Abstract
A molecule binds to a cytokine receptor and limits the cytokine from eliciting inflammatory responses by immune cells.
The cytokine tumor necrosis factor α (TNFα) is a major driver of rheumatoid arthritis and related inflammatory diseases. Therapeutic agents that block TNFα or mimic a soluble receptor for the cytokine are effective in these diseases, but whether other endogenous modulators of TNFα action exist that could be targets for therapeutic intervention is not clear. On page xxx of this issue, Tang et al. (1) show that progranulin, a secreted molecule with some cytokine-like properties, binds to TNF receptors and limits the action of TNFα in inflammatory arthritis.
The TNF family of cytokines in humans comprises 19 ligands, for which there are 35 receptors. TNFα is expressed mainly by endothelial and immune cells as a membrane protein that can be cleaved off the membrane by metalloproteinases. The cytokine acts through two receptors, TNFR1 and TNFR2. TNFR1 is broadly expressed and promotes an inflammatory response. TNFR2 is expressed predominantly by lymphocytes and stimulates lymphocyte activation. It also inhibits the development and function of “inducible” regulatory T cells (which suppress immune system activation) (2). Mouse models of inflammatory arthritis support a primary role for TNFR1, with some contribution by TNFR2, to the pathogenic role of TNFα (3, 4).
Progranulin (PGRN), also known as proepithelin or acrogranin, can be proteolyzed into small homologous subunits called granulins (GRNs) or epithelins. PGRN was originally identified as an autocrine growth factor for cancer cells and fibroblasts. Mutations in the human gene encoding PGRN are associated with some cases of frontotemporal dementia, a neurodegenerative disease (5). Other clues suggested that PGRN plays a role in inflammation. PGRN inhibits, whereas GRNs stimulate, the production of neutrophil-attracting chemokines. Cleavage of PGRN is promoted by enzymes including elastase, which is secreted by neutrophils. These findings suggested a role for GRN in amplifying acute inflammation, and PGRN in the resolution of inflammation and wound repair (6). Mice lacking PGRN have no overt immunological phenotype, but PGRN-deficient macrophages challenged with microbial lipopolysaccharide (and other agonists of Toll-like receptors expressed by macrophages) had increased proinflammatory cytokine production (7). But the receptors through which PGRN mediated these anti-inflammatory effects were unknown.
Tang et al. isolated TNFR2 in a screen for PGRN binding partners. Recombinant PGRN bound to TNFR1 and TNFR2 with nanomolar affinity and blocked interaction with TNFα. PGRN also inhibited inflammation in disease models that depend on TNFα. PGRN-deficient mice are hypersensitive to arthritis (induced by collagen), and treatment with PGRN reduced clinical and histological features of this disorder. In a model of systemic inflammation and arthritis (driven by human TNFα), PGRN-deficient mice developed more severe disease, and treatment with PGRN slowed arthritis progression.
The effects of PGRN may be complicated by its cleavage into GRNs. No individual GRN domain of PGRN bound to TNFR2, suggesting that the proinflammatory effects of GRNs may be mediated through other receptors. However, strong binding to TNFR2 was observed with a fusion of three partial GRN subunits. This fusion protein, called antagonist of TNF-TNFR signaling via targeting to TNF receptors (Atsttrin), exhibited more potent anti-inflammatory activity than PGRN, perhaps because it does not contain any complete GRN domains that have proinflammatory function.
Some aspects of how PGRN exerts its anti-inflammatory effects remain unclear. Tang et al. determined that the effects of Atsttrin in collagen-induced arthritis depend on TNFR2. However, PGRN deficiency also enhanced disease in mice that overexpress human TNFα, which is thought to interact solely with mouse TNFR1. Studies with mice lacking PGRN and one or both TNF receptors may resolve these discrepancies.
