Abstract
Setting
Two centres in Soweto and Cape Town, South Africa.
Objective
To assess the effects of timing of initiation of antiretroviral treatment (ART) and other factors on the risk of bacille Calmette-Guérin (BCG) related regional adenitis due to immune reconstitution inflammatory syndrome (BCG-IRIS) in human immunodeficiency virus (HIV) infected infants.
Design
HIV-infected infants aged 6–12 weeks with CD4 count ≥25% enrolled in the Children with HIV Early Antiretroviral Therapy (CHER) Trial received early (before 12 weeks) or deferred (after immunological or clinical progression) ART; infants with CD4 count <25% all received early ART. All received BCG vaccination after birth. Reactogenicity to BCG was assessed prospectively during routine study follow-up.
Results
Of 369 infants, 32 (8.7%) developed BCG-IRIS within 6 months of starting ART, 28 (88%) within 2 months after ART initiation. Of the 32 cases, 30 (93.8%) had HIV-1 RNA > 750 000 copies/ml at initiation. Incidence of BCG-IRIS was 10.9 and 54.3 per 100 person-years (py) among infants with CD4 count ≥25% at enrolment receiving early (at median age 7.4 weeks) vs. deferred (23.2 weeks) ART, respectively (HR 0.24, 95%CI 0.11–0.53, P < 0.001). Infants with CD4 count <25% receiving early ART had intermediate incidence (41.7/100 py). Low CD4 counts and high HIV-1 RNA at initiation were the strongest independent risk factors for BCG-IRIS.
Conclusions
Early ART initiation before immunological and/or clinical progression substantially reduces the risk of BCG-IRIS regional adenitis.
Keywords: BCG, immune reconstitution inflammatory syndrome, paediatric HIV
BACILLE CALMETTE-GUÉRIN (BCG) protects immunocompetent children against miliary tuberculosis (TB) and TB meningitis, but there are safety and efficacy concerns in human immunodeficiency virus (HIV) infection.1–4 The World Health Organization guidelines recommend avoiding BCG immunisation in HIV-infected infants due to the increased risk of dissemination.5,6 In South Africa, however, all neonates are still vaccinated, regardless of HIV exposure, as the prevalence of TB and HIV is high and HIV diagnosis before 6 weeks of age is not yet feasible.5,7–9
BCG-related adverse events are classified as local, regional and distant or disseminated disease.10,11 TB and BCG disease may occur concurrently.11,12 Complications occurring soon after initiation of antiretroviral treatment (ART) are usually ascribed to immune reconstitution inflammatory syndrome (IRIS).11,13 BCG-related regional adenitis due to IRIS (BCG-IRIS) was described in respectively 6% and 15% of children in two South African cohorts.12,14 However, these and other reports only included children starting ART following immunological or clinical decline.11,12,14,15 Guidelines based on the Children with HIV Early Antiretroviral Therapy (CHER) Trial recommend ART for all HIV-infected infants aged <12 months.5,16 As immunosuppression is a risk factor for IRIS, early ART initiation in infants should lower the risk of BCG-IRIS; this, however, has not been evaluated.12,13
We evaluated the effect of timing of ART initiation on the risk of BCG-IRIS among infants in the CHER trial. We also assessed other risk factors and described the clinical spectrum, management and outcome of cases.
Methods
Study population
In the main section of the CHER trial, Part A, HIV-infected infants aged 6–12 weeks with CD4 count ≥ 25% were randomised to deferred or immediate ART in two South African centres (the Perinatal HIV Research Unit in Soweto and the Children’s Infectious Disease Clinical Research Unit [KIDCRU] in Cape Town).16 In the deferred arm, ART was commenced when CD4% declined to below 25% or following the development of CDC Stage C or pre-defined severe Stage B disease. A small number of infants with CD4% < 25% at screening were recruited into Part B of the CHER trial and received immediate ART. All infants were given intradermal Danish SSI 1331 strain BCG in the right deltoid area during the first week of life. In June 2007, after a median follow-up of 40 weeks, the Data Safety Monitoring Board found that early treatment reduced mortality by 76%.16 All infants who started ART with subsequent follow-up by 20 June 2007 were included in the analysis, apart from those diagnosed with BCG-related regional adenitis before ART initiation in the deferred ART arm. Approval for the CHER study and for the publication of data from the study was obtained from the Institutional Review Boards at both sites.
