The changes in cholesteryl ester measured using 1H-NMR spectroscopy could be used as a molecular marker for meibomian gland dysfunction (MGD) and could potentially be applied to follow the efficacy of drug therapy of MGD.
Abstract
Purpose.
This study represents a first step toward the evaluation of possible compositional differences in meibum from normal donors (Mn) and donors with meibomian gland dysfunction (Md) by 1H-NMR spectroscopy. The results highlight the applicability of 1H-NMR spectroscopy for the quantitative analysis of waxes, cholesteryl esters, and glycerides in meibum lipid (ML).
Methods.
Meibum was obtained from 41 normal donors and 51 donors with meibomian gland dysfunction (MGD). 1H-NMR spectroscopy was used to quantify the amount of waxes, glycerides, and cholesteryl esters in human meibum.
Results.
The relative amount of cholesteryl esters in Mn increased with age and was 40% (P < 0.05) lower in Md. Interestingly, the relative levels of cholesteryl esters in infant meibum were comparable to those in Md. The relative amounts of glycerides were not affected significantly by age or MGD.
Conclusions.
The changes in cholesteryl ester could be used as a molecular marker for MGD and could potentially be applied to follow the efficacy of drug therapy in the treatment of MGD. The similarity of the levels of cholesteryl esters in infant meibum and Md suggests that the relative amounts of these meibum components alone are unlikely to be responsible for the increased stability of the infant tear film and decreased stability of the tear film with MGD. This study reveals the complexity of human MLs and the changes that occur with age and disease. Understanding the factors that lead to such variations is of utmost relevance in the design of effective therapies.
Meibum lipids (MLs) are important for tear film stability,1 and changes in their composition with meibomian gland dysfunction (MGD) may contribute to dry eye symptoms.2,3 Thin layer chromatography, high pressure liquid chromatography,4–14 gas chromatography with mass spectrometry (MS) detection,4–6,9–16 electron spray ionization tandem MS,17–19 and atmospheric pressure chemical ionization MS analysis20–25 have been applied to separate and quantify human meibum components. The ranges of values reported in the literature for the absolute and relative contents of waxes, cholesteryl esters, triglycerides, and phospholipids in human meibum are quite large.26
The mass spectrometric techniques used have provided a wealth of compositional and structural details that cannot be obtained with spectroscopic techniques such as Fourier transform infrared (FTIR) and nuclear magnetic resonance (NMR) spectroscopies. It is promising that current spectrometric lipidomic analytic methods could be used to quantify changes in human meibum with age, sex, dry eye symptoms, and types. However, as Robvosky et al.27 have noted, this is a challenging undertaking because “every possible fatty acid conjugate that is resolved chromatographically must be measured (potentially with separate internal standards) to accurately quantify total pools of the derived individual lipid classes.” The need of internal standards for accurate quantification is due to differences in ionization efficiency for the various lipid classes found in meibum.
Infrared and Raman spectroscopy have been applied to study hydrocarbon chain conformation in meibum and have provided limited information regarding meibum composition.28–36 These techniques may be applicable to high-throughput screening and FTIR spectroscopy has been applied recently as a diagnostic tool.32,33 The advantage of spectroscopic techniques is that the sample is not destroyed in the process of analysis and the same sample can be analyzed later by other techniques including mass spectrometry. NMR analysis has been used to identify and characterize new lipids in the human lens.37–42 No NMR studies have been performed to assess possible changes in human meibum with age or MGD. The results of this present study represent a first step toward the evaluation of possible compositional differences in human meibum from normal donors (Mn) and from those with MGD (Md) with the use of 1H-NMR spectroscopy.
Materials and Methods
Materials
Cyclohexane-d12, tetrahydrofuran (THF), and methanol (MeOH) were obtained from Sigma Chemical Company (St. Louis, MO).
Clinical Diagnosis
Subjects were recruited from the Kentucky Lion's Eye Center and the Robley Rex Veterans Affairs Medical Center in Louisville, KY. Normal status was assigned when the subject's meibomian gland orifices showed no evidence of keratinization or plugging with turbid or thickened secretions, and no dilated blood vessels were observed on the eyelid margin.
The diagnosis of MGD was made according to the criteria of Foulks and Bron.43 Plugging of the meibomian glands of at least five of ten orifices in the central portion of the upper eyelid was required for diagnosis of MGD. The secretion expressed by the meibomian gland had to be turbid, turbid with clumps, or paste-like. Both inflammation of the eyelid margin, as evidenced by swelling of the eyelid margin, and 2+ vascular injection of the posterior lid margin were necessary for diagnosis. The presence of telangiectasia of the posterior eyelid margin was confirmatory of chronic disease but not required for entry. Tear film stability was determined by instillation of sodium fluorescein into the tear film. Tear breakup time was <5 seconds for all Md subjects sampled.
Collection and Processing of Human Meibum
Written informed consent was obtained from all donors. Protocols and procedures were approved by the University of Louisville Institutional Review Board as well as the Robley Rex Veterans Affairs Institutional Review Board. All procedures were in accord with the Declaration of Helsinki. Meibomian gland expression was done by compressing the eyelid between cotton-tipped applicators, with strict attention to avoid touching the eyelid margin during expression. All four eyelids were expressed, and approximately 1 mg of meibum was collected per individual for direct spectroscopic study. The expressate was collected with a platinum spatula and immediately spread onto a AgCl window and into 0.5 mL of THF/MeOH, 3:1, vol:vol in a 9-mm microvial (secured with a Teflon cap; MicroLiter Analytical Supplies Inc., Suwanee, GA). Argon gas was bubbled onto the samples to prevent oxidation. Samples on the AgCl window and in the vial were capped and frozen under argon gas until analysis. Analyses were performed within 3 weeks of collection of the sample. Storage of the sample on AgCl windows for over 2 months under argon did not affect the sample.30 Before NMR analysis, the THF/MeOH in the microvial containing ML was evaporated with a stream of argon gas.
