the suspension buffer was used to prevent peptide dimerization through persulfide bond formation between Cys residues. The total lipid concentration was 0.6 mM and the peptide concentration was varied between 0.35%, 0.7%, and 3.0% total lipid.
The concentration of lipid and probes in stock solutions was determined as previously described (23). Fas peptide concentration was determined spectrophotometrically using ε(Fas, 280 nm, propanol) = 12,150 M−1 cm−1 (28).
Absorption and fluorescence
Absorption and steady-state fluorescence measurements were performed as described previously (29). Steady-state fluorescence anisotropy was calculated using
| (1) |
in which the different intensities (blank subtracted) are the steady-state vertical and horizontal components of the fluorescence emission with excitation vertical (IVV and IVH, respectively), and G is the instrumental correction factor. The excitation (λexc)/emission (λem) wavelengths were 295 nm/340 nm for steady-state anisotropy (〈r〉) and 280 nm/340 nm for emission and excitation spectra.
Trp fluorescence decays (excitation at λ = 295 nm) were measured at λ = 340 nm using the magic angle (54.7°) relative to the vertically polarized excitation beam produced by a frequency doubled Rhodamine 6G laser, as previously described (26,27). The data were analyzed with the TRFA software (Scientific Software Technologies Center, Minsk, Belarus). For a decay described by a sum of exponentials where αi is the normalized preexponential and τi is the lifetime of decay component i, the quantum-yield-weighted lifetime is given by
| (2) |
The time-resolved fluorescence anisotropies were determined as described previously (30). For the anisotropy decays, a nonassociative model was considered:
| (3) |
where i is the number of components in the anisotropy decay (in this study, i = 1 in the lo phase and i = 2 in the ld phase membranes), r∞ is the residual anisotropy, βi is the amplitude of component i, and θi is the rotational correlation time of the component i. The fundamental anisotropy (r0) is given by ∑iβi + r∞. The τi values obtained from the magic-angle decay were fixed during the analysis. The goodness of the fit was judged from the reduced χ2 values, the random distribution of the weighted residuals, and the correspondence between the experimental steady-state value of anisotropy and the integrated anisotropy decay.
Infrared spectroscopy
Details about sample preparation and instrumentation for ATR-FTIR are described in the Supporting Material.
Determination of Fas TMD phase behavior
The partition coefficient of Fas TMD between the lo and ld phases, , in POPC/PSM/Chol ternary mixtures was determined from the variation of the peptide's photophysical parameters with the molar fraction of the lo phase (Xlo). The molar fraction of each phase (Xi) was obtained from the tie line of the respective phase diagram (Fig. S1) (26), employing the lever rule. The partition coefficient (Kp) is an equilibrium constant that quantifies peptide distribution between the two phases present in the ternary mixtures (lo and ld). Kp was calculated from the Fas TMD quantum-yield-weighted lifetime according to (23)
| (4) |
where Xi is the phase mole fraction and is the quantum-yield-weighted lifetime of Fas TMD in phase i. Kp is obtained by fitting the previous equation to the experimental data as a function of Xi.
Results
Fas TMD steady-state fluorescence
The fluorescence emission spectra of Fas TMD (0.35 mol % of the total lipid) incorporated in liposomes made of POPC/PSM/Chol 72:23:5 (single ld phase) and 25:35:40 mol/mol/mol (single lo phase) (Fig. S1) MLVs are shown in Fig. 1 A. The photophysical data acquired result from the simultaneous excitation of the two Trp residues in Fas TMD and therefore correspond to the combined emission of Trp176 and Trp189. However, since they are symmetrically located, close to the opposite extremes of the helix, identical environments are being reported. The spectral position is sensitive to the lipid phase: in the ld phase, the emission maximum occurs near λ = 330 nm; in the lo phase, it is shifted to λ = 340 nm. The significantly blue-shifted emission obtained in ld liposomes contrasts with Trp emission in an aqueous environment (λ = 350 nm (31); Δλ = 20 nm), clearly showing that Fas TMD tryptophan residues are in a predominantly nonwater-solvating environment. In lo membranes, the less blue-shifted emission (Δλ = 10 nm relative to water) shows that Fas Trp are less protected from the aqueous solvent. In both cases, the peptide is membrane-bound, since after centrifugation of Fas TMD-containing liposomes in either pure ld or lo phases, Fas TMD was not detected in the aqueous supernatant (data not shown), as expected for the quite hydrophobic α-helical transmembrane segment of Fas (3,4).
Figure 1.
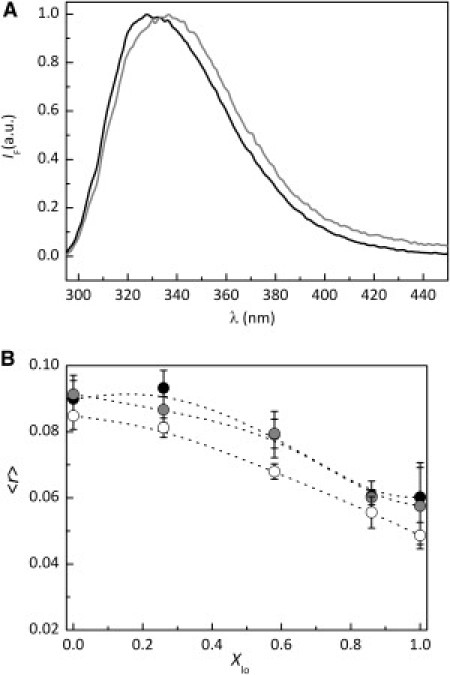
Fas TMD steady-state fluorescence (simultaneous excitation of Trp176 and Trp189). (A) Normalized and corrected fluorescence emission spectra of Fas TMD (0.35 mol % of total lipid) in POPC/PSM/Chol membranes in ld (black) and lo (gray) phases. (B) Steady-state fluorescence anisotropy (〈r〉) of Fas TMD in POPC/PSM/Chol liposomes as a function of Xlo. The data were acquired at room temperature, with λexc = 280 nm and λem = 340 nm, and the peptide concentrations were 0.35 mol % (solid circles), 0.7 mol % (gray circles), and 3.0 mol % (open circles) relative to the total lipid. The dashed lines are merely a guide to the eye. The error bars represent the standard deviations of at least three independent samples.
