Biointegration, one of the important problems facing keratoprostheses, was addressed by coating a model material with hydroxyapatite. The coating enhanced cell viability in vitro and biointegration ex vivo and reduced inflammation in vivo.
Abstract
Purpose.
Integration of keratoprosthesis with the surrounding cornea is very important in preventing bacterial invasion, which may cause ocular injury. Here the authors investigated whether hydroxyapatite (HAp) coating can improve keratoprosthesis (KPro) biointegration, using polymethyl methacrylate (PMMA)—the principal component of the Boston KPro—as a model polymer.
Methods.
HAp coatings were induced on PMMA discs after treatment with concentrated NaOH and coating with poly-dopamine (PDA) or polydopamine and then with 11-mercaptoundecanoic acid (11-MUA). Coatings were characterized chemically (Fourier transform infrared spectroscopy [FTIR], energy dispersive X-ray spectroscopy [EDX]) and morphologically (SEM) and were used as substrates for keratocyte growth in vitro. Cylinders of coated PMMA were implanted in porcine corneas ex vivo for 2 weeks, and the force required to pull them out was measured. The inflammatory reaction to coated discs was assessed in the rabbit cornea in vivo.
Results.
FTIR of the coatings showed absorption bands characteristic of phosphate groups, and EDX showed that the Ca/P ratios were close to those of HAp. By SEM, each method resulted in morphologically distinct HAp films; the 11-MUA group had the most uniform coating. The hydroxyapatite coatings caused comparable enhancement of keratocyte proliferation compared with unmodified PMMA surfaces. HAp coating significantly increased the force and work required to pull PMMA cylinders out of porcine corneas ex vivo. HAp coating of implants reduced the inflammatory response around the PMMA implants in vivo.
Conclusions.
These results are encouraging for the potential of HAp-coated surfaces for use in keratoprostheses.
According to the World Health Organization, corneal disease accounted for 8 million cases of blindness in 2009.1 Corneal allograft surgery is often successful in such cases, but in some patients and conditions the success rate is low. In a large outcome study, only 20% of regrafts remained clear for 5 years2; more recent reports have confirmed this trend.3,4 With repeated allograft failure, few treatments are available, and tissue-engineered corneas are not yet suitable for clinical use. Corneal prostheses (keratoprosthesis; KPro) are the only viable option for restoring sight.
There are several KPros on the global market that vary in terms of indications, surgical complexity regarding placement, and outcomes. The primary material used in the optic axis of many devices is polymethylmethacrylate (PMMA) because of its transparency, high mechanical strength, ease of processing, and low cost. Firm bonding of any KPro to the surrounding cornea is clinically critical; it is the main challenge in the development of most keratoprostheses. For example, in the case of the Boston KPro, which is relatively simple to construct and implant and which continues to have improved outcomes and expanded indications thanks to design enhancements and optimized postoperative management,5,6 the poor adhesion of PMMA to the surrounding tissue creates opportunities for bacteria to enter the eye, resulting in infection, leakage of aqueous humor, and even extrusion of the implant.7,8
Hydroxyapatite (HAp) is a main component of bone and teeth and has been widely used for surface modification of bone implants9 because it can bind electrostatically with charged biological molecules.10,11 HAp induces a relatively limited inflammatory and foreign body response12 and supports the adhesion and growth of human keratocytes (corneal fibroblasts) better than glass, polytetrafluoroethylene, and polyhydroxymethacrylate in vitro.13 These properties make it a desirable material for use in a KPro. However, the poor mechanical properties of bulk HAp ceramics make them challenging to work with.
Here we have developed or adapted a variety of related approaches to coating PMMA with HAp and assessed their effectiveness in enhancing integration with the cornea. HAp coating can be achieved through biomineralization using simulated body fluid (SBF).14–16 Deposition on PMMA can be enhanced by treatment with highly concentrated sodium hydroxide (NaOH), which produces negatively charged carboxyl groups on the surface.17 Coating with polydopamine, which can form on a wide range of surfaces,18 can also induce HAp deposition on various substrates in SBF.19 We have hypothesized that further coating the polydopamine-modified PMMA discs with 11-mercaptoundecanoic acid (11-MUA) would enhance apatite deposition. The carboxyl groups of 11-MUA can act as nucleation sites for Ca/P deposition.20 We have characterized these HAp-coated PMMAs in vitro, alone and in the presence of corneal fibroblasts. In addition to the cell-based experiments commonly performed in studies of biointegration, we have used a custom-made 3D printed device to determine whether coating with HAp improves the mechanical strength of the tissue interaction ex vivo. Finally, we studied the effect of coating with HAp on biocompatibility in vivo.
Materials and Methods
Preparation of SBF
SBF at 1.5× the concentration of regular SBF (1.5× SBF) was prepared as reported14,21 and was stored at 4°C.