After infrared analysis and solvent evaporation, ML was removed from the AgCl window using a series of solvents with different hydrophobicities to ensure that all lipid classes were extracted from the window. First, the AgCl window was placed with the ML side down into a 15-mL glass scintillation vial containing 1 mL of hexane, and purged with argon gas to avoid oxidation. A glass vial rather than a plastic one was used in all protocols to avoid plasticizer contamination. The vial was sonicated in an ultrasonic bath (Branson 1510; Branson Ultrasonics, Danbury, CT) for 10 minutes. The hexane was decanted into the microvial containing the ML rinsed from the spatula. The hexane was evaporated under a stream of nitrogen gas. Methanol (1.5 mL) was then added to the scintillation vial containing the AgCl window and then purged with argon gas. The vial was sonicated in an ultrasonic bath (Branson Ultrasonics) for 10 minutes. The methanol was decanted into the microvial containing the ML rinsed from the spatula and was evaporated under a stream of nitrogen gas. THF/MeOH (1.5 mL) was added to the scintillation vial containing the AgCl window and then purged with argon gas. The vial was sonicated in an ultrasonic bath (Branson Ultrasonics) for 10 minutes The microvial containing the extracted ML was lyophilized for 12 hours to remove trace amounts of organic solvents. Finally, deuterated cyclohexane (0.5 mL) was added to the sample and sonicated (Branson Ultrasonics) for 10 minutes in a bath sonicator. The solution was transferred to glass NMR tubes (Sigma Chemical Co.) and NMR spectra were collected.
NMR Spectral Measurements
Spectral data were acquired (Inova-500 spectrometer; Varian, Lexington, MA). The following parameters were used: 800 scans were acquired with a spectral width of 15 parts per million (ppm), 60° pulse, 4-K data points, 1.0-second delay time, and 2.049-second acquisition time at 25°C. Commercial software (GRAMS 386; Galactic Industries Corp., Salem, NH) was used for spectral deconvolution and curve fitting. The area of each band was used for the quantification of lipid composition. The same software was used to carry out principal component analysis (PCA) to detect subtle differences in the spectral data.
Statistics
Data are presented as the average ± SEM. Statistical significance was determined using Student's t-test or the correlation coefficient from the linear regression best fit. Values for which P < 0.05 were considered significantly different.
Results
NMR spectra of meibum from 51 donors with MGD (Md) were compared with those obtained for meibum from 44 normal donors (Mn). Donor characteristics are provided in Table 1.
Table 1.
Meibum Donor Grouping and Characteristics
| Group | Average Median Age (y) | Age Range (y) | Male:Female:Unknown | Race/Ethnicity, Relative to C = 1 | Number |
|---|---|---|---|---|---|
| Infant | 1/1 | 1–2 | 6:1:0 | B 0.24, H 0.2, A 0, U 0.24 | 7 |
| Child | 8/8 | 3–12 | 1.3:1:0 | B 0, H 0, A 0, U 0 | 7 |
| Adolescent/Adult | 44/52 | 13–88 | 8.0:1:0 | B 0.05, H 0, A 0.23 | 27 |
| MGD | 67/70 | 8–87 | 4.1:1:1.1 | B 0.13, H 0.03, A 0, U 0.042 | 51 |
MGD, meibomian gland dysfunction; C, Caucasian; B, Black; H, Hispanic; A, Asian; U, unknown. Infant, Child, Adolescent, and Adult groups were normal.
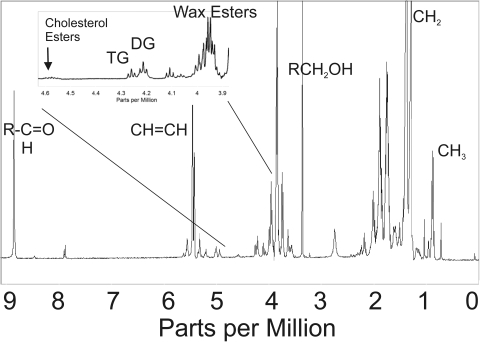
1H-NMR spectra of human meibum provide a wealth of information about the amount and chemical shift δ of protons (Fig. 1). The region below 1.39 ppm (CH2 band) shows 1H resonances associated with CH3 moieties. Resonances attributed to protons associated with carbon carbon double bonds appear between 5 and 6 ppm. Above 6 ppm, the resonances correspond to protons that are more deshielded due to the presence of nearby carbonyl groups. Detailed band assignments will appear in future reports.
Figure 1.
A typical 1H-NMR spectrum of human meibum.
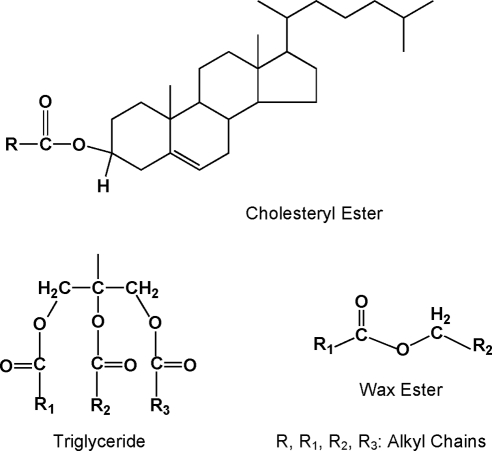
The region between 4 and 4.8 ppm is of interest to this study because it contains 1H resonances associated with the ester groups of cholesteryl esters, glycerides, and waxes (Fig. 1, inset). Waxes exhibit proton resonances between 3.9 and 4 ppm (Fig. 1), corresponding to CH2 protons (Fig. 2). Glycerides display resonances between 4.1 and 4.3 ppm (Fig. 1) also attributed to CH2 protons of the glycerol backbone (Fig 2). The broad band at 4.6 ppm (Fig. 1) is attributed to the single hydrogen attached to carbon 3 of cholesterol that is adjacent to the acyl linkage of the cholesteryl ester (Fig. 2). The region between 3.9 and 4.8 ppm was used to quantify the relative amount of cholesteryl esters, glycerides, and waxes.
Figure 2.
Structures of waxes, glycerides, and cholesterol esters discussed in the text are displayed. Hydrogen atoms that are unique to these compounds and are detected in 1H-NMR spectra of a standard mixture of model compounds and human meibum are shown.