Fas TMD steady-state fluorescence anisotropy data (Fig. 1 B) show that the peptide has different mobility in ld-rich and lo-rich membranes. For liposomes in a pure ld phase or with low Xlo, the values are higher, showing that the peptide is fairly immobile. A similar anisotropy value (∼0.09) was obtained by us in a previous study of a Trp-containing peptide strongly immobilized in membranes (30). Increasing Xlo, the steady-state anisotropy of Fas TMD decreases, reaching a value of ∼0.06 in liposomes in a pure lo phase. In these membranes, the peptide induced an extensive aggregation of the liposomes perceptible by the increased turbidity of the samples (not shown), as reflected by the higher experimental error values obtained (Fig. 1 B). The lower fluorescence anisotropy values obtained for Fas TMD in lo-rich membranes is somewhat surprising. It might be expected that a transmembrane peptide would present higher fluorescence anisotropy values in lo than in ld membranes, due to the higher order and less flexibility of the lo phase (26). Each peptide molecule contains two Trp residues, but emission depolarization by intrapeptide energy homotransfer is negligible. The distance separating the two Trp residues is 18 Å (assuming an ideal transmembrane α-helix), which is nearly two times larger than the critical radius (R0) for energy homotransfer between Trp residues (10 Å) (27). The lower steady-state anisotropy and less blue-shifted emission of Fas TMD in lo-rich membranes suggests that the peptide is more flexible and less protected from the aqueous solvent than in ld-rich membranes. Nonetheless, other processes could be responsible for the lower steady-state anisotropy of Fas TMD measured in the lo phase. For example, a variation of the Fas TMD fluorescence lifetime would affect Trp steady-state anisotropy, though we will show later that this is not the case. An alternative cause, in the case of peptide aggregate formation, could be emission depolarization due to interpeptide energy homotransfer.
To investigate the lateral distribution of the peptide, a study of the effect of Fas TMD concentration on its steady-state fluorescence anisotropy was carried out (Fig. 1 B). Doubling Fas TMD concentration to 0.7 mol % (relative to the total lipid) did not result in significant changes of the steady-state fluorescence anisotropy values. Only for 3.0 mol % of peptide was a perceptible decrease in Fas TMD steady-state anisotropy obtained in all mixtures studied. This suggests that for the highest Fas TMD concentration, emission depolarization by an intermolecular energy homotransfer might be occurring. At a peptide concentration as high as this, emission depolarization by energy homotransfer is expected to occur even for a random distribution of peptide (27). The fact that there are no significant changes between 0.35 and 0.7 mol % suggests that these concentrations of peptide are sufficiently low to consider an infinite-dilution regime. Nevertheless, it still could be speculated that Fas TMD low steady-state fluorescence anisotropy in lo-rich liposomes results from an efficient energy homotransfer process due to peptide aggregation in this phase. However, this hypothesis can be ruled out, as shown later.
Since Fas TMD emission and steady-state anisotropy suggest that the peptide has different solvent exposure and mobility in ld and lo-rich membranes, studies of Trp fluorescence quenching by the aqueous quencher acrylamide were performed to further investigate Fas TMD membrane organization (Fig. S2 and text in the Supporting Material). The bimolecular rate constant of the quenching process, kQ (Eqs. S1 and S2), recovered for Fas Trp in lo-rich liposomes (kQ = 1.24 × 109 M−1 s−1) is higher than the value obtained for the ld phase (kQ = 3.83 × 108 M−1 s−1). This clearly shows that the accessibility of acrylamide to Fas Trp is higher in the lo than in the ld phase. This is in agreement with Fas TMD lower steady-state anisotropy and less blue-shifted emission in the lo phase, showing that the peptide is in fact more flexible and less protected from the aqueous solvent in this phase compared to the ld phase.
Fas TMD fluorescence lifetime and preference for the ld phase
To obtain more detailed information about Fas TMD aggregation and conformation in the different lipid phases, time-resolved fluorescence measurements, namely, Trp fluorescence lifetimes and time-resolved anisotropy, were also conducted.
The quantum-yield-weighted lifetime (Eq. 2) of Fas TMD in POPC/PSM/Chol membranes is presented in Fig. 2 as a function of the lo-phase molar fraction (Xlo). Trp fluorescence decays are described by the sum of three exponentials (reduced χ2 ≤ 1.2). The peptide quantum-yield-weighted lifetime presents the same trend as the steady-state fluorescence anisotropy (Fig. 1 B). In ld-rich membranes, Trp quantum-yield-weighted lifetimes are high and decrease with increase of the Xlo. Increasing Fas TMD concentration to 0.7 mol % did not induce significant changes in Trp quantum-yield-weighted lifetimes, but when the concentration reached 3.0 mol %, Trp lifetimes became shorter for the membrane compositions with Xlo < 0.8 (Fig. 2). This decrease in Trp lifetimes for 3.0 mol % Fas can be ascribed to the presence of Cys and Lys residues in Fas TMD, which are known to be efficient quenchers of Trp fluorescence (32). At this high peptide concentration, an intermolecular dynamic self-quenching process is operative, reducing the Trp fluorescence emission lifetime.
Figure 2.