Follow-up and case-definition
Infants had follow-up visits for clinical review and CD4 measurements every 3 months.16 HIV-1 RNA at ART initiation was measured using standard Roche Amplicor (Roche, Branchburg, NJ, USA) monitor assay version 1.5 RNA, with an upper limit of detection of 750000 copies/ml.
Reactogenicity to BCG vaccination was assessed prospectively. The case definition for BCG-adenitis IRIS (BCG-IRIS) was the development of ipsilateral axillary lymph node enlargement ≥10 mm × 10 mm within 6 months of ART initiation, regardless of additional regional involvement and/or suppuration. Infants with BCG-IRIS and TB (either confirmed by isolation of Mycobacterium tuberculosis or presumptively diagnosed) occurring on overlapping time-frame were considered to have dual disease.11,17 A positive Mantoux test was defined as induration ≥5 mm. Speciation of M. tuberculosis was performed in Cape Town, but not in all cases from Soweto. Infants with suspected IRIS as well as dual disease were investigated and managed at the clinician’s discretion. Information on clinical presentation, diagnosis, treatment and outcome of infants fulfilling the BCG-IRIS case definition were manually extracted from clinical records and merged with data from the main database.
Statistical analysis
To estimate the incidence of BCG-IRIS, follow-up for each infant was considered from the date of ART initiation until the earliest of the date of the diagnosis of BCG-IRIS, the date of the last clinic visit or 6 months after starting treatment. Time to diagnosis of BCG-IRIS was estimated for each ART group using Kaplan-Meier methods. The effect of starting ART early compared to deferral until immunological or clinical progression on risk of BCG-IRIS was assessed based on infants in Part A of the study only, using Cox proportional hazards regression and without adjusting for other factors at ART initiation. Parts A and B were then combined to assess the effects of the following factors at initiation: age, sex, CD4% and absolute count, HIV-1 RNA viral load, and weight-for-age z-score (based on US standards for non-infected children).18 The ART group was not adjusted for in multivariable analyses. The individual effects of CD4% and count were estimated adjusting for other confounding factors, but not for each other. The effect of age was adjusted for CD4 count z-score rather than CD4% or absolute count, both of which decline with age.19 To allow for the possibility that CD4 and HIV-1 RNA levels are intrinsically different between HIV-infected male and female infants, the effect of sex was estimated with and without adjusting for CD4 and HIV-1 RNA levels at initiation.20,21 HIV-1 RNA values were categorised as < or > 750000 copies/ml in regression models, as 62% of children initiated ART at levels above the upper detection limit of the assay. Other covariates were analysed as continuous variables where appropriate, to increase power. Age was log-transformed to improve model fit. Non-linearity effects were assessed using a cubic spline term with knots at the 10th, 50th and 90th percentiles.22
Among infants with BCG-IRIS, the median time to resolution from diagnosis was estimated for the following treatment strategies, allowing for censored data:23 1) medical intervention with anti-mycobacterial treatment specifically aimed for BCG (combinations including ofloxacin or/and ethambutol) and/or steroids; 2) incision and drainage without medical intervention; and 3) no medical or surgical intervention. All analyses were undertaken using STATA (version 10.0, STATA Corporation, College Station, TX, USA).
Results
Analyses were based on 369 infants initiating ART (Table 1): 250 infants in Part A (early ART A, CD4% ≥ 25% at enrolment) and 40 in Part B (early ART B, CD4% < 25%) received early ART, and 79 infants received deferred ART in Part A (deferred ART, CD4% ≥ 25%). Of the 125 infants randomised to deferred ART, 46 were excluded: two developed BCG regional adenitis before initiating ART, 41 had not started ART (15 due to death and two lost to follow-up), and three initiated ART without subsequent follow-up by 20 June 2007. BCG-related complications were not noted as either the cause of death or contributing to death in any of the 15 infants who died prior to initiating treatment.16 Infants on early ART initiated ART at a median age of 7.4 weeks (interquartile range [IQR] 6.5–8.8) in Part A and 9.3 weeks (IQR 8.1–10.5) in Part B. In the deferred ART group, median age at initiation was 23.2 weeks (17.4–33.6), with nine (11%) <12 weeks of age. CD4% at ART initiation was similar in the deferred ART and early ART B groups (Table 1). Fifty-five per cent of infants in the early ART A group had HIV-1 RNA level >750000 copies/ml, compared to respectively 80% and 78% in the deferred ART and early ART B groups.