Coating of the PMMA Implants with HAp
PMMA discs (JG Machine Co. Inc, Woburn, MA) 10 mm in diameter and 1 mm thick were used as substrates for all in vitro experiments. The discs were washed with 70% ethanol solution and with deionized water in an ultrasonic bath then blow-dried in air. Three different methods were used to form HAp films on PMMA discs. In the first group, PMMA discs were immersed in 5M NaOH solution at room temperature for 10 minutes before they were placed into 1.5× SBF solution. In the second group, PMMA discs were immersed in a dilute aqueous solution of dopamine (2 mg/mL dopamine in 10 mM Tris buffer, pH 8.5) overnight before they were placed in 1.5× SBF solution. In the third group, the discs were immersed in dopamine solution overnight, followed by immersion in 11-MUA solution (50 μM in sodium phosphate buffer, pH 7.8) for 4 hours before they were placed in the 1.5× SBF solution. All samples were incubated in 1.5× SBF at 37°C for 14 days.21 The 1.5× SBF solution was replenished every 24 hours. After 14 days, the discs were removed from the 1.5× SBF solution, washed in 100 mL deionized water, and dried.
Fourier Transform Infrared Spectroscopy
Fourier transform infrared spectroscopy (FTIR; Bruker Alpha FTIR spectrophotometer; Bruker Corporation, Billerica, MA) was used to determine the functional groups on HAp coatings. Each sample was scanned over the range of 400 to 4000 cm−1 with a resolution of 4 cm−1. At least three samples per group were examined by FTIR; results between samples in the same group were essentially identical.
Scanning Electron Microscopy
The surface morphology of the discs was captured by scanning electron microscopy (SEM; JEOL 5600LV; JEOL Inc., Peabody, MA). All samples were coated with a thin film of gold-palladium before imaging.
Energy Dispersive X-Ray Spectroscopy Analysis
The elemental compositions of samples were analyzed with energy dispersive x-ray spectroscopy (FEI/Philips XL30FEG SEM; Philips Electric, Eindhoven, The Netherlands).
Culture of Keratocytes
Human corneal fibroblasts (a generous gift of James Zieske at Schepens Eye Institute, Harvard Medical School) from the fifth generation in the exponential phase of growth were used for cytotoxicity tests. Cells were grown to confluence in minimum essential medium (MEM; Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen), penicillin, and streptomycin (100 U/mL; Invitrogen) at 37°C in a humidified atmosphere containing 5% CO2.
Cell Culture and Assays
PMMA discs, either HAp-coated or noncoated, were placed in wells in a 24-well plate. Subsequently, 1 × 104 cells were seeded per well in MEM and allowed 24 hours to attach.
A viability/cytotoxicity assay using calcein-AM and ethidium homodiner-1 (EthD-1; Molecular Probes, Invitrogen) was performed on each of the test materials at 1, 4, and 7 days. Discs were washed with Dulbecco's phosphate-buffered saline (DPBS) and stained for 10 minutes at room temperature with DPBS containing 2 mM calcein-AM and 4 mM ethidium-homodimer-1. Cells were counted manually in five different high-powered fields on each of three discs in each group.
Mechanical Testing of Biointegration of Test Materials
Porcine eyes were obtained from a local abattoir, transported to the laboratory on ice in a moisture chamber, and processed for culture within 24 hours of animal death. The pig cornea was trephined with an 8-mm diameter blade (Acuderm Inc., Fort Lauderdale, FL). A 3-mm hole was punched through the center of the cornea, allowing the graft to be subsequently slid over a 3.35-mm diameter PMMA cylinder. The assembly was then placed individually in a 12-well culture plate filled with 2 mL Chen cornea organ culture medium22 consisting of penicillin-streptomycin (Modified Medium 199; Invitrogen), 5% dextran, and 25 mM Hepes, D,L-β-hydroxybutyrate (Sigma-Aldrich Corp., St. Louis, MO). The epithelium of the cornea was barely submerged in the medium. Specimens were incubated at 37°C in 5% carbon dioxide. Medium was changed every 24 hours.