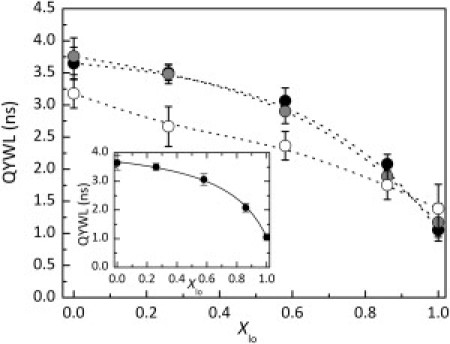
Fas TMD quantum-yield-weighted lifetimes as a function of Xlo in POPC/PSM/Chol membranes at room temperature with λexc = 295 nm and λem = 340 nm. The peptide concentration was 0.35 mol % (solid circles), 0.70 mol % (gray circles), and 3.0 mol % (open circles) of total lipid. The dashed lines serve as a guide to the eye. The error bars represent the standard deviations corresponding to at least three independent samples.(Inset) Nonlinear fit of Eq. 4 to the experimental data for 0.35 mol % peptide (from Fig. 2), with = 0.24 ± 0.02 (R2 = 0.998), where is the Fas TMD partition coefficient between the lo and ld phases in POPC/PSM/Chol membranes at room temperature.
On the other hand, the influence of the fluorescence lifetime on the steady-state anisotropy should be discussed. According to Perrin's equation (; see, e.g., Lakowicz (31)), a decrease in Trp fluorescence lifetime should lead to an increase in Trp steady-state fluorescence anisotropy. This is not the case for Fas TMD, since both the peptide fluorescence lifetime and steady-state anisotropy decrease when Xlo increases (Figs. 1 B and 2). Fas TMD steady-state anisotropy, emission spectra, and time-resolved fluorescence intensity data suggest that the peptide has a different membrane organization in the ld and lo phases. It is likely that the peptide changes from a transmembrane conformation in the ld phase to a nontransmembrane state in the lo phase.
Membrane composition affects peptide organization, and Fas TMD may also affect the membrane properties of the POPC/PSM/Chol mixtures. Thus, experiments were performed to evaluate Fas TMD effects on the lipid phase coexistence and the lipid domain size of these ternary mixtures (Supporting Material). It was found that Fas TMD does not affect the overall properties of the membrane at the hydrophobic core level, as reported by t-PnA fluorescence lifetime (Fig. S3 A). The fluorescence lifetime of t-PnA is very sensitive to alterations in the fraction and composition of the ordered domains, and its invariance in the absence and presence of 0.35 mol % Fas TMD shows that the phase-coexistence region of the POPC/PSM/Chol phase diagram is not affected by the presence of Fas TMD. However, the size of the lipid domains formed in these ternary lipid mixtures is affected by the presence of the peptide, as seen by the FRET efficiency between NBD-DPPE and Rho-DOPE (Fig. S3 B). In the presence of Fas TMD, the lower FRET efficiencies measured show that the domains formed are larger and also suggest that for membranes with high Xlo, Fas TMD might increase the membrane surface area (Supporting Material). Since the POPC/PSM/Chol phase-coexistence region is not changed by the presence of peptide, it is possible to determine the Fas TMD partition coefficient between the lo and ld phases from the fit of Eq. 4 to the quantum-yield-weighted lifetime variation of Fas Trp with Xlo for mixtures with 0.35 mol % of peptide (Fig. 2, inset). The value of = 0.24 ± 0.02 obtained states that Fas TMD has a strong preferential partition toward the ld phase.
Time-resolved fluorescence anisotropy: Fas TMD membrane dynamics
Time-resolved fluorescence anisotropy decays for Fas TMD in ld-rich and lo-rich membranes were also obtained (Fig. 3). Table 1 presents the parameters describing those decays according to Eq. 3. In ld membranes, the observed Fas TMD anisotropy decays were well described by two rotational correlation times (ϕ1 and ϕ2) with similar contributions and a limiting (residual) nonzero anisotropy. The residual anisotropy reflects the maximum degree of mobility of Fas TMD in the membrane (31). In lo-rich membranes, Fas TMD anisotropy decays were described by only one rotational correlation time (ϕ1) and a lower residual anisotropy (Fig. 3 and Table 1). For pure lo membranes, high-quality data could not be obtained due to the Fas-TMD-induced liposome aggregation mentioned previously, and for that reason, the data were acquired for the lipid mixture corresponding to Xlo = 0.86. Good agreement was obtained between the steady-state anisotropy values calculated from the integrated anisotropy decay and the experimentally determined values, both for lo-rich and ld membranes (Table 1). In both membrane types, the limiting anisotropy makes a large contribution to the peptide total anisotropy, reflecting Fas TMD association with the lipid membrane. However, the lower residual anisotropy value recovered for Fas TMD in lo-rich membranes, as compared with ld membranes, shows that Fas Trp has a higher degree of wobbling in lo-rich liposomes. Note that in the lipid mixture with Xlo = 0.86, more than half of the emission light (∼60%) arises from peptide molecules located in the residual ld phase as a result of Fas TMD higher partition and quantum yield in ld domains. Therefore, Fas TMD residual anisotropy in pure lo membranes should be even lower. The estimated residual anisotropy of 0.043 for pure lo membranes clearly reflects the less constricted configuration of Fas TMD in the lo phase compared to the ld phase. These results, together with the absence of a long correlation rotational time in Fas TMD anisotropy decay measured in these membranes, clearly show that the peptide has a different organization in the lo and ld phases, being less constrained in the lo phase. In this latter phase, Fas TMD is indeed in a nontransmembrane state as indicated by the previous fluorescence data.
Figure 3.