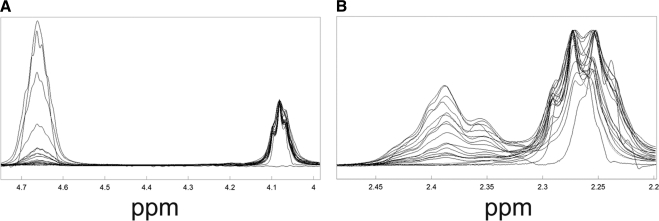
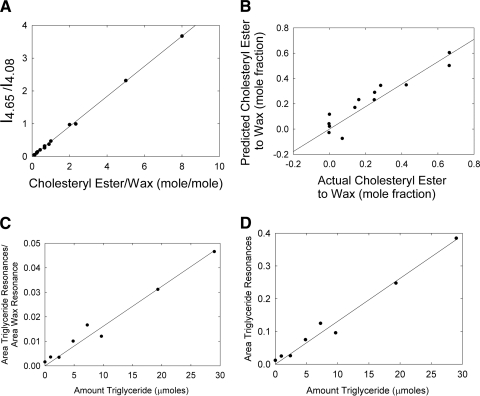
1H-NMR spectra of model lipid compounds in known quantities were acquired and, as expected, the area of the resonances at 4.6 and 2.3 ppm (Table 2) varied as the concentration of cholesteryl ester was changed (Fig. 3). A calibration curve was constructed by plotting the ratio of integrated areas of the resonances at 4.6 ppm (due to cholesteryl ester) and 4.1 ppm (due to wax) versus the molar ratio of the model cholestoryl ester and wax (Fig. 4A). The curve was linear with an intercept of 0, a slope of 0.459 mol/mol, and a correlation coefficient of 0.9995. A slope of 0.5 is expected since the band at 4.65 ppm arises from only one hydrogen per mole of cholesterol, whereas the band at 4.08 corresponds to two hydrogens per mole of wax. The difference between the expected value, 0.5, and the actual experimental value, 0.459, may be explained by the presence of impurities in the model compounds.
Table 2.
Standard Mixtures of Model Compounds Used in This Study
| Standard Number | Molar Percentage of Model Lipids |
||||
|---|---|---|---|---|---|
| Wax | Cholesterol Ester | Glycerides | Phospholipids | Hydrocarbons | |
| 1 | 80 | 20 | |||
| 2 | 80 | 11 | 4 | 5 | |
| 3 | 80 | 0 | 5 | 15 | |
| 4 | 20 | 40 | 6 | 4 | 30 |
| 5 | 32 | 40 | 8 | 10 | 10 |
| 6 | 70 | 20 | 10 | ||
| 7 | 70 | 10 | 20 | ||
| 8 | 86 | 14 | |||
| 9 | 100 | ||||
| 10 | 90 | 10 | |||
| 11 | 90 | 10 | |||
| 12 | 90 | 10 | |||
| 13 | 80 | 20 | |||
| 14 | 80 | 10 | 10 | ||
| 15 | 80 | 20 | |||
| 16 | 70 | 30 | |||
| 17 | 70 | 6 | 5 | 20 | |
| 18 | 70 | 30 | |||
| 19 | 60 | 15 | 5 | 5 | 15 |
| 20 | 60 | 10 | 5 | 5 | 20 |
| 21 | 60 | 40 | |||
| 22 | 50 | 8 | 2 | 40 | |
| 23 | 50 | 4 | 6 | 40 | |
| 24 | 50 | 50 | |||
| 25 | 30 | 20 | 2 | 8 | 40 |
| 26 | 30 | 4 | 6 | 60 | |
| 27 | 30 | 70 | |||
| 28 | 10 | 50 | 28 | 2 | 10 |
| 29 | 10 | 9 | 9 | 2 | 70 |
| 30 | 10 | 80 | 10 | ||
Wax: 50 mol % stearylpalmitate and oleyloleate. Cholesterol ester: cholesterylpalmitate.
Glycerides: 50 mol % 1-stearoyl-rac-glycerol, triolein. Phospholipids: 50 mol % dimyristoyl-phosphatidylcholine, palmitoylsphingomyelin. Hydrocarbons: n-tetradecane.
Figure 3.
NMR spectra of lipid standards containing various amounts of cholesteryl esters, waxes, glycerides, and phospholipids (Table 2). Key bands that change with cholesterol ester concentration are observed near 4.65 and 2.38 ppm.
Figure 4.
(A) Correlation curve between cholesteryl ester and wax (obtained from 1H-NMR resonance intensities). (B) The actual values for cholesteryl ester to wax molar ratios obtained from the mixtures of model lipid compounds versus those predicted using principal component analysis. (C) Correlation curve between triglycerides and wax obtained from 1H-NMR resonance intensities. The area of the resonance at 4.08 ppm was used to quantify the amount of wax. The resonances between 4.2 and 4.3 ppm were used to quantify the amount of triglyceride. A volume of 600 μL was measured. (D) The resonances between 4.2 and 4.3 ppm were used to quantify the amount of triglyceride. A volume of 600 μL was measured. Spectra were measured on the same day.
To evaluate all the possible spectral variations in the spectra collected for the model compounds (Table 2), we used PCA.44–46 PCA has been used to analyze infrared spectra of human meibum.32,33 PCA is a chemometric approach that enables the assessment of differences, even very subtle ones, in a set of spectra. Then, the differences are correlated to a “principal component” that is a variable, such as cholesteryl ester and wax content in our case. An eigenvector represents a constituent that changes its relative contribution from sample to sample. Using a press plot, we determined that only two eigenvectors were necessary to describe most of the variance in the NMR spectra of our model compounds. The actual values of cholesteryl ester to wax molar ratios were plotted versus those predicted using PCA analysis (Fig. 4B). They were linearly correlated, with a slope of 0.89 and correlation coefficient of r = 0.93. Like the curve in Figure 4A and for the same reasons, the actual values of cholesteryl ester to wax molar ratios were slightly lower than the predicted values, thus leading to the lower than unity slope.