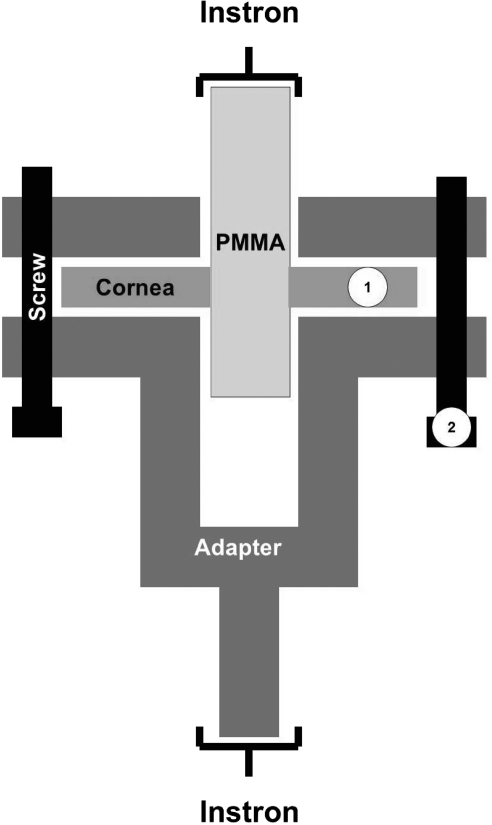
After 14 days, the specimens were removed from cultured media and placed in a custom-designed adapter (Fig. 1) to fit a universal testing machine (model 5542; Instron, Norwood, MA). In the adapter, the corneas were contained in a cavity between two plastic (acrylonitrile butadiene styrene; ABSPlus; Dimension Inc., Eden Prairie, MN) parts, while the PMMA cylinders protruded from the top of the adapter. Two screws maintained a fixed spacing (500–1500 μm) between the upper and lower parts of the adapter so the cornea would not be compressed. The adapter was produced by a printer (1200es 3D; Dimension Inc.). The Instron device grasped the adapter at the base and pulled on the cylinder from above at a rate of 5 mm/min and stopped when the PMMA cylinder was pulled out completely. The maximum load during testing was defined as maximum failure load.
Figure 1.
Schematic of the apparatus used for mechanical testing of corneal adhesion strength. ①, space for the cornea; ②, screws to maintain a fixed spacing (500–1500 μm) between the upper and lower parts of the adapter so as not to compress the cornea.
The PMMA cylinder was pulled out of the cornea at a constant velocity (10 mm/min), and the force required to maintain the constant pull-out velocity was measured, as described. The work required to pull cylinders out of the curve was calculated from the area under the force-displacement curves for that procedure.
After mechanical testing, the extracted cylinders were placed in 10% formalin, coated with platinum, and processed for scanning electron microscopic examination.
In Vivo Experiments
All animal studies were approved by the Administrative Panel on Laboratory Animal Care of the Massachusetts Eye and Ear Infirmary and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Six adult New Zealand rabbits, each weighing 3.5 to 5.5 kg, were used (one disc in one eye per animal).
Anesthesia was induced by intramuscular injections of ketamine 35 mg/kg and xylazine 5 mg/kg. Surgeries were performed on the right eye of each rabbit. Once the rabbits were anesthetized, proparacaine ophthalmic solution was given topically. A lamellar cornea pocket was made with a knife (Crescent; Katena Products Inc., Denville, NJ) to approximately two-thirds the thickness of the cornea to accommodate the discs of test material, whose diameters were 5 mm and whose thicknesses were 0.1 mm. The discs were inserted with forceps into the corneal lamellar pocket. Because of the superior position in the cornea under the eyelid, the flap was not sutured. There were two groups—plain PMMA and PMMA modified with HA (using the poly-dopamine and 11-MUA method)—and three rabbits in each group. Follow-up clinical examinations included slit-lamp examination to assess inflammatory reaction and neovascularization. After 1 month, the rabbits were euthanatized, and the corneal specimens were processed for histology (hematoxylin-eosin staining) and analyzed by light microscopy.
Statistical Analysis
In general, data are presented as mean ± SD and were analyzed by two-way analysis of variance (ANOVA) with F-tests used to assess treatment and time effects on live and dead cell counts. Differences in mechanical testing were tested for significance by the unpaired t-test. For all statistical tests, two-tailed values of P < 0.05 were considered statistically significant, and a Bonferroni adjustment was used for multiple group comparisons in comparing live and dead cells between HAp-treated and untreated PMMA discs. Statistical analysis was performed with the SPSS software package (version 18.0; SPSS Inc./IBM, Chicago, IL).