Table 1.
Children included in analysis: characteristics at ART initiation and subsequent diagnosis of BCG-related regional adenitis due to IRIS
| Deferred ART Group1 (CD4≥25% at enrolment) |
Early ART Part A Group (CD4≥25% at enrolment) |
Early ART Part B Group (CD4 <25% at enrolment) |
Overall | |
|---|---|---|---|---|
| Number of children | 792 | 250 | 40 | 369 |
| Female (%) | 45 (57%) | 145 (58%) | 19 (48%) | 209 (57%) |
| Characteristics at ART initiation | ||||
| Age (weeks) | ||||
| Median (IQR) | 23.2 (17.4 to 33.6) | 7.4 (6.5 to 8.8) | 9.3 (8.1 to 10.5) | 8.3 (7.0 to 11.0) |
| Range | 8.0 to 71.83 | 5.7 to12.0 | 6.1 to 12.0 | 5.7 to 71.7 |
| <12 weeks (%) | 9 (11%) | 250 (100%) | 40 (100%) | 299 (81%) |
| Median (IQR) CD4% | 21% (17 to 28%) | 35% (29 to 41%) | 20% (17 to 24%) | 31% (23 to 38%) |
| CD4% < 25% (%) | 53 (68%) | 19 (8%)4 | 29 (78%)4 | 101 (29%) |
| Median (IQR) CD4 count (cells/mm3) | 1039 (662 to 1528) | 2002 (1493 to 2745) | 1433 (653 to 2204) | 1779 (1136–2496) |
| Median (IQR) CD4 count z-score | −1.2 (−1.7 to −0.7) | −0.2 (−0.7 to 0.5) | −0.7 (−1.7 to 0.0) | −1.0 (−1.8 to −0.3) |
| Median (IQR) weight-for-age z-score | −1.1 (−2.1 to 0.0) | −0.8 (−1.5 to 0.0) | −1.1 (−2.2 to 0.0) | −0.9 (−1.7 to 0.0) |
| HIV-1 RNA > 750,000 copies/ml (%) | 53 (80%) | 138 (55%) | 31 (78%) | 222 (62%) |
| BCG regional adenitis diagnosed within 6 months after ART initiation | ||||
| Number of cases (%) | 13 (16%) | 12 (5%) | 7 (18%) | 32 (8%) |
| Rate per 100 person-years (95% CI) | 54.3 (31.5 to 93.4) | 10.9 (6.2 to 19.1) | 41.7 (19.9 to 87.6) | 21.2 |
| Estimated probability of developing BCG-IRIS5(95% CI) | 17.6% (10.6 to 28.3%) | 5.0% (2.9 to 8.8%) | 17.5% (8.8 to 33.2%) | 9.1% |
ART deferred until child fulfilled clinical or immunological criteria.
Excluded 2 children who developed BCG-related regional adenitis prior to ART initiation
6 children in the deferred ART group were older than 1 year at initiation.
Infants in the Early ART groups may have CD4 measured following enrolment prior to starting ART; CD4% had dropped to <25% for some in part A and increased to ≥25% for some in part B.
Estimated using Kaplan-Meir methods.
Of the 369 infants, 32 (8.7%) were diagnosed with BCG-related regional adenitis within 6 months of ART initiation: 13 (16%) in the deferred ART group, 12 (5%) in the early ART A group and seven (18%) in the early ART B group. Most cases (88%) occurred within 2 months of ART initiation, with only one after 3 months. All but two of the 32 cases had HIV-1 RNA level > 750 000 copies/ml at initiation.