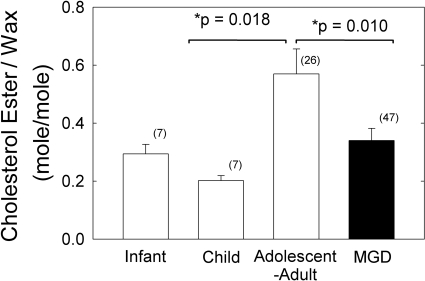
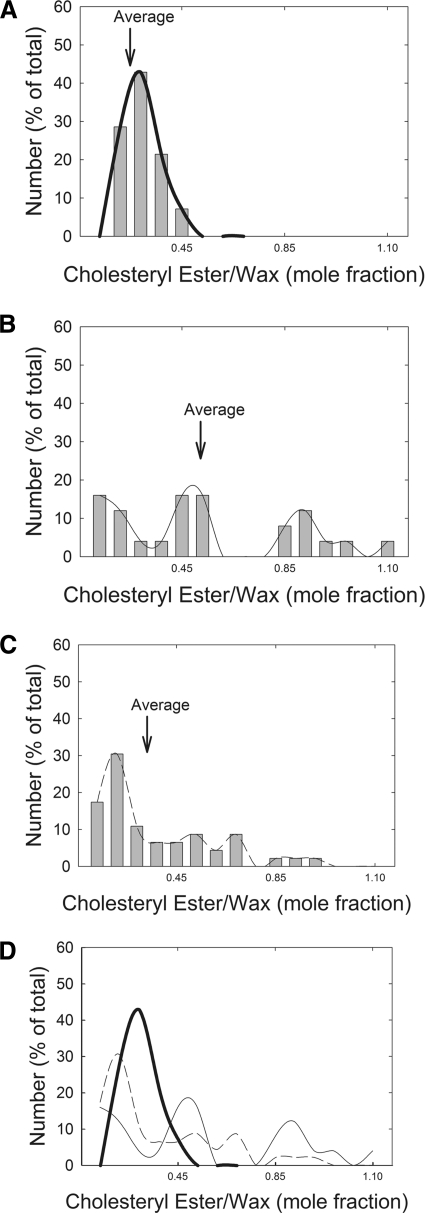
Because the ratio of the integrated areas (Fig. 4A) provided a standard curve with a correlation coefficient closer to unity compared with that obtained in the PCA-derived standard curve (Fig. 4B), we used the curve shown in Figure 4A to measure the mole fraction of cholesteryl ester to wax (CE:Wax) in human meibum samples. The average value obtained for CE:Wax was significantly higher, about twofold (P < 0.05), for the normal adolescent-to-adult group compared with the normal infant, child, and MGD groups (Fig. 5, Table 3). The median value for CE:Wax mole fraction was similar to the average values for Mn but slightly lower for Md (Table 3). The CE:Wax molar ratios for “Mn child” were relatively tightly distributed compared with the molar ratios for “Mn adolescent to adult” and Md (Fig. 6). The CE:Wax molar ratios for “Mn adolescent to adult” were distributed into three groups (Fig. 6B) and those for Md were distributed into two groups. The major group for Md centered near 0.15 molar ratio encompassed approximately 60% of the samples (Fig. 6C).
Figure 5.
Cholesteryl esters were found to increase with age and decrease with MGD.
Table 3.
Wax, Glyceride, and Cholesterol Ester Composition of Human Meibum
| Author/Study | Steroyl:Wax Ester Molar Ratio | Triglyceride:Wax Ester Molar Ratio | Number of Donors Sampled |
|---|---|---|---|
| Cory et al.7 | Not measured | 0.03 (TG:wax and SE) | 7 |
| Mathers et al.47 | 0.61 | 0.37 | 22 |
| Nicolaides et al.5 | 0.86 | 0.11 | 76 pooled |
| McCulley and Shine48 | 0.23 | 0.09 | ? |
| Tiffany13 | 0.78 (range, 0.38–1.2) | 0.87 | 4 |
| Robosky et al.28 | 0.54 | 0.073 | 1 |
| Shine et al.49 | Not measured | ||
| SBBL | 0.082 | 2 | |
| AR | 1 | 0.077 | 2 |
| AR-MKC | 0.157 | 6 | |
| Literature average | 0.60 | 0.13 w/o 0.87 | |
| This study human normal | 0.57 ± 0.09 | 0.19 ± 0.04 | 27 |
| Median | 0.50 | ||
| This study human child (<13 years old) | 0.24 ± 0.08 | 0.17 ± 0.01 | 14 |
| Median | 0.23 | ||
| This study human MGD | 0.34 ± 0.04 | 0.19 ± 0.02 | 48 |
| Median | 0.21 |
SBBL, seborrheic blepharitis; AR, acne rosacea; AR-MKC, AR with meibomian keratoconjunctivitis; MGD, meibomian gland dysfunction.
Figure 6.
Distribution of the mole fraction of cholesteryl esters to waxs. (A) Mn between 0 and 13 years of age. (B) Mn > 13 years of age. (C) Md. (D) Spline fit curves from (A), (B), and (C).
Triglyceride was quantified in a standard mixture with a detection limit of approximately 5 μmol (Fig. 4C). The standard curve produced by the ratio of the areas due to triglycerides and wax resonances as a function of their molar ratio was linear, r = 0.986. Even when the areas of the triglyceride resonances were not normalized to other resonances (Fig. 4D), the triglyceride standard curve was also linear, r = 0.988, suggesting that the magnetic field remains stable during the acquisition time. The two resonances between 4.2 and 4.3 ppm (Fig. 1) were assigned to the four CH2 protons associated with the glycerol backbone (Fig. 2). The relative amounts of glycerides were not significantly different among the groups (Table 3). The amount of glyceride in meibum was about two orders of magnitude above the detection limit of the instrument.
The intensity, width, and location of bands measured in our infrared and Raman spectroscopic studies of meibum are often sensitive to the conformation and the environment around the moieties associated with the band, thus complicating compositional analysis. Unlike vibrational and electronic spectroscopy, and if aggregation does not occur, the area of 1H-NMR resonances is proportional to the number of protons and is not affected by the surrounding environment. Therefore, quantitative studies based on 1H-NMR do not require standards for every different chain length and saturation. We acquired 1H-NMR spectra of eight different waxes spanning a wide range of chain lengths and saturation: steryl oleate, oleyl oleate, palmityl oleate, arachidyl oleate, steryl sterate, palmityl palmitate, myristyl laurate, and palmityl laurate. As expected, the ratios of the areas of the resonances at 3.98 and 0.88 ppm, assigned to the two protons of the first CH2 group in the alkyl chain of the waxes (Fig. 2) and to the six protons of the two terminal CH3, respectively, were not dependent on saturation or chain length, and their averaged ratio was 0.322 with SD of 0.008. This is close to the expected ratio of 0.333 (two to six protons). Palmityl laurate contains the shortest alkyl chain length (12 carbons), whereas arachidyl oleate has the longest alkyl chain (20 carbons). The ratios were 0.328 and 0.327, the shortest and longest alkyl chain waxes, respectively.