Results
Characterization of the Modified PMMA Surfaces
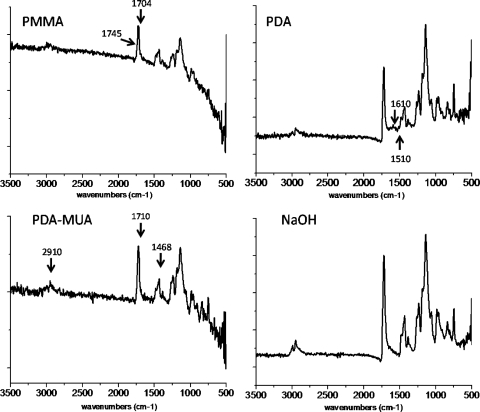
PMMA discs were treated with NaOH, polydopamine, or polydopamine and 11-MUA. The FTIR spectra demonstrated characteristic changes to the PMMA discs (Fig. 2). The unmodified PMMA showed a peak from 1700 to 1750 cm−1, which was overlapped by the peak at 1704 cm−1 (due to out-of-plane C=O stretching from the carbonyl group in PMMA dimerized by hydrogen bonding) and at 1745 cm−1 (C=O stretching of the carbonyl units that are not hydrogen bonded).23 The FTIR spectrum of dopamine-coated PMMA showed new peaks at 1610 cm−1 (the overlap of C-C resonance vibrations from the phenyl group from dopamine and N-H bending vibrations) and 1510 cm−1 (N-H shearing vibrations from the amine group in dopamine), indicating the presence of dopamine on the surface.24 11-MUA–modified polydopamine/PMMA showed the characteristic features of the alkyl chain in 11-MUA, the coexistence of the absorption band around 2910 cm−1 (from the asymmetric and symmetric stretch vibrations of the CH2 group) and the peak at 1468 cm−1 (from the absorption band of the deformation vibration of the CH2 group). Another important feature was the C=O stretching band of the carboxylic acid group from 11-MUA at 1710 cm−1. Immersion in NaOH did not change the FTIR spectrum, possibly because the absorbance of the carboxylic acid group (HOOC−, formed by the cleavage of a methyl group from PMMA by NaOH) overlapped with the ester group in PMMA.25
Figure 2.
FTIR spectra of pristine PMMA, PMMA-polydopamine (PDA), PMMA-polydopamine plus 11-MUA (PDA-MUA), and PMMA-NaOH (NaOH) before immersion in SBF solution.
Characterization of the HAp-Coated PMMA Surfaces
Morphology.
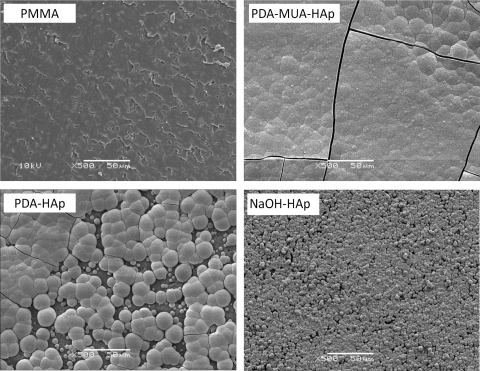
The discs described were coated with HAp by immersion in 1.5× SBF for 2 weeks. SEM (Fig. 3) of untreated PMMA discs showed no calcium phosphate growth. All the treated discs (NaOH, polydopamine, polydopamine and 11-MUA) were covered with tightly agglomerated crystallite. The coating of discs treated with polydopamine + 11-MUA was smoother than in the NaOH group and showed a more continuous coating than with the polydopamine method.
Figure 3.
SEM micrographs displaying surface morphology of PMMA disks after 2 weeks of immersion in 1.5× SBF. Pristine PMMA (PMMA), PMMA-polydopamine (PDA-HAp), PMMA-polydopamine plus 11-MUA (PDA-MUA-HAp), and PMMA-NaOH (NaOH-HAp). Labels indicate disc treatment before SBF. Scale bars, 50 μm.
Surface Chemistry.
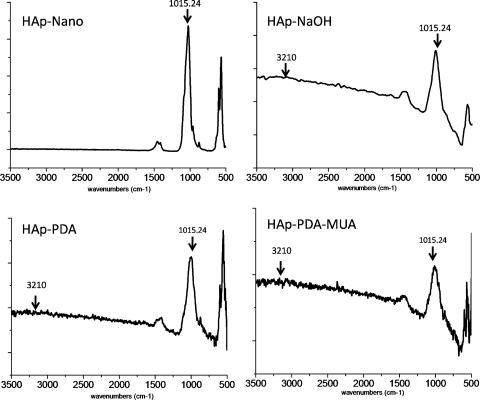
FTIR (Fig. 4) demonstrated characteristic features of HAp on the surfaces of all three coated modified PMMAs. Specifically, these were the IR spectral band around 1015.24 cm−1, which can be assigned to V3 stretching of P=O and P-O bonds in PO4−3,26,27 and to a very small peak at 3210 cm−1, which is attributable to stretching of the O-H bond in the OH group of the apatite.
Figure 4.
FTIR spectra of hydroxyapatite nanoparticles, PMMA-polydopamine (PDA), PMMA-polydopamine plus 11-MUA (PDA-MUA), and PMMA-NaOH after immersion in 1.5× SBF for 2 weeks. Compare with PMMA alone in Figure 1.
Elemental Analysis.
Study of the elemental composition by EDX of all three coated surfaces showed almost identical atomic ratios (1.63) of calcium to phosphorous that were close to the theoretical ratio for HAp (1.67). In contrast, only trace amounts of calcium or phosphorus signals were detected from PMMA surfaces.