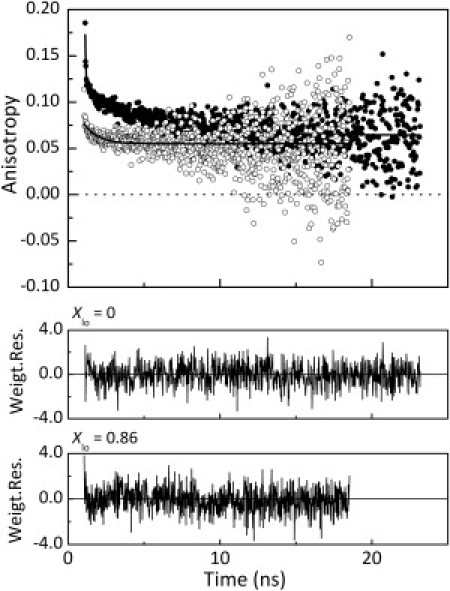
Time-resolved fluorescence anisotropy (r(t)) of Fas TMD (Trp176 and Trp189) incorporated in ld (solid circles) and lo-rich (Xlo = 0.86) (open circles) POPC/PSM/Chol membranes, obtained at room temperature with λexc = 295 nm and λem = 340 nm. (Upper) Circles are the experimental data points, and solid lines are the corresponding fits of Eq. 3. (Lower) Weighted residuals of each fit. The fitting parameters are shown in Table 1. Peptide concentration was 0.35 mol % total lipid.
Table 1.
Time-resolved fluorescence anisotropy parameters of Fas TMD in ld and lo-rich vesicles
| Xlo | 〈r〉exp | ϕ1 (ns) | β1 | ϕ2 (ns) | β2 | r∞ | χ2 | 〈r〉calc |
|---|---|---|---|---|---|---|---|---|
| 0 | 0.090 ± 0.006 | 0.033 | 0.067 | 4.325 | 0.045 | 0.064 | 1.098 | 0.088 |
| 0.86 | 0.061 ± 0.005 | —∗ | —∗ | 0.861 | 0.021 | 0.055 | 1.145 | 0.061 |
Parameters include rotational correlation times, ϕi, amplitudes, βi, and limiting anisotropy, r∞. Vesicles with Xlo = 0 are ld and those with Xlo = 0.86 are lo-rich; measurements were made at room temperature. Also shown are the experimentally measured steady-state fluorescence anisotropy, 〈r〉exp, and the steady-state anisotropy value calculated from the integration of the anisotropy decay, 〈r〉calc. Peptide concentration was 0.35 mol % relative to total lipid.
In lo-rich membranes, Fas TMD anisotropy decays were described by single-correlation rotational time (see text for further details).
ATR-FTIR measurements: Fas TMD membrane conformation
To gain further knowledge about Fas TMD membrane organization, ATR-FTIR studies were also performed. From these measurements, information about Fas TMD membrane orientation can be obtained. In Fig. 4 A are presented dichroic ATR-FTIR spectra for Fas TMD in ld and lo-rich membranes. The negative deviations observed in all spectra around 2850 cm−1 and 2920 cm−1 correspond to the lipid νs(CH2) and νas(CH2) signals (stretching), respectively, reflecting the parallel orientation of the lipid acyl chain to the bilayer normal. In other words, the expected parallel orientation of the lipid multilayer to the germanium crystal surface was successfully achieved (33,34). The positive deviations obtained around 1650 cm−1 and 1550 cm−1 correspond to the peptide amide I and amide II bands and reflect the contribution of Fas TMD to the infrared signal. The rescaled spectra in this region are presented in Fig. 4 B. For ld membranes (bottom line), the amide I signal has a maximum around 1652 cm−1, clearly showing that Fas TMD has a predominant transmembrane α-helical conformation (33,34). For lo-rich (Xlo = 0.86) and lo (Xlo = 1) membranes, the amide I signal is shifted to higher wavenumbers, with a maximum around 1680 cm−1. This shows that the peptide is in a nonhelical structure, although the main conformation cannot be precisely defined. It is possible that the lower stability of Fas TMD in these membranes results in a peptide population with different conformations that can be short helices, stressed helices, or turns. It is worth mentioning that β-sheet formation was not detected in the ATR spectra of any of these membranes, indicating that peptide aggregates are not forming. Altogether, the infrared results shows that in ld membranes Fas TMD adopts an α-helical transmembrane conformation (as expected for a TMD of a membrane protein), whereas in lo membranes, the peptide is in a nontransmembrane state, as supported by the fluorescence data.
Figure 4.
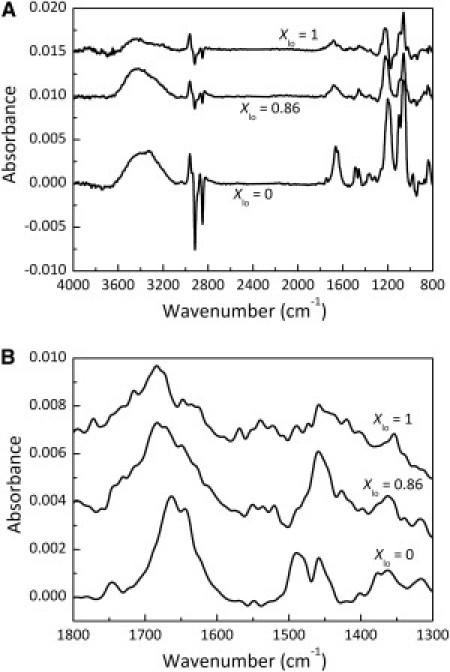
(A) Dichroic ATR-FTIR spectra of Fas TMD-POPC/PSM/Chol membranes in the ld, lo-rich (Xlo = 0.86), and lo phases. (B) Dichroic spectra rescaled to the amide I and amide II regions. The spectra are shifted along the ordinate for a better reading.