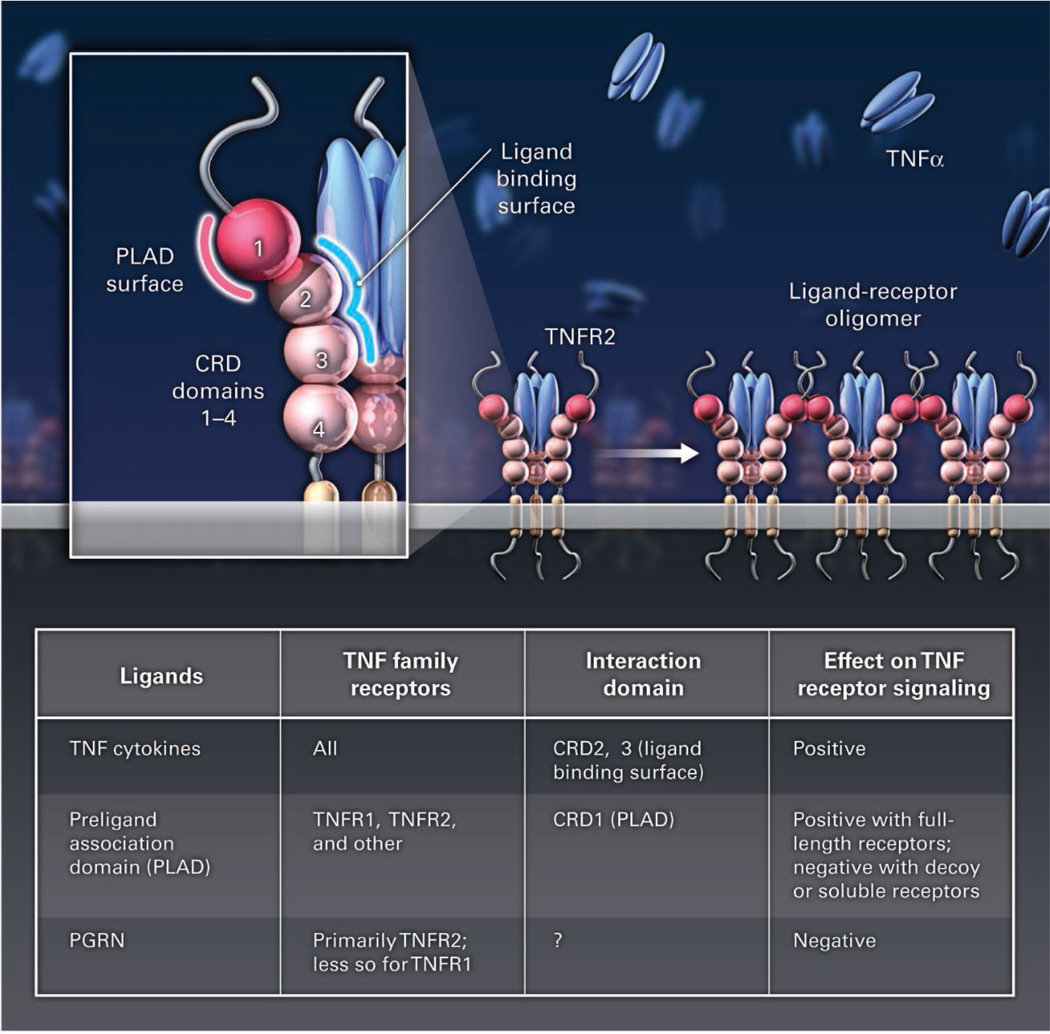
The extracellular regions of TNF receptors are elongated structures with multiple cysteine-rich repeat domains (CRDs). TNF-family ligands bind to receptors in a heterohexameric 3:3 complex in which each receptor subunit contacts two adjacent ligand subunits (see the figure) typically via CRD2 and CRD3, the “stalk” of the receptor. By contrast, the opposite side of the amino-terminal CRD1 contains the preligand association domain (PLAD), which mediates homotypic interactions among receptor chains and may also stabilize and propagate ligand-receptor contacts, thus producing higher-order oligomers critical for receptor signaling (8–11). Although interactions between receptors through the PLAD positively influence signaling, binding of decoy receptors or engineered soluble receptor homologs to the PLAD disrupts signaling by the targeted receptor (12, 13).
Figure 1.
Because PGRN and Atsttrin competitively bind TNF receptors, but apparently not TNFα itself, and potently block TNF-TNFR interactions in vitro, these molecules likely interact with the ligand-binding interface at CDR2 and CDR3. PGRN and Atsttrin are composed of multiple disulfide-stabilized GRN domains with some structural similarity to CRDs. Given their potential elongated shape, PGRN and Atsttrin could also interact with a surface on the opposite side of the ligand binding site of the receptor, including the PLAD, to induce allosteric changes that hinder TNFα binding. In this case, the mechanisms of action could inhibit both ligand binding and receptor oligomerization. The surfaces of GRN domains of PGRN and the CRDs of TNF receptors contain numerous positive and negative charges, which may facilitate either of these interactions. Also, the mechanisms of inhibition by PGRN versus Atsttrin may differ, the latter harboring multiple exposed, free cysteines. Structural studies of PGRN and Atsttrin bound to TNF receptors should resolve these issues. The work by Tang et al. suggests that there may be other endogenous regulators that dampen the dangerous consequences of excessive signaling by TNF receptors.
Footnotes
TNF signaling. TNF-α binds to TNFR2, which may lead to oligomerization of ligand-receptor complexes, helping to propagate signaling. Some regulators of TNF family receptor signaling are listed.
References
- 1.Tang W, et al. Science. 2011;332 xxx. [Google Scholar]
- 2.Valencia X, et al. Blood. 2006;108:253. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tada Y, et al. Clin. Immunol. 2001;99:325. doi: 10.1006/clim.2001.5027. [DOI] [PubMed] [Google Scholar]
- 4.Mori L, Iselin S, De Libero G, Lesslauer W. J. Immunol. 1996;157:3178. [PubMed] [Google Scholar]
- 5.Cruts M, et al. Nature. 2006;442:920. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, et al. Cell. 2002;111:867. doi: 10.1016/s0092-8674(02)01141-8. [DOI] [PubMed] [Google Scholar]
- 7.Yin F, et al. J. Exp. Med. 2010;207:117. doi: 10.1084/jem.20091568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan FK, et al. Science. 2000;288:2351. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RM, et al. Science. 2000;288:2354. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 10.Mukai Y, et al. Sci. Signal. 2010;3:ra83. doi: 10.1126/scisignal.2000954. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RM, et al. J. Cell Biol. 2004;167:735. doi: 10.1083/jcb.200406101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clancy L, et al. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18099. doi: 10.1073/pnas.0507329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng GM, Zheng L, Chan FK, Lenardo M. Nat. Med. 2005;11:1066. doi: 10.1038/nm1304. [DOI] [PubMed] [Google Scholar]