Cytotoxicity
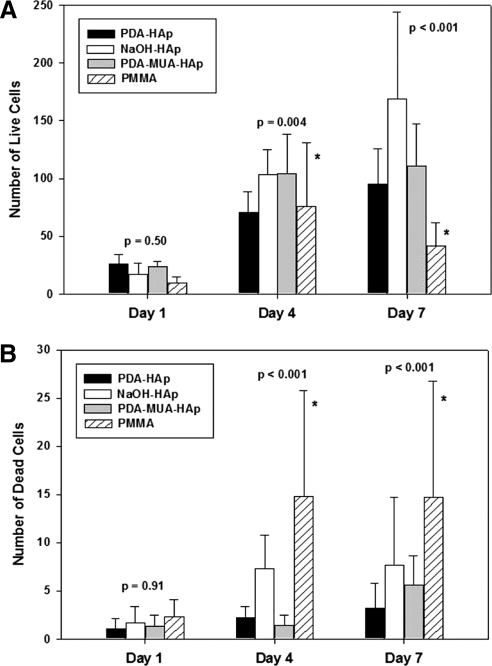
Corneal fibroblasts were cultured on HAp-coated discs of treated or untreated PMMA (n = 3 in each group), and cell viability was measured at predetermined intervals. There was no statistically significant difference in live cells between treated and untreated PMMA discs on day 1 (P = 0.50). ANOVA revealed overall group differences on day 4 (P = 0.004), with Bonferroni comparisons indicating significantly higher cell viability for NaOH-treated (P = 0.02) and PDA-MUA treated (P = 0.015) discs than for pure PMMA. On day 7 higher cell counts were observed on all Hap-coated samples compared with bare PMMA (all P < 0.001) (Fig. 5A). ANOVA indicated that live cell counts steadily increased between days 1 and 7 in all HAp groups (P < 0.001), whereas the number of cells decreased between days 4 and 7 on PMMA surfaces. Among HAp groups, NaOH-treated samples had a slightly higher average number of live cells (P < 0.05 compared with polydopamine + 11-MUA), albeit with a wide range.
Figure 5.
Cytotoxicity PMMA discs and their hydroxyapatite coatings. Live (A) and dead (B) dead cell counts. Data are means ± SD. n = 3 in each group. Note the difference in y-axis scales. (A, asterisks) Significantly less cell viability in unmodified PMMA than NaOH and PDA-MUA treatments on day 4 and significantly less live cell counts in PMMA compared with all HAp-treated discs on day 7. (B, asterisks) Higher dead cell counts on PMMA coatings compared with all HAp-treated coatings on days 4 and 7. P-values are provided in the text.
ANOVA indicated no differences in dead cell counts between treated and untreated discs on day 1 (P = 0.91). On days 4 and 7, dead cell counts were significantly higher on PMMA discs than on HAp-treated discs (all P < 0.001) (Fig. 5B). Mean numbers of dead cells were not significantly different between the HAp-coated discs on days 4 and 7.
Mechanical Integration
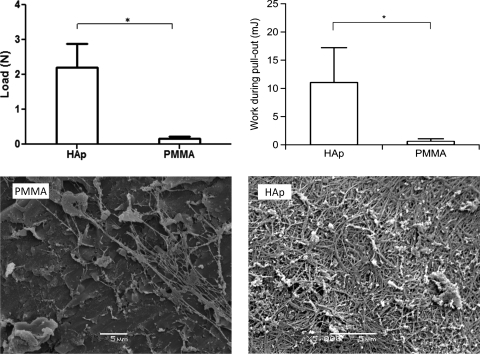
PMMA cylinders or HAp-coated PMMA cylinders were implanted in holes punched in porcine corneas. For the coated group, the polydopamine + 11-MUA group was chosen because it showed the most uniform HAp coating (Fig. 3). The corneas, with implanted cylinders, were maintained in organ culture for 2 weeks, after which corneas in both groups appeared similarly slightly swollen. The corneas were mounted in a custom-made device (Fig. 1), and the force required to pull the cylinders out of the corneas was measured (Fig. 6). The force required to pull a HAp-coated PMMA cylinder out of a cornea was 14.7 times greater than for an uncoated PMMA disc; the work performed to pull it out was 19 times greater. SEM of the cylinder surface after withdrawal from the cornea showed increased residual corneal tissue on the HAp-coated surfaces compared with plain PMMA.
Figure 6.
Top: force or work required to pull PMMA cylinders (uncoated or coated with HAp from the polydopamine + 11-MUA group) out of porcine corneas. Data are means ± SD. n = 4. *P < 0.05 for load; *P < 0.01 for work. Bottom: SEM images of cylinder surfaces after withdrawal from a cornea. Scale bar, 5 μm. The area shown for PMMA is particularly dense; that for HAp is representative.