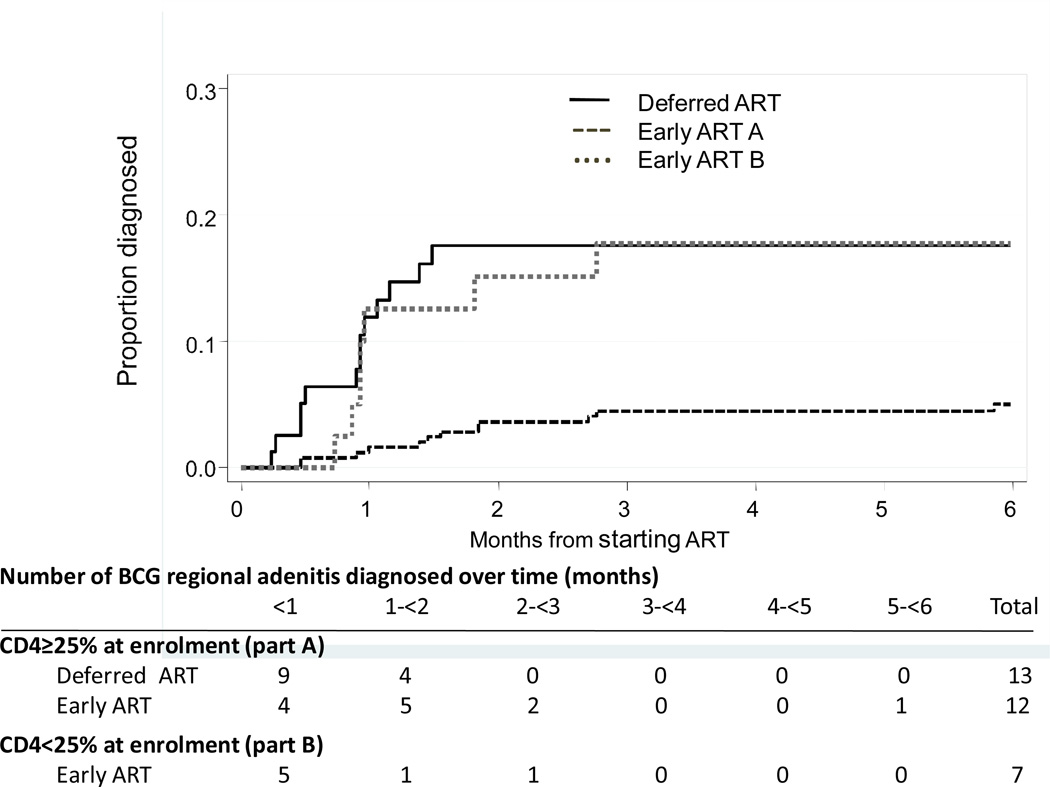
The overall incidence of BCG-IRIS was 21.2 per 100 person-years (py). In Part A, the rate was 10.9 per 100 py (95% confidence interval [CI] 6.2–19.1) in the early ART group compared to 54.3 (31.5–93.4) per 100 py in the deferred ART group (hazard ratio [HR] 0.24, 95%CI 0.11–0.53, P < 0.001). The early ART group from Part B experienced only a slightly lower rate (41.7/100 py, 95%CI 19.9–87.6) than the deferred ART group. Kaplan-Meier curves of time to BCG-IRIS are shown in the Figure.
Figure 1.
Kaplan-Meir curves of time from starting ART to diagnosis of BCG-related regional adenitis, by ART groups.
In multivariable analyses, low CD4 count (HR 0.89 per 100 cells/mm3, 95%CI 0.84–0.94, P < 0.001) and HIV-1 RNA > 750 000 copies/ml (HR 5.80, 95%CI 1.37–24.54, P = 0.017) were the strongest predictors of BCG-IRIS (Table 2). After adjusting for CD4 z-score and HIV-1 RNA, age appeared to show a non-linear effect (P value of cubic spline term = 0.015), with the risk of BCG-IRIS increasing with age until 12 weeks, but then decreasing from around 24 weeks (Table 2). Weak evidence for this initial increase in risk with age remained when including infants in the randomised early ART A group only (HR 1.28 per week, 95%CI 0.95–1.73, P = 0.110). After adjusting for age and HIV-1 RNA, low CD4% had only a weak effect (HR 0.81 per 5%, 95%CI 0.65–1.01, P = 0.058). Females had a lower risk of BCG-IRIS after adjusting for age only (HR 0.40, 95%CI 0.19–0.84, P = 0.015), although the sex difference was less apparent when further adjusted for CD4 count and HIV-1 RNA (HR 0.56, 95%CI 0.25–1.18, P = 0.129). There was no association with weight-for-age z-score after accounting for age and immunological and virological status.
Table 2.