Effect of ceramide on Fas TMD membrane organization
To investigate Cer effects on Fas plasma membrane organization, Fas peptide steady-state fluorescence anisotropy and fluorescence lifetimes were measured in POPC/PSM/Chol membranes with 2 mol % and 4 mol % PCer (Fig. 5). In membranes with Cer, Fas TMD steady-state anisotropy and quantum-yield-weighted lifetimes present the same trend of variation as in liposomes with no PCer. At first glance, PCer seems to affect both Fas Trp quantum-yield-weighted lifetimes and rotational dynamics. For a membrane with a given composition, the presence of PCer decreases Fas TMD steady-state anisotropy and increases its fluorescence lifetime. For instance, in the absence of PCer, and in membranes with Xlo = 0.26, Fas TMD has a steady-state fluorescence anisotropy of 0.093 ± 0.005 and a fluorescence lifetime of 3.50 ± 0.12 ns. For membranes with the same composition but containing 2 mol % PCer, those values are 0.080 ± 0.003 and 3.92 ± 0.02 ns, respectively. When a modified Perrin equation, which takes into account the limiting anisotropy contribution, is used to correct Fas TMD steady-state fluorescence anisotropy for the increase in peptide lifetimes, a value of 0.093 is obtained (r0 = 0.270 (35)). Calculating all values of Fas TMD steady-state anisotropy that would be obtained hypothetically if there were no changes in the fluorescence lifetime in membranes with Cer resulted in anisotropy values similar to those obtained in membranes without Cer (Fig. 4 A, inset). This shows that PCer does not affect Fas TMD steady-state anisotropy, but slightly increases its fluorescence lifetimes.
Figure 5.
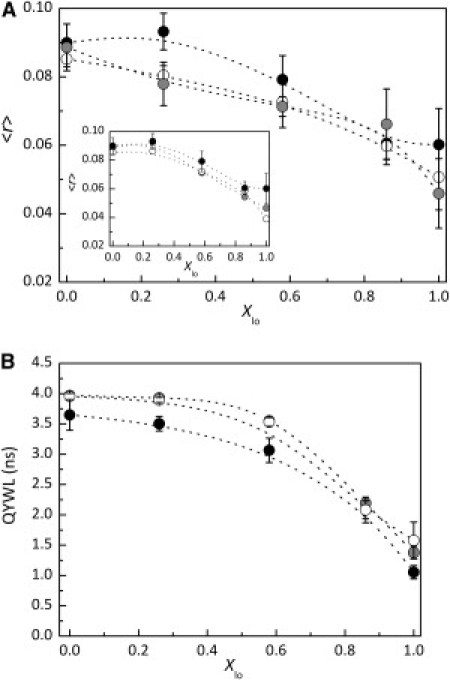
Ceramide has minor effects on Fas TMD membrane organization. (A) Steady-state fluorescence anisotropy (〈r〉) of Fas TMD in POPC/PSM/Chol membranes containing 0 (solid circles), 2 (gray circles), and 4 (open circles) mol % PCer. (Inset) Fas peptide steady-state anisotropy values in membranes containing PCer calculated assuming no alterations in the fluorescence lifetime. (B) Quantum-yield-weighted lifetimes of Fas TMD in the membranes in A. Errors bars represent standard deviations of at least three independent samples.
Discussion
Fas TMD membrane organization and dynamics
In this work, we have exploited the intrinsic fluorescence of the two tryptophan residues (Trp176 and Trp189) of Fas TMD reconstituted into POPC/PSM/Chol (raft model) membranes with or without Cer, to better understand how lipid rafts and Cer modulate Fas conformation and membrane lateral distribution. Further evidence regarding peptide structure and organization was obtained from ATR-FTIR. In vivo, Fas is expressed in plasma membrane as preassembled trimers (1,2). Fas trimerization is maintained mainly by interactions between each extracellular (via the preligand receptor association domain (PLAD)) and intracellular domains (by the death domains of Fas and FADD) (2,4). TMD interactions are unlikely to occur, since they are hindered by the bulkier ecto and endodomains of the receptor and therefore play a minor role in Fas association. In this study, the absence of intermolecular static-quenching (data not shown), which could be a likely process in the case of dimer (or trimer) formation, shows that Fas TMD does not aggregate. This is also supported by the ATR-FTIR data. Only for the highest Fas concentration studied (3 mol %) was a small extent of interaction detected. This is shown by Trp lower quantum-yield-weighted lifetimes in ld-rich membranes when compared to diluted samples (Fig. 2), which can be interpreted as a result of a dynamic self-quenching process. The effect of collisional self-quenching on the fluorescence lifetime of a molecule with a complex decay and its relation with the diffusion coefficient (D) is shown in the Supporting Material (Eqs. S9–S11). Assuming a random peptide distribution in the membrane, a value of D = 11 × 10−9 cm2 s−1 is obtained. This value is in the same order of magnitude of those typically found for TMD protein diffusion in an ld phase (D = 10–70 × 10−9 cm2 s−1 (27,36,37)), showing that Fas TMD does not exhibit significant aggregation in these membranes, being mostly monomeric and hence in a similar configuration as in vivo.
Our results show that the membrane state modulates Fas TMD organization. In ld-rich membranes, Fas TMD is in a transmembrane α-helical conformation with a severely restricted rotational dynamics and a lateral diffusion typical of the ld phase. In lo-rich membranes, Fas TMD is likely located near the membrane-water interface, where its Trps are more exposed to the aqueous solvent and much less constrained. As a result, compared to the case for ld membranes, Fas Trps present red-shifted emission, shorter fluorescence lifetimes, and lower steady-state anisotropy. An alternative explanation for these results is the formation of transmembrane peptide clusters in the lo phase, which might lead to efficient fluorescence self-quenching and energy homotransfer processes. However, our results do not support this hypothesis. Trp red-shifted emission and higher accessibility to acrylamide in lo-rich membranes show that Fas TMD is less protected from water than in ld membranes. Furthermore, neither α-helical conformation nor aggregate formation could be detected by ATR-FTIR for lo-rich membranes (Fig. 4 B). The observation of Fas-TMD-induced liposome aggregation in lo-rich membranes also points to a Fas TMD location near the membrane-water interface, since membrane aggregation mediated by interfacial located hydrophobic peptides is one way of decreasing hydrophobic residue exposure to the polar solvent (38). Time-resolved fluorescence anisotropy data (Fig. 3 and Table 1) also supports the postulated nontransmembrane state of Fas TMD in the lo phase (Fig. 3 and Table 1). Independent of peptide cluster formation, if Fas TMD was located inside the bilayer in lo-rich membranes, its rotational freedom would be low due to the higher order and lower flexibility of the lo phase (26). Thus, its anisotropy decay would be characterized by a high limiting anisotropy, which should be higher than its limiting anisotropy in ld membranes. This is not the case, since the peptide residual anisotropy in the lo phase is lower than in the ld phase. Altogether, these results highlight the nontransmembrane conformation of Fas TMD in lo-rich membranes.