Matrix-assisted laser desorption/ionization, time of flight mass spectrometry was used to detect free cholesterol in all our samples.50 Free cholesterol was <5% of the cholesteryl esters, thus suggesting that cholesteryl esters were not hydrolyzed in our samples (data not shown).
Milligram quantities of model phospholipids were analyzed by 1H-NMR spectroscopy under the same conditions used for the meibum samples. No phospholipid was detected because glycero- and sphingophospholipids are not soluble in cyclohexane.
Discussion
The major findings of this study are that meibum from normal adolescents and adults contains molar ratios of wax to cholesteryl ester to glyceride of 1:0.57:0.19 and those cholesteryl esters were found to be 40% lower in Md compared with that in Mn. The predominance of wax in meibum was suggested by infrared and Raman spectroscopy.30,35,36 Five groups have reported the ratio of wax to cholesteryl ester in human meibum (Table 3). Our value for CE:Wax, 0.57 (mol:mol), is approximately equal to the average value of 0.60 reported in the literature and is closest to the ratio evaluated by Robosky and colleagues,27 n = 1, and Mathers and Lane,47 n = 22, and within the range reported by Tiffany,13 n = 4 (Table 3). SD values were not provided in any of the previous studies. Our value for CE:Wax is much higher than that reported by McCulley and Shine.48
In one study, meibum samples from normal donors were classified into two groups.9 The cholesteryl esters were low in four meibum samples from one group, 6 μg per 790 μg of meibum, and approximately eightfold higher in four other samples from another group.9 Normal donor information profiles were not given in the publication. In the present study, for normal adolescents to adults, the cholesterol ester to wax ratios could be divided into three groups, with a low, medium, and high ratio, 0.1, 0.5, and 0.8 mol:mol, respectively. Our data support the large range of values reported by Tiffany13 and Shine and McCulley.9 The studies in Table 3 did not provide SDs or ranges for their values.
CE:Wax was significantly higher for the normal adolescent to adult group compared with that for the normal infant and child groups and MGD group. Could these differences account for the tear film stability and meibum conformational differences observed with age30–32,51–63 and MGD?33,34,64–66 Probably not for the following reasons: cholesteryl esters do not change the conformation of wax in model systems34; therefore, the 40% decrease observed in meibum cholesteryl esters associated with MGD is unlikely to account for the decreased meibum hydrocarbon chain order (decreased viscosity) observed with age32,35 or increased meibum hydrocarbon chain order (increased viscosity) with MGD.34 It is more likely that saturation34 and/or proteins33 play a greater role in the degree of hydrocarbon chain order in human meibum.34 Cholesteryl esters in human meibum contain long-chain hydrocarbons.67 Longer chain hydrocarbons tend to order membranes. Therefore, the loss of cholesteryl esters with MGD would tend to raise lipid order rather than decrease it, opposite to the reported trends.
The relative amount of cholesteryl esters in infant meibum is comparable to that in Md. Therefore, differences in the amount of cholesteryl esters alone are unlikely to be responsible for the greater stability of the infant tear film relative to that of the tear film from both donors with MGD and adolescents and adults. It is possible that the specific features of cholesteryl esters (chain length, branching, and degree of unsaturation) may change with age and disease. For this reason, age and/or disease-related changes in the specific species of cholesteryl esters should be evaluated.
Compositional differences49 and hydrocarbon chain order have been used to monitor the efficacy of drugs and as markers for MGD.28 Changes in cholesteryl ester could be used in a similar manner. The quality rather than the quantity of meibum is more of a factor in MGD.68
Seven groups have reported the ratio of wax to glyceride in human meibum (Table 2). Our value for glyceride:wax, 0.19 (mol:mol), is within the range of values reported in the literature, which averaged 0.13. Glycerides represented only 11% of the constituents we quantified. The relative amount of total glycerides or areas of the resonances corresponding to diglyceride and trigyceride did not change with age or MGD. Therefore, glycerides are unlikely to account for the decreased meibum hydrocarbon chain order (decreased viscosity) observed with age32,35,36 or increased meibum hydrocarbon chain order (increased viscosity) with MGD.34 The triglyceride-to-wax ratios were higher in samples from donors with both acne rosacea and meibomian keratoconjunctivitis compared with donors with seborrheic blepharitis or acne rosacea without meibomian keratoconjunctivitis. However, the difference was not statistically significant due to the limited number of donors in the latter two groups.49 The diglyceride-to-wax ratio decreased significantly after 3 and 6 months of treatment with minocycline.49 Triglycerides from a group of donors with meibomian keratoconjunctivitis contained 20-carbon fatty acids that were more saturated compared with Mn.10
Although NMR spectroscopy has been applied in other biological systems to quantify waxes, cholesteryl esters, triglycerides, saturation, CH2, CH3, and CH—C O moieties,27,69–84 no such studies had been performed before to assess the changes in human meibum with age or MGD. In this report, we have focused on only a narrow spectral region encompassing three resonances. There is, however, a wealth of extra information available in the rest of the 1H-NMR spectral range. The analysis of these regions and the interpretation of the changes with age and disease will be presented in future reports.
O moieties,27,69–84 no such studies had been performed before to assess the changes in human meibum with age or MGD. In this report, we have focused on only a narrow spectral region encompassing three resonances. There is, however, a wealth of extra information available in the rest of the 1H-NMR spectral range. The analysis of these regions and the interpretation of the changes with age and disease will be presented in future reports.