Associations between factors at ART initiation and BCG-related regional adenitis due to IRIS
| Factors | Rate per 100 person-years (number of cases/PY) |
Univariable analyses | Multivariable analyses | ||
|---|---|---|---|---|---|
| Hazard Ratio1 (95% CI) |
P-value | Hazard Ratio1 (95% CI) |
P-value | ||
| Gender | |||||
| Male | 33.5 (21/62.8) | 1 | 0.010 | 1 | |
| Female | 12.4 (11/88.8) | 0.38 (0.19–0.80) | 0.56 (0.26–1.18)3 | 0.129 | |
| Age (weeks) | |||||
| < 8 | 8.0 (6/75.1) | 1 | <0.001 | 1 | 0.029 |
| 8 to 11 | 23.2 (13/56.1) | 4.92 (2.20–11.01) | 2.56 (1.17–5.64) | ||
| 12 to 23 | 99.8 (10/10.0) | 9.16 (3.05–27.56) | 2.46 (0.79–7.61) | ||
| ≥ 24 | 29.0 (3/10.3) | 4.90 (1.51–15.90)2 | 1.07 (0.26–4.40)2 | ||
| CD4% | 0.68 (0.55–0.83) per 5% | <0.001 | 0.81 (0.65–1.01) per 5% | 0.058 | |
| < 20% | 60.8 (12/19.7) | ||||
| 20 to 24% | 40.7 (7/17.2) | ||||
| 25 to 29% | 11.2 (3/26.9) | ||||
| ≥ 30% | 11.3 (9/79.4) | ||||
| CD4 count cells/mm3 | 0.86 (0.81–0.91) per 100 cells/mm3 | <0.001 | 0.89 (0.84–0.94) per 100 cells/mm3 | <0.001 | |
| < 1000 | 72.1 (17/23.6) | ||||
| 1000 to 1999 | 19.1 (11/57.6) | ||||
| ≥ 2000 | 4.8 (3/62.1) | ||||
| HIV-1 RNA (copies/ml) | |||||
| < 750,000 | 3.4 (2/59.1) | 1 | 1 | ||
| > 750,000 | 33.7 (30/88.9) | 9.49 (2.27–39.70) | 0.002 | 5.80 (1.37–24.54) | 0.017 |
| Weight-for-age z-score | 0.67 (0.49–0.91) per unit | 0.010 | 0.80 (0.60–1.08) per unit | 0.144 | |
| < −2 | 57.6 (11/19.1) | ||||
| −2 to < −1 | 17.2 (8/46.6) | ||||
| −1 to < 0 | 18.2 (8/43.9) | ||||
| ≥ 0 | 10.5 (4/38.0) | ||||
In Cox regression models, age, CD4%, CD4 count and weight-for-age z-score were analysed as continuous variables while HIV-1 RNA was defined as < or > 750,000 copies/ml.
Age was log transformed and fitted with a cubic spline (p-value for cubic term=0.015). To present the estimated effect of age, we derived from the fitted model the hazard at the approximate median age value within each of the age groups defined (age 7 weeks for the group <8 weeks, 9.5 for 8–11 weeks, 20 for 12–23 weeks and 36 for ≥ 24 weeks), and then calculated the corresponding hazard ratios.
The estimated hazard ratio for females compared to males was 0.40 (95% CI 0.19–0.84; p=0.015) when adjusted for age only (to allow for the possibility that the effect of gender is mediated through CD4 and HIV-1 RNA), and 0.56 (0.26–1.18; p=0.129) when CD4 count and HIV-1 RNA were further adjusted for.
Clinical signs and symptoms
The lymph nodes progressed to suppuration in 17 (53%) of the 32 cases, with fistulae occurring in 14 (44%). Eight (25%) had concurrent BCG and TB disease; two initiated ART while on TB treatment and six were diagnosed with TB within 6 months after ART initiation and prior to resolution of BCG adenitis. In five cases the diagnosis of TB was based on history of contact, symptoms suggestive of TB and radiographic features. In one case, M. tuberculosis complex was cultured from a site other than a regional arm lymph node. Fine needle aspiration of a regional axillary lymph node was performed in 28 (88%) infants. Organisms were isolated in 25 cases, of which 16 (64%) grew M. tuberculosis complex, with BCG confirmed in six. No other pathogens were identified. The clinical features nevertheless supported BCG adenitis IRIS in the remainder. Of 17 cases with BCG IRIS but without TB, 14 (82%) had reactive skin tests, with eight having an induration ≥ 10 mm.