Biocompatibility
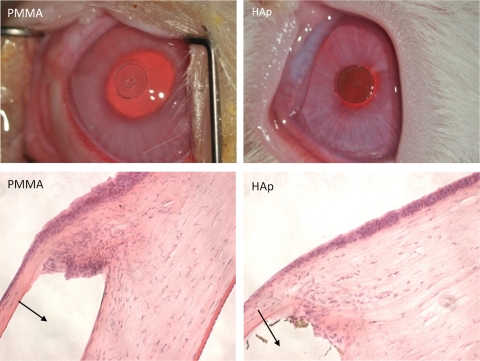
PMMA discs 3 mm in diameter, pristine or coated with HAp (polydopamine + 11-MUA group), were implanted in the lamellae of the corneas of New Zealand rabbits. The implanted discs can be seen clearly after the surgery (Fig. 7). On gross examination, there were inflammatory reactions 7 to 10 days after surgery, including conjunctival vascular congestion, mild local cornea edema, and local neovascularization, which disappeared after 2 weeks. Histology 1 month after implantation revealed a more vigorous inflammatory/foreign body response to the unmodified PMMA disc than to HAp-coated ones (n = 3 in each group).
Figure 7.
Top: representative photographs of discs on postoperative day 7. Scale bar, 3 mm. Bottom: photomicrographs of hematoxylin- eosin–stained histologic sections 1 month after implantation. Arrows: location of the implant in the histologic samples. Scale bars, 200 μm. The HAp-coated discs were from the polydopamine + 11-MUA group.
Discussion
The Boston KPro, which is made primarily of PMMA is, as mentioned, vulnerable to infection. The device spans the cornea from the surface to the anterior chamber and is loosely held in place by corneal tissue. In many instances, anecdotally, sudden intraocular infection (endophthalmitis) can rapidly occur, especially after contact lens application or other manipulation that might push a bolus of bacteria into the eye. Bacterial endophthalmitis could result in total destruction and blindness of the eye. Such a calamity can happen particularly in autoimmune diseases (e.g., Stevens-Johnson syndrome, ocular pemphigoid, atopy) in which there is a tendency for tissue to melt that can lead to leakage around the KPro. Prophylaxis with topical antibiotics is very helpful28 but not completely protective. Additionally, poor compliance with such a regimen exacerbates the problem.
For all these reasons, any method that can enhance the adhesion of corneal tissue around the KPro on a long-term basis might reduce the incidence of infection. Alteration of the surface of the device to allow better biointegration could be of clinical benefit. Here we have modified the PMMA surface with HAp, which is widely used in orthopedic surgery and which has been proposed at least twice before as a KPro coating. Thus, Leon et al.29 implanted a penetrating type of KPro that was coated by coral HAp in a patient. Brittleness of the coral was cited as a problem, but it seems otherwise to have been well tolerated. Mehta et al.,13 when testing surfaces of various materials in tissue culture, found that HAp promoted superior keratocyte adhesion and proliferation.
We have modified a polymeric surface (PMMA) with HAp using three different approaches. The first was a previously reported method of forming HAp on PMMA by treatment with a concentrated base.17 The second was also a PMMA coating with polydopamine, and we used it to enhance HAp deposition.19 Deposition of HAp on polydopamine is initiated by the strong binding between calcium ion and the catechol group of dopamine.19,30 Polydopamine-based methods are advantageous in that they can be easily applied to numerous other substrates, because of the adhesive properties of polydopamine.18 The third method was an alternative approach. We formed a coating with polydopamine, which then readily binds the thiol group in 11-MUA.20 We added 11-MUA to polydopamine, resulting in the most uniform HAp coating on PMMA. The addition of 11-MUA might have further facilitated the HAp deposition process through the additional ionic interaction between calcium ions and the carboxyl groups on 11-MUA.
Biointegration involves the establishment of seamless physical connections between the tissue and the implant, which results in an increase in mechanical binding strength. Mechanical binding strength between the tissue and the implant has been used as an important measure of biointegration in orthopedic implants.31 However, in the case of prosthetic devices that interface with soft tissues (e.g., keratoprostheses), mechanical binding has not received much attention. Here, we have developed a simple method to measure the mechanical binding strength of an implant to the cornea. All HAp coatings not only enhanced cell viability on PMMA but also greatly increased the mechanical strength of binding of PMMA to corneal tissue ex vivo. This may be of benefit in preventing extrusion, particularly in response to trauma or transient increases in intraocular pressure. Ideally, this will reflect better tissue integrity and will translate to a lesser risk of bacterial translocation into the eye. PMMA itself is widely used and well tolerated as a KPro material; the fact that HAp coating did not adversely affect biocompatibility in vivo and appeared to reduce the inflammatory reaction to the PMMA is reassuring.