In the ld phase, Fas TMD anisotropy decay was described by two correlation times, ϕ1 and ϕ2 (Table 1). The shorter correlation time (ϕ1) can be attributed to movements of the Trp indole ring, and its value is similar to the short correlation times of the Trp-containing TMD of acetylcholine receptor γM4 inserted into lipid bilayers (30). The peptide longer correlation time (ϕ2) results from movements involving several amino acid residues. NH groups of Trp indole moiety are potentially capable of forming hydrogen bonds with the hydroxyl groups of surrounding molecules, drastically decreasing molecular rotation (39). In ld membranes, Fas-TMD-hindered rotations, reflected by its high limiting anisotropy, indicate that its Trp residues have strong interactions with the lipid. This is in accordance with the remaining fluorescence (blue-shifted emission, less fluorescence quenching by acrylamide, and long fluorescence lifetimes) and infrared data (α-helical signal in ATR spectra), which already suggested that Fas Trp would be in a hydrophobic environment.
Lipid rafts, ceramide, and Fas TMD membrane lateral distribution
Fas recruitment to lipid rafts, where it aggregates with other Fas receptors and other proteins (FADD and procaspase-8), is a crucial step of apoptotic signaling (5–8). Our results demonstrate that Fas TMD plays a minor role in receptor translocation to lipid rafts. In addition, it does not induce alterations in the POPC/PSM/Chol phase diagram (Fig. S3). The peptide partition coefficient between raft and nonraft regions ( = 0.24 ± 0.02) (Fig. 2, inset) shows that the Fas TMD concentration is around four times higher in the ld (nonraft) phase than in the lo (raft) phase. The in vivo translocation of Fas to lipid rafts must therefore occur by a different process. Studies have shown that native Fas is palmitoylated at a Cys residue of the cytoplasmatic region proximal to the membrane, and this posttranslational modification was identified as the main mechanism localizing Fas to lipid rafts (12,13). In this study, the less efficient localization of Fas TMD to raft regions results from the absence of palmitoylation and from the strong hydrophobic mismatch between the peptide and lo bilayers. These membranes have a bilayer thickness of 40 Å, with a hydrophobic region of 30 Å (42). Being a 21-residue-long hydrophobic peptide with α-helical structure (3,4), Fas TMD has a total length of ∼32 Å and is thus 8 Å shorter than lo membranes. This strong negative hydrophobic mismatch is responsible for Fas TMD avoiding lo domains and for the conformational change detected for lo-rich membranes. In this case, the free energy for Fas TMD insertion into the thick lo bilayer is much higher than the entropy-driven forces that minimize the hydrogen-bonding network reorganization of water caused by unfavorable interactions of Fas TMD hydrophobic residues with polar water molecules (hydrophobic effect). As a result, Fas TMD adopts a nontransmembrane conformation and probably translocates to a position near the membrane surface. This is not expected to occur in vivo since the transmembrane state of TMD will be stabilized by the bulkier charged exo- and cytoplasmatic domains of the receptor. On the other hand, ld membranes have a thickness of 36 Å, with a hydrophobic region of 26 Å (42). Between Fas TMD and ld bilayers there is only a slight negative hydrophobic mismatch (∼4 Å) that is not strong enough to drive the peptide from a transmembrane conformation to a nontransmembrane state. Therefore, in a situation where there is ld-lo phase separation, similar to plasma membrane rafts (nonpalmitoylated) in cells, Fas TMD higher preference for the thinner/disordered membranes will drive the receptor to those membrane regions.
It has been reported that Cer drives the fusion of small rafts into large signaling platforms, facilitating Fas clustering and thus improving Fas signaling (14,15). Although studies in model systems have shown that Cer is not able to induce the formation of large domains (22,23), doubt remains about the ability of this lipid to change Fas membrane localization and organization. Our results show that Cer does not affect Fas TMD membrane organization but does increase Fas Trp fluorescence lifetimes. This reflects the increased compactness of membranes containing Cer (18–20). Furthermore, Fas TMD fluorescence parameters in mixtures containing Cer-rich gel (4 mol % PCer, Xlo ≤ 0.58 (23)) are essentially the same as for those in which no gel is formed (2 mol % PCer) (23) (Fig. 5), showing that the peptide conformation and lateral distribution are not affected by the formation of Cer-rich gel. These results are in accordance with a previous study that shows that in the absence of any death stimuli, Cer cannot induce Fas translocation to lipid rafts (8). By increasing membrane order, Cer reduces the lateral diffusion of membrane proteins and lipids, trapping and clustering those molecules (25). In lipid rafts with Cer, Fas oligomer diffusion will be reduced, minimizing complex dissociation and receptor translocation out of those regions. As a result, formation of large and stable apoptotic protein clusters is enhanced, resulting in efficient triggering of apoptosis. This seems to be the general mechanism by which Cer traps and clusters raft-associated proteins and lipids, namely Fcγ receptor II (43,44), CD40 (45), GPI-PLAP and ganglioside GM1 (25), and Fas (14,15). Therefore, Cer is apparently not involved in Fas trimer recruitment to lipid rafts, but enhances Fas clustering with other Fas trimers and other proteins of the apoptotic machinery in those membrane domains.