Treatment and outcome
Treatment of adenitis varied widely (Table 3). At last follow-up, adenitis had resolved in 29 of 32 cases, including all of the 10 infants who received no medical or surgical intervention. Two of the remaining three infants died before resolution, one of diarrhoeal disease and the other, with dual BCG and TB disease, after relocating to another province. This child had culture-confirmed M. tuberculosis complex disease that was not further speciated. Median time to resolution was 4.0 months (IQR 3.2–7.5), and was similar across the treatment strategies.
Table 3.
Management of children with BCG-related regional adenitis due to IRIS
| Management | Number of children (%) |
BCG adenitis resolved by last follow-up |
Median (IQR) number of months to resolution from diagnosis |
|---|---|---|---|
| No medical or surgical intervention | 10 (31%) | 10 | 3.9 (3.2–9.5) |
| Anti-mycobacterial therapy and/or steroids | 151 (47%) | 13 | 3.8 (2.2–6.8) |
| Incision and drainage only | 7 (22%) | 6 | 4.0 (2.3–4.6) |
| Overall | 32 (100%) | 292 | 4.0 (3.2–7.5) |
Four children were treated with anti-mycobacterial therapy, 9 with steroids and 2 with both anti-mycobacterial therapy and steroids. Incision and drainage was performed in 8 of the 15 children.
BCG adenitis had not resolved by last follow-up in one child and two died before resolution.
Discussion
We evaluated the incidence of BCG-related regional adenitis IRIS in HIV-infected infants followed from early in life and starting ART at different ages and CD4 values. We found that starting early ART prior to CD4 depletion or clinical progression at a median age of 7 weeks resulted in a 4-fold reduction in risk of BCG-IRIS compared to deferring ART.
Low CD4 count and high HIV-1 RNA viral load at ART initiation were the strongest risk factors for BCG-IRIS. However, CD4% had only a weak effect after adjusting for age and viral load, possibly because the majority of infants initiated ART early and at high CD4% (71% had CD4% ≥ 25%). The higher prognostic value of absolute CD4 count compared to percentage is consistent with an analysis of untreated children in the United States and Europe, where CD4 count was more predictive of progression to acquired immune deficiency syndrome or death, even in infancy.24 HIV-1 RNA level at ART initiation was >750000 copies/ml in 30 (94%) of the 32 infants who developed BCG-IRIS, compared to only 60% of those who did not. The role of elevated viral load in the development of BCG-IRIS could be mediated through increased immune activation. Viral load was also shown to be an independent risk factor by Nuttall et al.14 However, in another South African cohort in which IRIS events were combined (BCG-related and other conditions), no evidence of this association was observed.12
Age at ART initiation was independently associated with risk of BCG-IRIS, although the relationship was non-linear (P for nonlinearity 0.015); risk of IRIS increased with age until around 12 weeks, with some decrease later in infancy. There remained weak evidence of this early increase in risk when the analysis was restricted to infants randomised to early ART. This age trend has not been reported before and could potentially be due to the increase in BCG organism load in the immediate weeks after vaccination. As the subsequent decreased risk of BCG-IRIS in older infants at ART initiation was based on sparser data, this could have been due to chance. It could also be partly explained by a ‘survivor bias’ effect, as all infants starting treatment from the age of 12 weeks onwards were from the deferred ART arm only; those initiating at older ages would likely be those with better HIV prognosis and thus a lower risk of BCG-IRIS, as the more severely affected infants will have reached the criteria for starting ART or died earlier in infancy. A lower risk of IRIS at older age at ART initiation was also reported previously.12,14 These studies of older children could also have been affected by survivor bias, as ART initiation depended on immunological and clinical criteria. Finally, although we attempted to estimate the effect of age adjusting for viral load (analysed as < or > 750000 copies/ml), ‘residual’ confounding may have occurred, contributing to the observed trends with age at initiation among both the younger and older infants; HIV-1 RNA viral load increases sharply with age during the first weeks of life, peaking at around 3 months.21
In our study, we adopted a clinical case definition for BCG-IRIS; HIV-1 RNA viral load was not available at the time of diagnosis. Given the early onset of symptoms and the cost of the viral load and CD4 assays, robust clinical case definitions are appropriate in lower-resource settings. Although local disease was severe, we did not observe any cases of proven distant or disseminated BCG, possibly because the CHER trial children were intensively monitored so that disseminated disease did not have time to develop; the exception was the child who defaulted from follow-up and who subsequently died. The study did not mandate fine-needle aspiration for diagnostic purposes, as right axillary adenitis after ART initiation was considered to be characteristic of BCG-IRIS. BCG-IRIS is more common than TB IRIS in HIV-infected South African infants.12 We found a relatively high proportion (25%) of BCG-IRIS cases with concurrent TB disease. Six of the eight dual cases developed TB within 6 months of ART. The overlapping clinical time period and robust Mantoux skin test response observed in these infants on ART complicates the diagnosis of TB.