An important question is whether tissue adherence to substrates is caused by direct cell adhesion or by substrate interaction with the extracellular matrix (ECM), or both. A hybrid view is that the cells are crucial, though primarily insofar as they produce ECM. Here, we observed improved cell survival (which may require improved adhesion, but we did not demonstrate that rigorously such as by atomic force microscopy) in HAp-coated PMMA discs along with increased strength of adhesion. This study was not designed to address the contributions to adhesive properties from the ECM or how they compare with those from cells. It is also difficult to extrapolate findings from published reports because these mostly address integration with hard tissues such as bone and teeth.32 It is possible that the enhanced performance of the coated discs was caused by the accretion to HAp of proteins such as growth factors that promote cell adherence and ECM synthesis.33–35 Although the mechanism is unknown, these results are encouraging regarding the potential clinical applicability of HAp coatings in keratoprostheses. The technique shown here of measuring adhesion between tissue and KPro and thereby quantifying biointegration could be further applicable to a range of surfaces, including other inorganic compounds and metals as well as varying surface characteristics such as roughness and porosity. Findings of increased mechanical binding might well translate to tighter tissue adherence to the KPro and thereby reduce the risk for devastating infection. HAp coating of the Boston KPro stem now seems ready for clinical testing.
In conclusion, PMMA discs coated with HAp greatly improved cell viability, implant adhesion to tissue, and biocompatibility compared with unmodified PMMA.
Footnotes
Supported by National Institute of General Medical Sciences Grant GM073626 (DSK) and the Boston KPro Fund, Massachusetts Eye and Ear Infirmary (DSK).
Disclosure: L. Wang, None; K.J. Jeong, None; H.H. Chiang, None; D. Zurakowski, None; I. Behlau, None; J. Chodosh, None; C.H. Dohlman, None; R. Langer, None; D.S. Kohane, None
References
- 1. World Health Organization 10 Facts about Blindness and Visual Impairment. Geneva, Switzerland: WHO; 2011. http://www.who.int/features/factfiles/blindness/en/index.html Accessed August 20, 2011 [Google Scholar]
- 2. Dandona L, Naduvilath TJ, Janarthanan M, Ragu K, Rao GN. Survival analysis and visual outcome in a large series of corneal transplants in India. Br J Ophthalmol. 1997;81(9):726–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bersudsky V, Blum-Hareuveni T, Rehany U, Rumelt S. The profile of repeated corneal transplantation. Ophthalmology. 2001;108(3):461–469 [DOI] [PubMed] [Google Scholar]
- 4. Thompson RW, Jr, Price MO, Bowers PJ, Price FW., Jr Long-term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110(7):1396–1402 [DOI] [PubMed] [Google Scholar]
- 5. Dohlman CH, Dagher MH, Khan BF, Sippel K, Aquavella JV, Graney JM. Introduction to the use of the Boston keratoprosthesis. Expert Rev Ophthalmol. 2006;1(1):41–48 [Google Scholar]
- 6. Aldave AJ, Kamal KM, Vo RC, Yu F. The Boston type I keratoprosthesis: improving outcomes and expanding indications. Ophthalmology. 2009;116(4):640–651 [DOI] [PubMed] [Google Scholar]
- 7. Nouri M, Terada H, Alfonso EC, Foster CS, Durand ML, Dohlman CH. Endophthalmitis after keratoprosthesis: incidence, bacterial causes, and risk factors. Arch Ophthalmol. 2001;119(4):484–489 [DOI] [PubMed] [Google Scholar]
- 8. Garcia JP, Jr, de la Cruz J, Rosen RB, Buxton DF. Imaging implanted keratoprostheses with anterior-segment optical coherence tomography and ultrasound biomicroscopy. Cornea. 2008;27(2):180–188 [DOI] [PubMed] [Google Scholar]
- 9. de Groot K, Geesink R, Klein CP, Serekian P. Plasma sprayed coatings of hydroxylapatite. J Biomed Mater Res. 1987;21(12):1375–1381 [DOI] [PubMed] [Google Scholar]
- 10. Colman A, Byers MJ, Primrose SB, Lyons A. Rapid purification of plasmid DNAs by hydroxyapatite chromatography. Eur J Biochem. 1978;91(1):303–310 [DOI] [PubMed] [Google Scholar]
- 11. Schroder E, Jonsson T, Poole L. Hydroxyapatite chromatography: altering the phosphate-dependent elution profile of protein as a function of pH. Anal Biochem. 2003;313(1):176–178 [DOI] [PubMed] [Google Scholar]