Implications for Fas-mediated cell death
To trigger apoptosis, Fas needs to oligomerize and cluster with intracellular apoptotic proteins to activate the apical protease caspase-8 (1,2). However, these processes only occur after Fas translocation into lipid rafts, where the apoptotic proteins are concentrated (5–7), making this process the central step of Fas early (membrane) signaling events (1,2). Our results suggest that Fas TMD does not participate in receptor translocation into lipid rafts. In fact, our results show that Fas TMD has a marked preference toward nonraft regions. This feature of the Fas receptor may correspond to a protective mechanism of cells against apoptosis triggering in the absence of death stimuli. Fas has an intrinsic ability to cocluster (either by its extracellular PLAD domain or by its cytoplasmatic death domain (4)) and starts apoptosis in the absence of its ligand (FasL) and even without an intact extracellular domain (46). To overcome the natural facility of Fas to cap, the protein is equipped with a TMD that has a low ability to localize into membrane regions where the probability of apoptosis triggering by receptor oligomerization is enhanced. This hypothesis is supported by studies that show that Fas needs to be palmitoylated to translocate into lipid rafts (12,13), and it has been suggested that the regulation of Fas palmitoylation determines protein localization into or out of rafts (4,11). Moreover, differences in Fas palmitoylation were thought to be responsible for the constitutive presence of Fas in lipid rafts of some cells (type I), whereas in most resting cells (type II), Fas is excluded from those regions (4,11). Thus, it seems that in the absence of death stimuli, the proapoptotic attribute of Fas to cluster with cell death machinery proteins located in lipid rafts is hampered by its low partition to those membrane regions, which results from the lack of palmitoylation and the short length of the Fas TMD.
Altogether, this study highlights the role of Fas TMD as a regulator of Fas translocation to lipid rafts, the main stage of Fas signaling early events. The TMD of Fas strongly stalls receptor localization into lipid rafts, suggesting that its length and sequence may have a protective role against unspecific Fas-mediated apoptosis.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia grants PTDC/QUI-BIQ/099947/2008 and PEst-OE/QUI/UI0612/2011 and fellowship BD/36635/2007 (to B.M.C.).
Supporting Material
References
- 1.Algeciras-Schimnich A., Shen L., Peter M.E. Molecular ordering of the initial signaling events of CD95. Mol. Cell. Biol. 2002;22:207–220. doi: 10.1128/MCB.22.1.207-220.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peter M.E., Krammer P.H. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 3.Mollinedo F., Gajate C. Fas/CD95 death receptor and lipid rafts: new targets for apoptosis-directed cancer therapy. Drug Resist. Updat. 2006;9:51–73. doi: 10.1016/j.drup.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Ramaswamy M., Cleland S.Y., Siegel R.M. Many checkpoints on the road to cell death: regulation of Fas-FasL interactions and Fas signaling in peripheral immune responses. In: Kalthoff H., editor. Death Receptors and Cognate Ligands in Cancer. Springer; Heidelberg: 2009. pp. 17–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajate C., Mollinedo F. The antitumor ether lipid ET-18-OCH3 induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood. 2001;98:3860–3863. doi: 10.1182/blood.v98.13.3860. [DOI] [PubMed] [Google Scholar]
- 6.Hueber A.O., Bernard A.M., He H.T. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep. 2002;3:190–196. doi: 10.1093/embo-reports/kvf022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheel-Toellner D., Wang K., Lord J.M. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem. Biophys. Res. Commun. 2002;297:876–879. doi: 10.1016/s0006-291x(02)02311-2. [DOI] [PubMed] [Google Scholar]
- 8.Gajate C., Del Canto-Jañez E., Mollinedo F. Intracellular triggering of Fas aggregation and recruitment of apoptotic molecules into Fas-enriched rafts in selective tumor cell apoptosis. J. Exp. Med. 2004;200:353–365. doi: 10.1084/jem.20040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 10.London E., Brown D.A. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts) Biochim. Biophys. Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 11.Muppidi J.R., Siegel R.M. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat. Immunol. 2004;5:182–189. doi: 10.1038/ni1024. [DOI] [PubMed] [Google Scholar]
- 12.Feig C., Tchikov V., Peter M.E. Palmitoylation of CD95 facilitates formation of SDS-stable receptor aggregates that initiate apoptosis signaling. EMBO J. 2007;26:221–231. doi: 10.1038/sj.emboj.7601460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakrabandhu K., Hérincs Z., Hueber A.O. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 2007;26:209–220. doi: 10.1038/sj.emboj.7601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cremesti A., Paris F., Kolesnick R. Ceramide enables fas to cap and kill. J. Biol. Chem. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- 15.Grassmé H., Cremesti A., Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- 16.Hannun Y.A., Obeid L.M. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 17.Cremesti A.E., Goñi F.M., Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS Lett. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- 18.Castro B.M., de Almeida R.F., Prieto M. Formation of ceramide/sphingomyelin gel domains in the presence of an unsaturated phospholipid: a quantitative multiprobe approach. Biophys. J. 2007;93:1639–1650. doi: 10.1529/biophysj.107.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nybond S., Bjorkqvist Y.J.E., Slotte J.P. Acyl chain length affects ceramide action on sterol/sphingomyelin-rich domains. Biochim. Biophys. Acta. 2005;1718:61–66. doi: 10.1016/j.bbamem.