Interferon-gamma release assays were undertaken in some infants but were too few to permit interpretation; negative results do not exclude TB.25
Clinical trial data to guide appropriate therapy for BCG disease in HIV-infected children are lacking.13 Although no conclusions about the efficacy of different approaches can be drawn due to potential selection bias and the small number of cases, we observed minimal difference in time to resolution of adenitis between treatment strategies. Nearly a third of the cases resolved spontaneously, suggesting that regional disease may sometimes only require monitoring and symptomatic relief, consistent with anecdotal evidence.13,26 The value of anti-mycobacterial treatment for regional adenitis remains unclear, with potential drug-drug interactions with antiretroviral drugs and risk of compromising adherence complicating decision making.13,14 Danish strain BCG is resistant to low-dose isoniazid, pyrazinamide and ethionamide.27
Conclusion
Early initiation of ART before immunological and clinical progression reduces the risk of BCG-IRIS substantially among HIV-infected infants vaccinated with BCG at birth. Standardised treatment protocols for BCG adenitis based on evidence from clinical trials are required.
Acknowledgements
The authors thank the Comprehensive International Program of Research on AIDS–South Africa (CIPRA-SA) executive committee, the Children with HIV Early Antiretroviral Therapy data and safety monitoring board and the clinical management teams at the sites as well as the participants and their families.
The CHER study team would like to thank GSK and the departments of health of the Western Cape and Gauteng for providing antiretroviral medications. Support for this study was provided by the US National Institute of Allergy and Infectious Diseases (NIAID) of the US National Institutes for Health, through the CIPRA network, Grant U19 AI53217.
Footnotes
Previous presentation of data: 15th Conference on Retroviruses and Opportunistic Infections, 3–6 February 2008, Boston, MA, USA.
Disclaimer: The content of this presentation does not necessarily reflect the views or policies of NIAID, nor does mention of trade names, commercial projects or organisations imply endorsement by the US Government.
References
- 1.Bhat GJ, Diwan VK, Chintu C, et al. HIV, BCG and TB in children: a case control study in Lusaka, Zambia. J Trop Pediatr. 1993;39:219–223. doi: 10.1093/tropej/39.4.219. [DOI] [PubMed] [Google Scholar]
- 2.Mansoor N, Scriba TJ, de Kock M, et al. HIV-1 infection in infants severely impairs the immune response induced by bacille Calmette-Guerin vaccine. J Infect Dis. 2009;199:982–990. doi: 10.1086/597304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on child-hood tuberculous meningitis and miliary tuberculosis world wide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006;367:1173–1180. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 4.Azzopardi P, Bennett CM, Graham SM, Duke T. Bacille Calmette-Guérin vaccine-related disease in HIV-infected children: a systematic review. Int J Tuberc Lung Dis. 2009;13:1331–1344. [PubMed] [Google Scholar]
- 5.Revised BCG vaccination guidelines for infants at risk for HIV infection. Wkly Epidemiol Rec. 2007;82:193–196. [PubMed] [Google Scholar]
- 6.World Health Organization. Recommendations for a public health approach. Geneva, Switzerland: WHO; 2007. [Accessed June 2011]. Antiretroviral therapy for HIV infection in infants and children: towards universal access. www.who.int/hiv/topics/paediatric/en/index.html. [PubMed] [Google Scholar]
- 7.Third stock taking report on children and AIDS. Geneva, Switzerland: UNAIDS; 2008. [Accessed June 2011]. Joint United Nations Programme on HIV/AIDS. http://data.unaids.org/pub/Report/2008/2008 1201_3rd_stocktaking_en.pdf. [Google Scholar]
- 8.Cotton MF, Schaaf HS, Lottering G, et al. Tuberculosis expo- sure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis. 2008;12:225–227. [PubMed] [Google Scholar]