- 12. Ohgushi H, Goldberg VM, Caplan AI. Heterotopic osteogenesis in porous ceramics induced by marrow cells. J Orthop Res. 1989;7(4):568–578 [DOI] [PubMed] [Google Scholar]
- 13. Mehta JS, Futter CE, Sandeman SR, et al. Hydroxyapatite promotes superior keratocyte adhesion and proliferation in comparison with current keratoprosthesis skirt materials. Br J Ophthalmol. 2005;89(10):1356–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kokubo T, Kushitani H, Sakka S, Kitsugi T, Yamamuro T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J Biomed Materials Res. 1990;24(6):721–734 [DOI] [PubMed] [Google Scholar]
- 15. Mao CB, Li HD, Cui FH, Ma CL, Feng QL. Oriented growth of phosphates on polycrystalline titanium in a process mimicking biomineralization. J Crystal Growth. 1999;206(4):308–321 [Google Scholar]
- 16. Oyane A, Kim HM, Furuya T, Kokubo T, Miyazaki T, Nakamura T. Preparation and assessment of revised simulated body fluids. J Biomed Materials Res A. 2003;65A(2):188–195 [DOI] [PubMed] [Google Scholar]
- 17. Tanahashi M, Yao T, Kokubo T, et al. Apatite coated on organic polymers by biomimetic process-improvement in its adhesion to substrate by NaOH treatment. J Appl Biomater. 1994;5(4):339–347 [DOI] [PubMed] [Google Scholar]
- 18. Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryu J, Ku SH, Lee H, Park CB. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv Functional Materials. 2010;20(13):2132–2139 [Google Scholar]
- 20. Tanahashi M, Matsuda T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J Biomed Materials Res. 1997;34(3):305–315 [DOI] [PubMed] [Google Scholar]
- 21. Kokubo T, Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27(15):2907–2915 [DOI] [PubMed] [Google Scholar]
- 22. Chen CH, Rama P, Chen SC, Sansoy FN. Efficacy of organ preservation media enriched with nonlactate-generating substrate for maintaining tissue viability: a transplantation study. Transplantation. 1997;63(5):656–663 [DOI] [PubMed] [Google Scholar]
- 23. Nyquist RA, Platt AE, Priddy DB. Infrared studies of styrene-acrylic acid and styrene-acrylamide copolymers at variable temperature. Appl Spectrosc. 1982;36(4):417–420 [Google Scholar]
- 24. Jiang JH, Zhu LP, Li XL, Xu YY, Zhu BK. Surface modification of PE porous membranes based on the strong adhesion of polydopamine and covalent immobilization of heparin. J Membr Sci. 2010;364(1–2):194–202 [Google Scholar]
- 25. Kang IK, Kwon BK, Lee JH, Lee HB. Immobilization of proteins on poly (methyl methacrylate) films. Biomaterials. 1993;14(10):787–792 [DOI] [PubMed] [Google Scholar]
- 26. He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Materials. 2003;2(8):552–558 [DOI] [PubMed] [Google Scholar]
- 27. Antonakos A, Liarokapis E, Leventouri T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials. 2007;28(19):3043–3054 [DOI] [PubMed] [Google Scholar]
- 28. Durand ML, Dohlman CH. Successful prevention of bacterial endophthalmitis in eyes with the Boston keratoprosthesis. Cornea. 2009;28(8):896–901 [DOI] [PubMed] [Google Scholar]
- 29. Leon CR, Barraquer JI, Jr, Barraquer JI., Sr Coralline hydroxyapatite keratoprosthesis: first human case. Ann Inst Barraquer (Barc). 2001;30:4 [Google Scholar]
- 30. Holten-Andersen N, Mates TE, Toprak MS, Stucky GD, Zok FW, Waite JH. Metals and the integrity of a biological coating: the cuticle of mussel byssus. Langmuir. 2009;25(6):3323–3326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petrie TA, Raynor JE, Reyes CD, Burns KL, Collard DM, Garcia AJ. The effect of integrin-specific bioactive coatings on tissue healing and implant osseointegration. Biomaterials. 2008;29(19):2849–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jeong KJ, Kohane DS. Surface modification and drug delivery for biointegration. Ther Deliv. 2011;2:737–752 [DOI] [PubMed] [Google Scholar]
- 33. Begley CT, Doherty MJ, Mollan RA, Wilson DJ. Comparative study of the osteoinductive properties of bioceramic, coral and processed bone graft substitutes. Biomaterials. 1995;16(15):1181–1185 [DOI] [PubMed] [Google Scholar]
- 34. Labat B, Chamson A, Frey J. Effects of gamma-alumina and hydroxyapatite coatings on the growth and metabolism of human osteoblasts. J Biomed Mater Res. 1995;29(11):1397–1401 [DOI] [PubMed] [Google Scholar]
- 35. Kilpadi KL, Chang PL, Bellis SL. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J Biomed Mater Res. 2001;57(2):258–267 [DOI] [PubMed] [Google Scholar]