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Busto J.V., Sot J., Alonso A. Cholesterol displaces palmitoylceramide from its tight packing with palmitoylsphingomyelin in the absence of a liquid-disordered phase. Biophys. J. 2010;99:1119–1128. doi: 10.1016/j.bpj.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro B.M., Silva L.C., Prieto M. Cholesterol-rich fluid membranes solubilize ceramide domains: implications for the structure and dynamics of mammalian intracellular and plasma membranes. J. Biol. Chem. 2009;284:22978–22987. doi: 10.1074/jbc.M109.026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva L.C., Futerman A.H., Prieto M. Lipid raft composition modulates sphingomyelinase activity and ceramide-induced membrane physical alterations. Biophys. J. 2009;96:3210–3222. doi: 10.1016/j.bpj.2008.12.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva L.C., de Almeida R.F., Prieto M. Ceramide-domain formation and collapse in lipid rafts: membrane reorganization by an apoptotic lipid. Biophys. J. 2007;92:502–516. doi: 10.1529/biophysj.106.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sot J., Ibarguren M., Alonso A. Cholesterol displacement by ceramide in sphingomyelin-containing liquid-ordered domains, and generation of gel regions in giant lipidic vesicles. FEBS Lett. 2008;582:3230–3236. doi: 10.1016/j.febslet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Chiantia S., Ries J., Schwille P. Role of ceramide in membrane protein organization investigated by combined AFM and FCS. Biochim. Biophys. Acta. 2008;1778:1356–1364. doi: 10.1016/j.bbamem.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 26.de Almeida R.F.M., Fedorov A., Prieto M. Sphingomyelin/phosphatidylcholine/cholesterol phase diagram: boundaries and composition of lipid rafts. Biophys. J. 2003;85:2406–2416. doi: 10.1016/s0006-3495(03)74664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Almeida R.F.M., Loura L.M.S., Barrantes F.J. Cholesterol modulates the organization of the γM4 transmembrane domain of the muscle nicotinic acetylcholine receptor. Biophys. J. 2004;86:2261–2272. doi: 10.1016/S0006-3495(04)74284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pace C.N., Vajdos F., Gray T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995;4:2411–2423. doi: 10.1002/pro.5560041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Almeida R.F.M., Loura L.M.S., Prieto M. Nonequilibrium phenomena in the phase separation of a two-component lipid bilayer. Biophys. J. 2002;82:823–834. doi: 10.1016/S0006-3495(02)75444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Almeida R.F.M., Loura L.M.S., Barrantes F.J. Structure and dynamics of the γM4 transmembrane domain of the acetylcholine receptor in lipid bilayers: insights into receptor assembly and function. Mol. Membr. Biol. 2006;23:305–315. doi: 10.1080/09687860600703613. [DOI] [PubMed] [Google Scholar]
- 31.Lakowicz J. Springer; New York: 2006. Principles of Fluorescence Spectroscopy. [Google Scholar]
- 32.Chen Y., Barkley M.D. Toward understanding tryptophan fluorescence in proteins. Biochemistry. 1998;37:9976–9982. doi: 10.1021/bi980274n. [DOI] [PubMed] [Google Scholar]
- 33.Goormaghtigh E., Cabiaux V., Ruysschaert J.M. Secondary structure and dosage of soluble and membrane proteins by attenuated total reflection Fourier-transform infrared spectroscopy on hydrated films. Eur. J. Biochem. 1990;193:409–420. doi: 10.1111/j.1432-1033.1990.tb19354.x. [DOI] [PubMed] [Google Scholar]
- 34.Goormaghtigh E., Raussens V., Ruysschaert J.M. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim. Biophys. Acta. 1999;1422:105–185. doi: 10.1016/s0304-4157(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 35.Valeur B., Weber G. Resolution of the fluorescence excitation spectrum of indole into the 1La and 1Lb excitation bands. Photochem. Photobiol. 1977;25:441–444. doi: 10.1111/j.1751-1097.1977.tb09168.x. [DOI] [PubMed] [Google Scholar]
- 36.Ramadurai S., Holt A., Poolman B. Lateral diffusion of membrane proteins. J. Am. Chem. Soc. 2009;131:12650–12656. doi: 10.1021/ja902853g. [DOI] [PubMed] [Google Scholar]
- 37.Ramadurai S., Holt A., Poolman B. Influence of hydrophobic mismatch and amino acid composition on the lateral diffusion of transmembrane peptides. Biophys. J. 2010;99:1447–1454. doi: 10.1016/j.bpj.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holt A., Killian J.A. Orientation and dynamics of transmembrane peptides: the power of simple models. Eur. Biophys. J. 2010;39:609–621. doi: 10.1007/s00249-009-0567-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dutt G.B. Rotational diffusion of nondipolar probes in Triton X-100 micelles: role of specific interactions and micelle size on probe dynamics. J. Phys. Chem. B. 2002;106:7398–7404. [Google Scholar]
- 40.Reference deleted in proof.
- 41.Reference deleted in proof.
- 42.Gandhavadi M., Allende D., McIntosh T.J. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys. J. 2002;82:1469–1482. doi: 10.1016/S0006-3495(02)75501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korzeniowski M., Shakor A.B.A., Sobota A. Fc γRII activation induces cell surface ceramide production which participates in the assembly of the receptor signaling complex. Cell. Physiol. Biochem. 2007;20:347–356. doi: 10.1159/000107520. [DOI] [PubMed] [Google Scholar]
- 44.Abdel Shakor A.B., Kwiatkowska K., Sobota A. Cell surface ceramide generation precedes and controls FcγRII clustering and phosphorylation in rafts. J. Biol. Chem. 2004;279:36778–36787. doi: 10.1074/jbc.M402170200. [DOI] [PubMed] [Google Scholar]
- 45.Grassmé H., Jendrossek V., Gulbins E. Ceramide-rich membrane rafts mediate CD40 clustering. J. Immunol. 2002;168:298–307. doi: 10.4049/jimmunol.168.1.298. [DOI] [PubMed] [Google Scholar]
- 46.Papoff G., Hausler P., Ruberti G. Identification and characterization of a ligand-independent oligomerization domain in the extracellular region of the CD95 death receptor. J. Biol. Chem. 1999;274:38241–38250. doi: 10.1074/jbc.274.53.38241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