- 9.Hesseling AC, Cotton MF, Jennings T, et al. High incidence of tuberculosis among HIV-infected infants: evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis. 2009;48:108–114. doi: 10.1086/595012. [DOI] [PubMed] [Google Scholar]
- 10.Bannister C, Bennett L, Carville A, et al. Evidence behind the WHO guidelines: hospital care for children: what is the evidence that BCG vaccination should not be used in HIV-infected children? J Trop Pediatrics. 2008;55:78–82. doi: 10.1093/tropej/fmp018. [DOI] [PubMed] [Google Scholar]
- 11.Hesseling AC, Rabie H, Marais BJ, et al. Bacille Calmette-Guerin vaccine-induced disease in HIV-infected and HIV-uninfected children. Clin Infect Dis. 2006;42:548–558. doi: 10.1086/499953. [DOI] [PubMed] [Google Scholar]
- 12.Smith K, Kuhn L, Coovadia A, Meyers T. Immune reconstitution inflammatory syndrome among HIV-infected South African infants initiating antiretroviral therapy. AIDS. 2009;23:1097–1107. doi: 10.1097/QAD.0b013e32832afefc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulware DR, Callens S, Pahwa S. Pediatric HIV immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS. 2008;3:461–467. doi: 10.1097/COH.0b013e3282fe9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuttall JJ, Davies M, Hussey G, et al. Bacillus Calmette-Guérin (BCG) vaccine-induced complications in children treated with highly active antiretroviral therapy. Int J Infect Dis. 2008;12:e99–e105. doi: 10.1016/j.ijid.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Puthanakit T, Oberdorfer P, Punjaisee S, et al. Immune reconstitution syndrome due to bacillus Calmette-Guérin after initiation of antiretroviral therapy in children with HIV infection. Clin Infect Dis. 2005;41:1049–1052. doi: 10.1086/433177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Violari A, Cotton M, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Geneva, Switzerland: WHO; 2007. [Accessed June 2011]. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification for HIV-related disease in adults and children. www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;246:1–190. [PubMed] [Google Scholar]
- 19.Wade AM, Ades AE. Incorporating correlations between measurements into the estimation of age-related reference ranges. Stat Med. 1998;17:1989–2002. doi: 10.1002/(sici)1097-0258(19980915)17:17<1989::aid-sim891>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.European Collaborative Study. Are there gender and race differences in cellular immunity patterns over age in infected and uninfected children born to HIV-infected women? J Acquir Immune Defic Syndr. 2003;33:635–641. doi: 10.1097/00126334-200308150-00013. [DOI] [PubMed] [Google Scholar]
- 21.Gray L, Cortina-Borja M, Newell ML. Modeling HIV-RNA viral load in vertically infected children. Stat Med. 2004;23:769–781. doi: 10.1002/sim.1615. [DOI] [PubMed] [Google Scholar]
- 22.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 23.Cleves M. An introduction to survival analysis using Stata. College Station, TX, USA: Stata Press; 2004. [Google Scholar]
- 24.Boyd K, Dunn DT, Castro H, et al. Discordance between CD4 cell count and CD4 cell percentage: implications for when to start antiretroviral therapy in HIV-1 infected children. AIDS. 2010;24:1213–1217. doi: 10.1097/QAD.0b013e3283389f41. [DOI] [PubMed] [Google Scholar]
- 25.Davies MA, Connell T, Johannisen C, et al. Detection of tuberculosis in HIV-infected children using an enzyme-linked immunospot assay. AIDS. 2009;23:961–969. doi: 10.1097/QAD.0b013e32832956ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabie H, Meyer T, Cotton MF. Immune reconstitution inflammatory syndrome in children. Southern African J HIV Med. 2009;10:70–75. [Google Scholar]
- 27.Ritz N, Tebruegge M, Connell T, et al. Susceptibility of Myco-bacterium bovis BCG vaccine strains to antituberculous antibiotics. Antimicrob Agents Chemother. 2009;53:316–318. doi: 10.1128/AAC.01302-08. [DOI] [PMC free article] [PubMed] [Google Scholar]