Abstract
A clever twist on presenting spirometry results can boost smoking cessation rates
Practice changer
Perform spirometry on patients who smoke—even if they’re asymptomatic—and show them their lung age—that is, the average age of a nonsmoker with a forced expiratory volume at 1 second (FEV1) equal to theirs. Doing so can help patients kick the habit.1
Strength of recommendation
B: Based on a single well-done randomized controlled trial (RCT).
Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598-600.
ILLUSTRATIVE CASE
A 48-year-old man comes to your office for a routine physical. He has a 30 pack-year smoking history. When you talk to him about smoking cessation, he tells you he’s tried to stop more than once, but he can’t seem to stay motivated. You find no evidence of chronic lung disease and do not perform spirometry screening. (The US Preventive Services Task Force does not recommend spirometry for asymptomatic patients.) But could spirometry have therapeutic value in this case?
Smoking is the leading modifiable risk factor for mortality in the United States,2 and smoking cessation is the most effective intervention. Nortriptyline, bupropion, nicotine replacement agents, and varenicline are effective pharmacological treatments.3 Adding counseling to medication significantly improves quit rates (estimated odds ratio [OR]=1.4; 95% confidence interval [CI], 1.2-1.6).3 Nonetheless, physicians’ efforts to help patients stop smoking frequently fail.
But another option has caught—and held—the attention of researchers.
The promise of biomarkers
It has long been suspected that presenting smokers with evidence of tobacco’s harmful effect on their bodies—biomarkers—might encourage them to stop. Biomarkers that have been tested in randomized controlled trials (RCTs) include spirometry, exhaled carbon monoxide measurement, ultrasonography of carotid and femoral arteries, and genetic susceptibility to lung cancer, as well as combinations of these markers. But the results of most biomarker studies have been disappointing. A 2005 Cochrane Database review found insufficient evidence of the effectiveness of these markers in boosting quit rates.4
Lung age, a biomarker that’s easily understood
Lung age, a clever presentation of spirometry results, had not been tested in an RCT prior to the study we summarize below. Defined in 1985, lung age refers to the average age of a nonsmoker with a forced expiratory volume at 1 second (FEV1) equal to that of the person being tested (FIGURE 1). The primary purpose was to make spirometry results easier for patients to understand, but researchers also envisioned it as a way to demonstrate the premature lung damage suffered as a consequence of smoking.5
FIGURE 1.
Translating FEV1 into lung age1

STUDY SUMMARY: Graphic display more effective than FEV1 results
FAST TRACK
For every 14 smokers who are told their lung age and shown a graphic display of this biomarker, 1 additional smoker will quit
This study was a well-done, multicenter RCT evaluating the effect on tobacco quit rates of informing adult smokers of their lung age.1 Smokers ages 35 and older from 5 general practices in England were invited to participate. The authors excluded patients using oxygen and those with a history of tuberculosis, lung cancer, asbestosis, bronchiectasis, silicosis, or pneumonectomy. The study included 561 participants with an average of 33 pack-years of smoking, who underwent spirometry before being divided into an intervention or a control group. The researchers used standardized instruments to confirm the baseline comparability of the 2 groups.
Subjects in both groups were given information about local smoking cessation clinics and strongly encouraged to quit. All were told that their lung function would be retested in 12 months.
The controls received letters with their spirometry results presented as FEV1. In contrast, participants in the intervention group received the results in the form of a computer-generated graphic display of lung age (FIGURE 2), which was further explained by a health care worker. They also received a letter within 1 month containing the same data. Participants were evaluated for smoking cessation at 12 months, and those who reported quitting received confirmatory carbon monoxide breath testing and salivary cotinine testing. Eleven percent of the subjects were lost to follow-up.
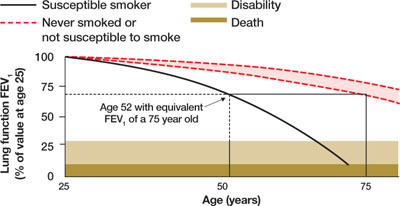
FIGURE 2.
Lung age helps spirometry pack a bigger punch
Drawing a vertical line from the patient’s age (on the horizontal axis) to reach the solid curve representing the lung function of the “susceptible smoker” and extending the line horizontally to reach the curve with the broken lines representing “never smokers” graphically shows the patient’s lung age and the accelerated decline in lung function associated with smoking. The patient shown here is a 52-year-old smoker with FEV1 equivalent to a 75-year-old nonsmoker.