Footnotes
Supported in part by Public Health Service/National Eye Institute Research Grant EY017094-01, the Kentucky Lions Eye Foundation, and an unrestricted grant from Research to Prevent Blindness Inc. Much of this material is the result of work supported with resources and use of the facilities at the Robley Rex Veterans Affairs Medical Center, Louisville, Kentucky. GNF is a member of the part-time staff of the Surgical Service, Robley Rex Veteran Affairs Medical Center, Louisville, Kentucky. The opinions and conclusions reported in this study are not those of the Department of Veterans Affairs.
Disclosure: R.K. Shrestha, None; D. Borchman, None; G.N. Foulks, None; M.C. Yappert, None; S.E. Milliner, None
References
- 1. Bron AJ, Tiffany JM. The meibomian glands and tear film lipids. Structure, function, and control. Adv Exp Med Biol. 1998;438:281–295 [DOI] [PubMed] [Google Scholar]
- 2. Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52:369–374 [DOI] [PubMed] [Google Scholar]
- 3. Bron AJ, Sci FM, Tiffany JM. The contribution of meibomian disease to dry eye. Ocul Surf. 2004;2:149–165 [DOI] [PubMed] [Google Scholar]
- 4. Nicolaides N, Santos EC. The di- and tri-esters of the lipids of steer and human meibomian glands. Lipids. 1985;20:454–467 [DOI] [PubMed] [Google Scholar]
- 5. Nicolaides N, Kaitaranta JK, Rawdah TN, Macy JI, Boswell FM, 3rd, Smith R. Meibomian gland studies: comparison of steer and human lipids. Invest Ophthalmol Vis Sci. 1981;20:522–536 [PubMed] [Google Scholar]
- 6. Nicolaides N, Ruth EC. Unusual fatty acids in the lipids of steer and human meibomian gland excreta. Curr Eye Res. 1982/1983;2:93–98 [DOI] [PubMed] [Google Scholar]
- 7. Cory CC, Hinks W, Burton JL, Shuster S. Meibomian gland secretion in the red eyes of rosacea. Br J Dermatol. 1973;89:25–27 [DOI] [PubMed] [Google Scholar]
- 8. Dougherty JM, Osgood JK, McCulley JP. The role of wax and sterol ester fatty acids in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:1932–1937 [PubMed] [Google Scholar]
- 9. Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–2280 [PubMed] [Google Scholar]
- 10. Shine WE, McCulley JP. Meibomian gland triglyceride fatty acid differences in chronic blepharitis patients. Cornea. 1996;15:340–346 [DOI] [PubMed] [Google Scholar]
- 11. Shine WE, McCulley JP. Polar lipids in human meibomian gland secretions. Curr Eye Res. 2003;26:89–94 [DOI] [PubMed] [Google Scholar]
- 12. Andrews JS. Human tear film lipids: I. Composition of the principal non-polar component. Exp Eye Res. 1970;10:223–227 [DOI] [PubMed] [Google Scholar]
- 13. Tiffany JM. Individual variations in human meibomian lipid composition. Exp Eye Res. 1978;27:289–300 [DOI] [PubMed] [Google Scholar]
- 14. Shine WE, McCulley JP. Role of wax ester fatty alcohols in chronic blepharitis. Invest Ophthalmol Vis Sci. 1993;34:3515–3521 [PubMed] [Google Scholar]
- 15. Harvey DJ, Tiffany JM, Duerden JM, Pandher KS, Mengher LS. Identification by combined gas chromatography-mass spectrometry of constituent long-chain fatty acids and alcohols from the meibomian glands of the rat and a comparison with human meibomian lipids. J Chromatogr. 1987;414:253–263 [DOI] [PubMed] [Google Scholar]
- 16. Joffre C, Souchier M, Grégoire S, et al. Differences in meibomian fatty acid composition in patients with meibomian-gland dysfunction and aqueous-deficient dry eye. Br J Ophthalmol. 2008;92:116–119 [DOI] [PubMed] [Google Scholar]
- 17. Nichols KK, Ham BM, Nichols JJ, Ziegler C, Green-Church KB. Identification of fatty acids and fatty acid amides in human meibomian gland secretions. Invest Ophthalmol Vis Sci. 2007;48:34–39 [DOI] [PubMed] [Google Scholar]
- 18. Saville JT, Zhao Z, Willcox MD, Ariyavidana MA, Blanksby SJ, Mitchell TW. Identification of phospholipids in human meibum by nano-electrospray ionisation tandem mass spectrometry. Exp Eye Res. 2011;92:238–240 [DOI] [PubMed] [Google Scholar]
- 19. Chen J, Green-Church KB, Nichols KK. Shotgun lipidomic analysis of human meibomian gland secretions with electrospray ionization tandem mass spectrometry. Invest Ophthalmol Vis Sci. 2010;51:6220–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butovich IA, Uchiyama E, Di Pascuale MA, McCulley JP. Liquid chromatography-mass spectrometric analysis of lipids present in human meibomian gland secretions. Lipids. 2007;42:765–776 [DOI] [PubMed] [Google Scholar]
- 21. Butovich IA, Uchiyama E, McCulley JP. Lipids of human meibum: mass-spectrometric analysis and structural elucidation. J Lipid Res. 2007;48:2220–2235 [DOI] [PubMed] [Google Scholar]
- 22. Butovich IA. Cholesteryl esters as a depot for very long chain fatty acids in human meibum. J Lipid Res. 2009;50:501–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Butovich IA. On the lipid composition of human meibum and tears: comparative analysis of nonpolar lipids. Invest Ophthalmol Vis Sci. 2008;49:3779–3789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009;50:2471–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Butovich IA. Lipidomic analysis of human meibum using HPLC-MSn. Methods Mol Biol. 2009;579:221–246 [DOI] [PubMed] [Google Scholar]
- 26. Butovich IA, Millar TJ, Ham BM. Understanding and analyzing meibomian lipids: a review. Curr Eye Res. 2008;33:405–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robosky LC, Wade K, Woolson D, et al. Quantitative evaluation of sebum lipid components with nuclear magnetic resonance. J Lipid Res. 2008;49:686–692 [DOI] [PubMed] [Google Scholar]