Source: Parkes G et al. BMJ. 2008;336:598-600. Reproduced with permission from the BMJ Publishing Group.
Quit rates higher when patients know lung age
At 1 year, verified quit rates were 13.6% in the intervention group and 6.4% in the control group (a difference of 7.2%, 95% CI, 2.2%-12.1%; P=.005). This means that for every 14 smokers who are told their lung age and shown a graphic display of this biomarker, 1 additional smoker will quit after 1 year.
Contrary to what might be expected, the investigators found that quitting did not depend on the degree of lung damage. Patients with both normal and abnormal lung age quit smoking at similar rates.
WHAT’S NEW: Lung age resonates more than spirometry alone
FAST TRACK
Simply using spirometry to encourage smoking cessation is not sufficient
This is the first RCT demonstrating that informing smokers of their lung age can help them quit, and the first well-designed study to demonstrate improved cessation rates using a physiological biomarker. The research also suggests that successful quitting may have less to do with spirometry results—the level of severity of lung damage it shows—than with the way the results are presented. Giving patients information about their lung function in an easily understandable format, the authors observe, appears to result in higher quit rates.
CAVEATS: Young smokers weren’t studied
The study did not test to see if this intervention would work in younger adults, as only those 35 years of age and older were enrolled. This is a single study, and it is possible that the findings cannot be generalized to other groups or are due to unmeasured confounding factors. However, the intervention is unlikely to cause any significant harm, so we see no risks associated with it other than the cost of spirometry.
CHALLENGES TO IMPLEMENTATION: Time and expense of spirometry
We suspect the biggest challenges to implementing this recommendation in clinical practice are the expense of obtaining a spirometer (TABLE), staff training for those practices without one, and the time needed for the intervention. The average time to perform spirometry on study participants was 30 minutes; a health care worker spent, on average, another 15 minutes reviewing results with each member of the intervention group.
TABLE.
Spirometry: equipment costs
| The initial cost of a spirometer varies widely, depending on the sophistication of the equipment and the available options and features. Additional costs—for disposable mouthpieces, line filters, nose clips, and hoses, for example—are low. A sampling of reasonably priced models well suited for office use is shown below. All of these models meet American Thoracic Society criteria for spirometry, and all calculate lung age. | ||
| SPIROMETER MANUFACTURER/MODEL | PRICE | SUPPLIER |
| Futuremed Discovery-2 | $2,125 | medsupplier.com |
| Micro Medical MicroLoop | $1,780 | Miami-med.com |
| Micro Medical SpiroUSB | $1,580 | Miami-med.com |
| NDD EasyOne Frontline | $1,000 | medsupplier.com |
| SDI Diagnostics Spirolab II | $2,600 | med-electronics.com |
Another challenge: Not all spirometers calculate lung age or can create a graphic similar to FIGURE 2. However, any FEV1 measurement, whether it is generated by formal pulmonary function testing or by an inexpensive hand-held meter, can easily be converted to lung age using the formula shown in FIGURE 1. If desired, the same elements—the patient’s age, height, and gender as well as FEV1—could also be used to create a computer-generated graphic display.
The PURLs Surveillance System is supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
PURLs methodology This study was selected and evaluated using FPIN’s Priority Updates from the Research Literature (PURL) Surveillance System methodology. The criteria and findings leading to the selection of this study as a PURL can be accessed at www.jfponline.com/purls.
Contributor Information
Kristen Deane, Department of Family and Community Medicine, University of Missouri – Columbia.
James J. Stevermer, Department of Family and Community Medicine, University of Missouri – Columbia.
John Hickner, Department of Family Medicine, The University of Chicago.
References
- 1.Parkes G, Greenhalgh T, Griffin M, Dent R. Effect on smoking quit rate of telling patients their lung age: the Step2quit randomised controlled trial. BMJ. 2008;336:598–600.. doi: 10.1136/bmj.39503.582396.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245.. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- 3.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical practice guideline. Rockville, MD: US Department of Health and Human Services, Public Health Service; May 2008. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use08.pdf. Accessed July 6, 2008. [Google Scholar]
- 4.Bize R, Burnand B, Mueller Y, Cornuz J. Biomedical risk assessment as an aid for smoking cessation. Cochrane Database Syst Rev. 2005;(4):CD004705.. doi: 10.1002/14651858.CD004705.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Morris JF, Temple W. Spirometric “lung age” estimation for motivating smoking cessation. Prev Med. 1985;14:655–662.. doi: 10.1016/0091-7435(85)90085-4. [DOI] [PubMed] [Google Scholar]