- 28. Foulks GN, Borchman D, Yappert MC, Kim SH, McKay JW. Topical azithromycin therapy of meibomian gland dysfunction: clinical response and lipid alterations. Cornea. 2010;29:781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Foulks GN, Borchman D. Meibomian gland dysfunction: the past, the present, future. Eye Contact Lens. 2010;36:249–253 [DOI] [PubMed] [Google Scholar]
- 30. Oshima Y, Sato H, Zaghloul A, Foulks GN, Yappert MC, Borchman D. Characterization of human meibum lipid using Raman spectroscopy. Curr Eye Res. 2009;34:824–835 [DOI] [PubMed] [Google Scholar]
- 31. Borchman D, Foulks GN, Yappert MC, et al. Physical changes in human meibum with age as measured by infrared spectroscopy. Ophthalmic Res. 2010;44:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borchman D, Foulks GN, Yappert MC. Confirmation of changes in human meibum lipid infrared spectra with age using principal component analysis. Curr Eye Res. 2010;35:778–786 [DOI] [PubMed] [Google Scholar]
- 33. Borchman D, Foulks GN, Yappert MC. Changes in human meibum lipid with meibomian gland dysfunction using principal component analysis. Exp Eye Res. 2010;91:246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borchman D, Foulks GN, Yappert MC, et al. Human meibum lipid conformation and thermodynamic changes with meibomian-gland dysfunction. Invest Ophthalmol Vis Sci. 2011;52:3805–3817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Borchman D, Foulks GN, Yappert MC, Tang D, Ho DV. Spectroscopic evaluation of human tear lipids. Chem Phys Lipids. 2007;147:87–102 [DOI] [PubMed] [Google Scholar]
- 36. Borchman D, Foulks GN, Yappert MC, Ho DV. Temperature-induced conformational changes in human tear lipids hydrocarbon chains. Biopolymers. 2007;87:124–133 [DOI] [PubMed] [Google Scholar]
- 37. Huang L, Grami V, Marrero Y, et al. Human lens phospholipid changes with age and cataract. Invest Ophthalmol Vis Sci. 2005;46:1682–1689 [DOI] [PubMed] [Google Scholar]
- 38. Estrada R, Puppato A, Borchman D, Yappert MC. Re-evaluation of the phospholipid composition in membranes of adult human lenses by 31-P NMR and MALDI-MS. Biochim Biophys Acta Biomembr. 2010;1798:303–311 [DOI] [PubMed] [Google Scholar]
- 39. Ferguson-Yankey SR, Borchman D, Taylor KG, DuPre DB, Yappert MC. Conformational studies of sphingolipids by NMR spectroscopy. I. Dihydrosphingomyelin. Biochem Biophys Acta. 2000;1467:307–325 [DOI] [PubMed] [Google Scholar]
- 40. Borchman D, Byrdwell WC, Yappert MC. Regional and age-dependent differences in the phospholipid composition of human lens membranes. Invest Ophthalmol Vis Sci. 1994;35:3938–3942 [PubMed] [Google Scholar]
- 41. Byrdwell WC, Borchman D, Porter RA, Taylor KG, Yappert MC. Separation and characterization of the unknown phospholipid in human lens membranes. Invest Ophthalmol Vis Sci. 1994;35:4333–4343 [PubMed] [Google Scholar]
- 42. Merchant TE, Lass JH, Meneses P, Greiner JV, Glonek T. Human crystalline lens phospholipid analysis with age. Invest Ophthalmol Vis Sci. 1991;32:549–555 [PubMed] [Google Scholar]
- 43. Foulks GN, Bron AJ. Meibomian-gland dysfunction: a clinical scheme for description, diagnosis, classification and grading. Ocul Surf. 2003;1:17–36 [DOI] [PubMed] [Google Scholar]
- 44. Malinowski ER, Howery DG. Factor Analysis in Chemistry. New York: Wiley; 1980 [Google Scholar]
- 45. Malinowski ER. Theory of the distribution of error eigenvalues resulting from principal component analysis with applications to spectroscopic data. J Chemometrics. 1987;1:33–40 [Google Scholar]
- 46. Sutter JM, Kalivas JH, Lang PM. Which principal component to utilize for principal component regression. J Chemometrics. 1992;6:217–225 [Google Scholar]
- 47. Mathers WD., Lane JA. Meibomian gland lipids, evaporation, and tear film stability. Adv Exp Med Biol. 1998;438:349–360 [DOI] [PubMed] [Google Scholar]
- 48. McCulley JP, Shine WE. A compositional based model for the tear film lipid layer. Trans Am Ophthalmol Soc. 1997;95:79–93 [PMC free article] [PubMed] [Google Scholar]
- 49. Shine WE, McCulley JP, Pandya AG. Minocycline effect on meibomian gland lipids in meibomianitis patients. Exp Eye Res. 2003;76:417–420 [DOI] [PubMed] [Google Scholar]
- 50. Rujoi M, Borchman D, Vorobyov I, Estrada R, Yappert MC. Isolation and lipid characterization of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Invest Ophthalmol Vis Sci. 2003;44:1634–1642 [DOI] [PubMed] [Google Scholar]
- 51. Mantelli F, Tiberi E, Micera A, Lambiase A, Visintini F, Bonini S. MUC5AC overexpression in tear film of neonates. Graefes Arch Clin Exp Ophthalmol. 2007;245:1377–1381 [DOI] [PubMed] [Google Scholar]
- 52. Isenberg SJ, Del Signore M, Chen A, Wei J, Guillon J. The lipid layer and stability of the preocular tear film in newborns and infants. Ophthalmology. 2003;110:1408–1411 [DOI] [PubMed] [Google Scholar]
- 53. Bacher LF. Factors regulating eye blink rate in young infants. Optom Vis Sci. 2010;87:337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lawrenson JG, Birhah R, Murphy PJ. Tear-film lipid layer morphology and corneal sensation in the development of blinking in neonates and infants. J Anat. 2005;206:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sforza C, Rango M, Galante D, Bresolin N, Ferrario VF. Spontaneous blinking in healthy persons: an optoelectronic study of eyelid motion. Ophthalmic Physiol Opt. 2008;28:345–353 [DOI] [PubMed] [Google Scholar]
- 56. Cho P, Yap M. Age, gender, and tear break-up time. Optom Vis Sci. 1993;70:828–831 [DOI] [PubMed] [Google Scholar]
- 57. Mohidin N, Bay TC, Yap M. Non-invasive tear break-up time in normal Malays. Clin Exp Optom. 2002;85:37–41 [DOI] [PubMed] [Google Scholar]
- 58. Ozdemir M, Temizdemir H. Age- and gender-related tear function changes in normal population. Eye. 2010;24:79–83 [DOI] [PubMed] [Google Scholar]
- 59. Maïssa C, Guillon M. Tear film dynamics and lipid layer characteristics: effect of age and gender. Cont Lens Anterior Eye. 2010;33:176–182 [DOI] [PubMed] [Google Scholar]
- 60. Sun WS, Baker RS, Chuke JC, Rouholiman BR. Age-related changes in human blinks. Invest Ophthalmol Vis Sci. 1997;38:92–99 [PubMed] [Google Scholar]
- 61. Lavezzo MM, Schellini SA, Padovani CR. Eye blink in newborn and preschool-age children. Acta Ophthalmol. 2008;86:275–278 [DOI] [PubMed] [Google Scholar]
- 62. Craig J, Tomlinson A. Age and gender effects on the normal tear film. Adv Exp Med Biol. 1998;438:411–415 [DOI] [PubMed] [Google Scholar]
- 63. Henderson JW, Prough WA. Influence of age, sex on flow of tears. Arch Ophthalmol. 1950;43:224–231 [DOI] [PubMed] [Google Scholar]
- 64. Tsubota K, Hata S, Okusawa Y, Egami F, Ohtsuki T, Nakamori K. Quantitative videographic analysis of blinking in normal subjects and patients with dry eye. Arch Ophthalmol. 1996;114:715–720 [DOI] [PubMed] [Google Scholar]
- 65. Tsubota K. Tear dynamics and dry eye. Prog Retinal Eye Res. 1998;17:565–596 [DOI] [PubMed] [Google Scholar]
- 66. Tomlinson A, Khanal S. Assessment of tear film dynamics: quantification approach. Ocul Surf. 2005;3:81–95 [DOI] [PubMed] [Google Scholar]
- 67. Butovich IA, Wojtowicz JC, Molai M. Human tear film and meibum. Very long chain wax esters and (O-acyl)-omega-hydroxy fatty acids of meibum. J Lipid Res. 2009;50:2471–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ashraf Z, Pasha U, Greenstone V, et al. Quantification of human sebum on skin and human meibum on the eye lid margin using sebum tape, spectroscopy and chemical analysis. Curr Eye Res. 2011;36:553–562 [DOI] [PubMed] [Google Scholar]
- 69. Verardo G, Pagani E, Geatti P. A thorough study of the surface wax of apple fruits. Anal Bioanal Chem. 2003;376:659–667 [DOI] [PubMed] [Google Scholar]
- 70. Frost DJ, Barzilay J. Proton magnetic resonance identification of nonconjugated cis-unsatureated fatty acids and esters. Anal Chem. 1971;43:1316–1318 [Google Scholar]
- 71. Scano P, Marincola FC, Locci E, Lai A. 1H and 13C NMR studies of melon and head blubber of the striped dolphin (Stenella coeruleoalba). Lipids. 2006;41:1039–1048 [DOI] [PubMed] [Google Scholar]
- 72. Tulloch AP. Beeswax: structure of the esters and their component hydroxyl acids and diols. Chem Phys Lipids. 1971;6:235–265 [Google Scholar]
- 73. Wineburg JP, Swern D. NMR chemical shift reagents in structural determination of lipid derivatives: II. Methyl petroselinate and methyl oleate. J Am Oil Chem Soc. 1971;49:267–273 [DOI] [PubMed] [Google Scholar]
- 74. Ke PJ, Ackman RG, Hooper DL. NMR determination of wax esters in marine lipids. Anal Chem Acta. 1974;69:253–258 [Google Scholar]
- 75. Purcell JM, Morris SG, Susi H. Proton magnetic resonance spectra of unsaturated fatty acids. Anal Chem. 1966;38:588–592 [Google Scholar]
- 76. Johnson LF, Shoolery JN. Determination of unsaturation and average molecular weight of natural fats by nuclear magnetic resonance. Anal Chem. 1962;34:1136–1139 [Google Scholar]
- 77. Glass CA, Dutton HJ. Determination of beta-olefinic methyl groups in esters of fatty acids by nuclear magnetic resonance. Anal Chem. 1964;36:2401–2404 [Google Scholar]
- 78. Harlan JW. Applications of nuclear magnetic resonance spectroscopy in the fat and oil industry. J Am Oil Chem Soc. 1964;41:13–14 [Google Scholar]
- 79. Tulloch AP. Solvent effects on the nuclear magnetic resonance spectra of methyl hydroxysterates. J Am Oil Chem Soc. 1966;43:670–674 [Google Scholar]
- 80. Haywood RM, Claxson WD, Hawkes GE, et al. Detection of aldehydes and their conjugated hydroperoxydiene precursors in thermally-stressed culinary oils and fats: investigations using high resolution proton NMR spectroscopy. Free Radic Res. 1995;22:441–482 [DOI] [PubMed] [Google Scholar]
- 81. Miyake Y, Yokomizo K, Matsuzaki N. Determination of unsaturated fatty acid composition by high-resolution nuclear magnetic resonance spectroscopy. J Am Oil Chem Soc. 1998;75:1091–1094 [Google Scholar]
- 82. Aursand M, Rainuzzo JR, Grasdalen H. Quantitative high-resolution 13C and 1H nuclear magnetic resonance of ω3 fatty acids from white muscle of Atlantic salmon (Salmo salar). J Am Oil Chem Soc. 1993;70:971–981 [Google Scholar]
- 83. Guillén MD, Ruiz A. Oxidation process of oils with high content of linoleic acyl groups and formation of toxic hydroperoxy- and hydroxyalkenals. A study by 1H nuclear magnetic resonance. J Sci Food Agric. 2005;85:2413–2420 [Google Scholar]
- 84. Siddiqui N, Sim J, Silwood CJL, Toms H, Iles RA, Grootveld M. Multicomponent analysis of encapsulated marine oil supplements using high-resolution 1H and 13C NMR techniques. J Lipid Res. 2003;44:2406–2427 [DOI] [PubMed] [Google Scholar]